Abstract
Over the latest decade, the role of microRNAs (miRNAs/miRs) has received more attention. miRNAs are small non-coding RNAs that may serve a role as oncogenes or tumor suppressor genes. Certain miRNAs regulate the apoptosis pathway by influencing pro- or anti-apoptotic genes. We hypothesized that increases in the expression of B cell lymphoma 2 (BCL2) and BCL2-like 1 (BCL2L1) genes, which have been reported in various types of cancer tissues, may be due to the downregulation of certain miRNAs. The present study aimed to identify miRNAs that target BCL2 and BCL2L1 anti-apoptotic genes in prostate cancer (PCa) clinical tissue samples. Certain candidate miRNAs were selected bioinformatically and their expression in PCa samples was analyzed and compared with that in benign prostatic hyperplasia (BPH) tissue samples. The candidate miRNAs that targeted BCL2 and BCL2L1 genes were searched in online databases (miRWalk, microRNA.org, miRDB and TargetScan). A total of 12 miRNAs that target the 3′-untranslated region of the aforementioned genes and/or for which downregulation of their expression has previously been reported in cancer tissues. A total of 30 tumor tissue samples from patients with PCa and 30 samples tissues from patients with BPH were obtained and were subjected to reverse transcription-quantitative polymerase chain reaction for expression analysis of 12 candidate miRNAs, and the BCL2 and BCL2L1 genes. Additionally, expression of 3 finally selected miRNAs and genes was evaluated in prostate cancer PC3 and DU145 cell lines and human umbilical vein endothelial cells. Among 12 miRNA candidates, the expression of miR-1266, miR-185 and miR-30c-2 was markedly downregulated in PCa tumor tissues and cell lines. Furthermore, downregulation of these miRNAs was associated with upregulation of the BCL2 and BCL2L1 genes. An inverse association between three miRNAs (miR-1266, miR-185 and miR-30c-2) and two anti-apoptotic genes (BCL2 and BCL2L1) may be considered for interventional miRNA therapy of PCa.
Keywords: microRNA 1266-5p, microRNA 185-5p, miR-30c-2, prostate cancer, tumor suppressor
Introduction
Prostate Cancer (PCa) is the most common type of cancer in men >40 years of age (1). Over the past decade, the age at mortality and the rate of mortality caused by PCa have declined. After lung cancer, PCa is the second leading cause of cancer-associated mortality in men (2). In 2015, the American Cancer Society announced that >220,000 men were diagnosed with PCa; it was anticipated that 25,000 of these men would succumb as a result of their cancer (3).
An increase in the serum level of prostate-specific antigen (PSA) or an abnormal digital rectal examination result may be warning signs for PCa. There is no general consensus on the normal PSA level in men of different ages and therefore, its use remains undetermined for an accurate and early diagnosis (4). One of the main difficulties in treating PCa is the resistance of cancer cells to current therapy methods (5).
MicroRNAs (miRNAs/miRs) are non-coding ribonucleic acids, 18–25 nucleotides in length, that are highly conserved across species. These molecular structures are involved in controlling physiological and pathological cellular processes. miRNAs primarily bind to the 3′-untranslated region (3′-UTR) of target mRNA(s) through complementary base pairing, which will cause degradation of mRNA or halt its translation (6–8).
As of yet, as demonstrated in the miRBase (www.mirbase.org), >21,264 miRNAs have been identified. With regards to cancer, miRNAs may be oncogenic or tumor suppressors. Therefore, it is reasonable to assume that cancer may be controlled by altering the expression of miRNAs (9).
In recent decades, the role of miRNAs has been studied in numerous types of human and animal cancer. The expression of miRNAs has been studied in cancerous and healthy cells, and different resulting profiles have revealed that miRNAs may control certain cell cycle pathways, including apoptosis (10–12). Defective apoptosis pathways may be considered one of the most important features of cancer cells (13). At present, two main pathways for apoptosis have been discovered in cells. The extrinsic pathway involves receptors [first apoptosis signal receptor (FAS), complement receptor 4/5 (CR4/5), and tumor necrosis factor receptor (TNFR)] on the cell surface, which, after binding with their specific ligands (FAS ligand, TNF-related apoptosis-inducing ligand and threose nucleic acid), oligomerize the FAS-associated death domain (FADD) and activate apoptosis (14).
The second pathway is a mitochondrial pathway that is regulated by B cell lymphoma 2 (BCL2) family proteins. BCL2 family proteins include pro-apoptotic [BCL2-associated X protein (BAX) and BCL2 homologous killer protein (BAK)] and anti-apoptotic [BCL2 and BCL2-like 1 (BCL2L1)] members. The balance between pro- and anti-apoptotic proteins regulates the permeability of the mitochondrial membrane (15–17). Although the exact mechanisms of mitochondrial proteins remain clear, conformational changes in BAK and BAX lead to pore formation in the outer mitochondrial membrane and cytochrome C release (18), which eventually leads to apoptosis. BCL2L1, which is a member of the BCL2 protein group, serves a role in regulating mitochondrial apoptosis through regulating the release of pro-apoptotic factors from the mitochondria (15). Mitochondrial apoptosis pathway stimulation is induced by DNA damage or cytotoxic drugs that eventually cause cytochrome C to be released from the mitochondria through the permeated membrane and apoptosis to take place (16,17). The BCL2L1 gene is transcribed to a few isoforms. The longest of these is translated to a 233-amino acid protein with anti-apoptotic function (15,19,20).
Considering the role of miRNAs in the suppression of anti-apoptotic genes, it can be assumed that downregulation of these miRNAs may be associated with the initiation or maintenance of cancer (21).
The present study analyzed the association between the expression of BCL2 and BCL2L1 anti-apoptosis genes and the expression of the miRNAs that target them. Using bioinformatics and literature review, a few miRNAs that had been validated for having or were predicted to have inhibitory effects on the BCL2 and BCL2L1 genes by targeting their 3′-UTR were nominated. Among them, 12 miRNAs were selected based on their features, including being bound to more than one anti-apoptotic gene, having more complementary base pairing nucleotides in the seed region, being predicted by miRNA algorithm tools and novelty. Finally, expression profiles of the 12 selected miRNAs taken from the PCa tissue samples were examined and compared with those taken from the benign prostatic hyperplasia (BPH) tissue samples. Simultaneously, expression profiles of the target genes (BCL2 and BCL2L1) were analyzed.
Materials and methods
miRNA selection
Four databases were used to identify 12 miRNAs. One score was given for each target prediction by any of the databases and the miRNAs with the 12 highest overall scores were selected. miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) provides a validated and predicted miRNA-target interaction database of human, mouse and rat genes. It aggregates and compares results from other miRNA-to-mRNA databases. miRanda (http://34.236.212.39/microrna/getDownloads.do) is an online database for experimentally observed microRNA expression patterns and predicted microRNA targets. miRDB (www.mirdb.org/) is also an online database for predicted miRNA targets in animals. TargetScan (www.targetscan.org/) miRNA target predictor software has context score contribution to include seed pairing stability and target site.
Clinical samples
The present study was approved by the Pasteur Institute of Iran Ethical Review Board. Written informed consent was obtained from all participants prior to the clinical samples being obtained. PCa tissues (n=30) were collected following radical prostatectomy from untreated patients with PCa who were 48–80 years old (mean, 65.66 years). Within 1 h of prostatectomy, the specimens were dissected by a uropathologist (Hashemi Nejad Clinical Research Developing Center, Tehran, Iran) and were stored at −80°C until RNA extraction. Only samples containing >70% tumor cells were included in the study. The Gleason score, pathological stage and histological diagnosis were assessed according to the guidelines of the Union for International Cancer Control 2002 (22). As a control group, RNA was prepared from prostate tissue samples obtained from patients undergoing radical surgery for BPH (n=30). The absence of cancer cells in the prostate tissue was verified. In all cases, tissue samples were randomly selected. Clinical samples were obtained from the Department of Pathology, Hashemi Nejad Hospital (Tehran, Iran) between 2014 and 2015 (Table I).
Table I.
Patient demographics and clinicopathological features.
| Variable | No. | % |
|---|---|---|
| Age, years | ||
| 40-49 | 2 | 6.66 |
| 50–59 | 7 | 23.33 |
| 60–69 | 10 | 33.33 |
| 70–79 | 9 | 30 |
| >80 | 2 | 6.66 |
| Mean | 65.66 | |
| Median | 65.5 | |
| Maximum | 80 | |
| Minimum | 48 | |
| TNM pT | ||
| 3a | 2 | 6.6 |
| 3b | 3 | 10 |
| 2b | 2 | 6.7 |
| 2c | 23 | 76.7 |
| TNM pN | ||
| 0 | 27 | 90 |
| 1 | 3 | 10 |
| TNM pM | ||
| x | 27 | 90 |
| 0 | 2 | 6.66 |
| 1 | 1 | 3.33 |
| Gleason score | ||
| 7 | 19 | 63.3 |
| <7 | 8 | 26.7 |
| >7 | 3 | 10 |
| PSA, ng/ml | ||
| >10 | 21 | 70 |
| 4–10 | 9 | 30 |
| <10 | 0 | 0 |
TNM, Tumor-Node-Metastasis; pT, local extension of primary tumor; pN, regional lymph node; pM, distant metastasis status; PSA, prostate-specific antigen.
Cell lines
The human PCa PC3 and DU145 cell line, and human umbilical vein endothelial cells (HUVECs) were purchased from the Cell Bank of the Pasteur Institute of Iran (Tehran, Iran). Cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham MA, USA) at 37°C with 5% CO2.
RNA extraction
Tissues
To begin with, 10–50 mg samples of frozen muscle in pieces were homogenized in 1 ml 100% Triazole (Merck KGaA, Darmstadt, Germany) in 15 ml centrifuge tubes. During the homogenization process, the tubes were kept cold using liquid nitrogen. The tubes were then left at room temperature for 5 min to permit complete dissociation of nucleoprotein complexes. Subsequently, 100 µl 1-Bromo-3-chloropropane was added to each tube, vortexed for 10 sec and incubated at room temperature for 10 min. Following centrifugation at 20,817 × g -for 15 min at 4°C, the mixture separated into 3 layers; the lower phenol-red chloroform phase, the relatively turbid middle interphase and the colorless upper aqueous phase. The latter was carefully transferred into unused 1.5 ml tubes. Following the addition of an equal volume of isopropanol and brief vortexing, the samples were stored at 4°C for 24 h, which was followed by centrifugation at 6,797 × g, for 1 h at 4°C. The precipitate RNA was visualized as a small palette. Subsequently, the supernatant was carefully removed and the palette was washed with 1 ml 75% ethanol and centrifuged at 6,797 × g for 5 min at 4°C. The supernatant was removed and the pellet was air dried for 2–3 min and re-dissolved in 100 µl diethyl pyrocarbonate-treated water. The quantity and quality of the purified RNA samples were determined spectrophotometrically (A260/280 >2.0; A260/230 >1.8), using a NanoDrop ND-2000 Spectrophotometer (Thermo Fisher Scientific, Inc.).
Cell lines
Total RNA was extracted from cells using a miRN easy Mini kit (Qiagen GmbH, Hilden, Germany). In brief, cells were seeded (to ~90% confluency, ~100,000 cells/well) onto 24-well plates for 24 h, and were then disrupted by the addition of Qiazole (Qiagen GmbH,), prior to the samples being separated to three phases by the addition of chloroform (Merck KGaA). Ethanol (70%; Merck KGaA) was added to the aqueous phase to precipitate RNA; finally, according to the manufacturer's protocol, following washing and centrifuging, total RNA was extracted.
cDNA synthesis
microRNA
Due to its specific structure, first strand synthesis of cDNA was performed using an miScript II RT kit (Qiagen GmbH, Hilden, Germany), which first adds a poly-A tail to the 3′ overhang of molecules and then incorporates a tagged sequence upstream to the poly-T sequence for the universal reverse primer used in subsequent reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
mRNA
A PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan) was used for reverse transcription of molecules, according to the manufacturer's protocols.
RT-qPCR
microRNAs
RT-qPCR for the analysis of selected microRNAs was performed in a Rotor-Gene 6000 (Corbett Life Science; Qiagen GmbH, Hilden, Germany) with 7 µl qPCR Master mix 2×, 1 µl universal reverse-primer sequence (as mentioned earlier), in addition to a specific forward primer (Table II). The SNORD47 (U47) and U6 microRNAs were used as reference genes. The thermocycling conditions were as follows: 95°C for 15 min, 40 cycles of 94°C for 15 sec, 55°C for 30 sec and 70°C for 30 sec. The quantitative cycle (CQ) was defined as a fractional cycle at which the fluorescence of the sample passes through the defined threshold. Analysis of the RT-qPCR data was performed using the 2−∆∆Cq method, which is defined as follows:
Table II.
Primers for 13 selective microRNAs (forward)a and genes (forward and reverse).
| MicroRNA/gene | Sequence |
|---|---|
| 1301–3p | 5′-TTGCAGCTGCCTGGGAGTGACTTC-3′ |
| 193b-3p | 5′-AACTGGCCCTCAAAGTCCCGCT-3′ |
| 206 | 5′-TGGAATGTAAGGAAGTGTGTGG-3′ |
| 1266-5p | 5′-CCTCAGGGCTGTAGAACAGGGCT-3′ |
| 133b | 5′-TTTGGTCCCCTTCAACCAGCTA-3′ |
| 653-5p | 5′-GTGTTGAAACAATCTCTACTG-3′ |
| 143-3p | 5′-TGAGATGAAGCACTGTAGCTC-3′ |
| 219a-5p | 5′-TGATTGTCCAAACGCAATTCT-3′ |
| 185-5p | 5′-TGGAGAGAAAGGCAGTTCCTGA-3′ |
| 126-3p | 5′-TCGTACCGTGAGTAATAATGCG-3′ |
| 126-5p | 5′-CATTATTACTTTTGGTACGCG-3′ |
| 30c-2-3p | 5′-CTGGGAGAAGGCTGTTTACTCT-3′ |
| BCL2 forward | 5′-GGATCCAGGATAACGGAGGC-3′ |
| BCL2 reverse | 5′-GGCAGGCATGTTGACTTCAC-3′ |
| BCL2L1 forward | 5′-CCTTGGATCCAGGAGAACGGC-3′ |
| BCL2L1 reverse | 5′-GGGAGGGTAGAGTGGATGGTC-3′ |
| GAPDH forward | 5′-AACGGGAAGCTTGTCATCAATGGAAA-3′ |
| GAPDH reverse | 5′-GCATCAGCAGAGGGGGCAGAG-3′ |
The reverse primer sequence was not provided by the manufacturer. BCL2, B cell lymphoma 2; BCL2L1, BCL2-like 1.
Δ Cq (sample)=Cq (target microRNA sample)-Cq (reference microRNA sample)
Δ Cq (control)=Cq (target microRNA control)-Cq (reference microRNA control)
ΔΔ Cq=Δ Cq (sample)-Δ Cq (control)
Normalized target gene expression level=2−ΔΔCq
mRNAs
First strand cDNAs were subjected to RT-qPCR using SYBR® Premix Ex Taq™ (Tli RNase H Plus; Takara Biotechnology Co., Ltd., Dalian, China). Reactions (20 ml) were used containing forward and reverse primers for the BCL2, BCL2-XL target genes and the GAPDH gene as the normalizer (Table II). The thermocycling conditions were as follows: 1 cycle at 95°C for 30 sec; 40 cycles at 95°C for 5 sec and 60°C for 20 sec. Analysis of the RT-qPCR results was performed as described earlier. All primers were synthesized by TAG Copenhagen A/S (Copenhagen, Denmark).
Statistical analysis
The relative expressions of 12 miRNAs in PCa and BPH tissue samples were compared using a Student's t-test with Welche's correction. Data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference. GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) was used for calculations and drawing graphs.
Results
miRNA selection
The results obtained from the four aforementioned databases are presented in Table III. A total of 12 miRNAs were selected based on the highest scores. Furthermore, previously validated miRNAs that were differentially downregulated in cancer cells were considered.
Table III.
Selection of microRNAs using 4 algorithmsa.
| Name | Gene | miRanda | miRDB | miRWalk | Target scan | Individual score | Overall score |
|---|---|---|---|---|---|---|---|
| miR-30C-2-3P | BCL2 | 1 | 0 | 1 | 1 | 3 | 6 |
| BCL2L1 | 1 | 0 | 1 | 1 | 3 | ||
| miR-126-3P | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 | ||
| miR-126-5P | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 | ||
| miR-185-5P | BCL2 | 1 | 0 | 1 | 1 | 3 | 5 |
| BCL2L1 | 1 | 0 | 0 | 1 | 2 | ||
| miR-219a-5P | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 | ||
| miR-1301-3P | BCL2 | 1 | 1 | 0 | 1 | 3 | 4 |
| BCL2L1 | 1 | 0 | 0 | 0 | 1 | ||
| miR-193b-3P | BCL2 | 0 | 0 | 0 | 0 | 0 | 3 |
| BCL2L1 | 1 | 0 | 1 | 1 | 3 | ||
| miR-653b-5P | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 | ||
| miR-143-3P | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 | ||
| miR-133b | BCL2 | 0 | 0 | 0 | 0 | 0 | 3 |
| BCL2L1 | 1 | 0 | 1 | 1 | 3 | ||
| miR-1266-5p | BCL2 | 1 | 0 | 1 | 1 | 3 | 6 |
| BCL2L1 | 1 | 0 | 1 | 1 | 3 | ||
| miR-206 | BCL2 | 1 | 0 | 1 | 1 | 3 | 3 |
| BCL2L1 | 0 | 0 | 0 | 0 | 0 |
1, microRNA targets specified gene; 0, microRNA does not target specified gene. miR, microRNA; BCL2, B cell lymphoma 2; BCL2L1, BCL2-like 1.
Expression analysis in clinical samples
Initially, to evaluate RT-qPCR proliferation efficiency, which was measured as the ability to double the quantity of the target molecules with each cycle, of selected miRNAs, 4 serial dilutions of a pooled cDNA sample were tested with each set of primers (data not shown).
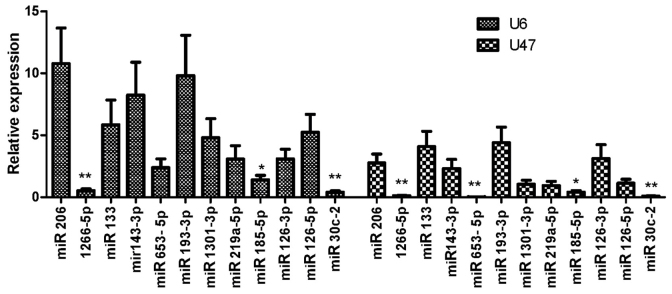
The expression of candidate miRNAs in PCa cancer and BPH tissues was evaluated by RT-qPCR and the results were normalized to U6 and U47 miRNA expression and mentioned earlier. Among the 12 miRNAs that were evaluated for expression in clinical samples, miR-1266-5p, miR-185-5p and miR-30c-2-3p were downregulated in PCa samples compared with BPH samples (Fig. 1).
Figure 1.
The expression of candidate microRNAs in prostate cancer tissues compared with benign prostatic hyperplasia tissues (normalized to U6 and U47). *P<0.05 and **P<0.01, compared with benign prostatic hyperplasia tissue. miR, microRNA.
Furthermore, expression analysis of the BCL2 and BCL2L1 genes revealed that the two genes were upregulated in PCa samples compared with expression in BPH samples (Fig. 2).
Figure 2.
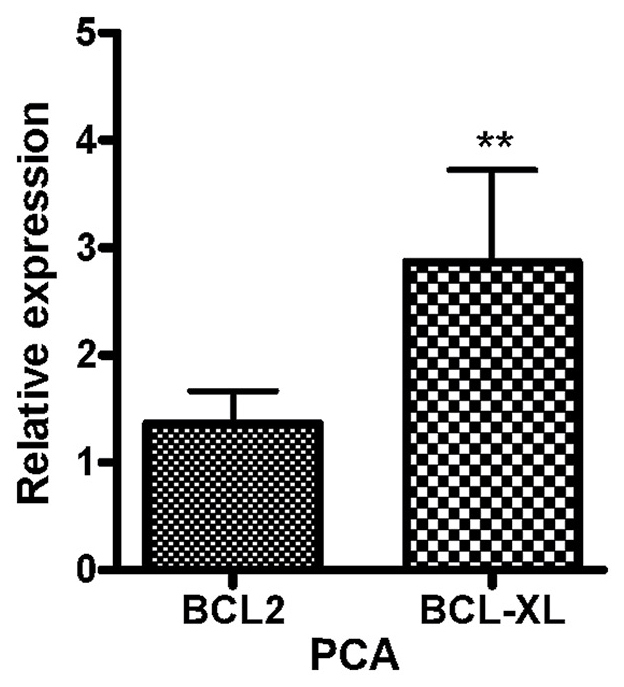
Relative expression of BCL-2 and BCL2L1 genes in prostate cancer tissues compared with benign prostatic hyperplasia tissues (normalized to GAPDH). **P<0.01. BCL-2, B-cell lymphoma 2; BCL2L1, BCL-2-Like 1.
Expression analysis in PC3 and DU145 cell lines compared with HUVECs
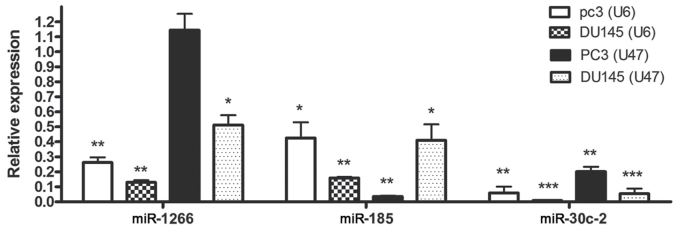
The expression of miR-185-5p and miR-30c-2-3p was downregulated in DU145 and PC3 cells compared with expression in HUVECs when U6 or U47 housekeeping genes were used as the normalizers. With respect to PC3 cells, miR-1266-5p expression was also significantly downregulated when U47 was employed as the normalizer (Fig. 3).
Figure 3.
Relative expression of miR-1266, miR-185 and miR30C (normalized to U6 and U47) in PC3 and DU145 cells lines, compared with human umbilical vein endothelial cells. *P<0.05, **P<0.01 and ***P<0.001, compared with human umbilical vein endothelial cells. miR, microRNA.
Expression of the BCL2 gene was upregulated in the DU145 cells compared with expression in the HUVECs; however, expression of this gene in the PC3 cells was not significantly altered. On the other hand, expression of the BCL2L1 gene was increased in the DU145 and PC3 cells compared with expression in the HUVECs (Fig. 4).
Figure 4.
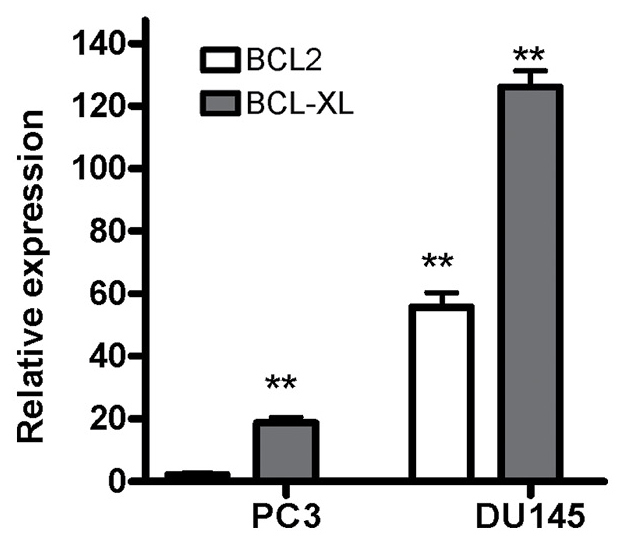
Relative expression of BCL2 and BCL2L1 genes (normalized to GAPDH) in PC3 and DU145 cell lines, compared with human umbilical vein endothelial cells. BCL-2, B-cell lymphoma 2; BCL2L1, BCL-2-Like 1. **P<0.01, compared with human umbilical vein endothelial cells.
In conclusion, the expression of 3 miRNAs (miR-1266-5p, miR-185-5p and miR-30c-2-3p) was downregulated in clinical PCa tissue samples and PCa cell lines.
Discussion
The principal aim of cancer treatment is the complete removal of cancer cells without causing changes to the rest of the body. Over the last few decades, microRNAs have demonstrated potential in cancer treatment. These molecules are able to regulate the expression of proteins involved in different important cellular pathways, including the cell cycle and apoptosis. Downregulation of certain miRNAs has been observed in numerous types of cancer (23,24), and molecular therapy studies have revealed the usefulness of miRNA overexpression in cancer treatment in vitro and in vivo (25).
The present study aimed to determine whether the downregulation of miRNAs targeting two anti-apoptotic genes (BCL2 and BCL2L1) may be associated with the molecular pathogenesis of PCa. In order to examine this, a few miRNA candidates were selected using bioinformatics tools, and their expression was analyzed in clinical tissue samples obtained from patients with PCa and compared with their expression in BPH tissue samples. Among 12 miRNA candidates, 3 exhibited downregulation that was counter-regulated with the expression of two target genes.
The results of the present study are supported by those of previous studies, which evaluated the expression of selected miRNAs in various cancer tissues. In 2014, Chen et al (23) demonstrated that miR-1266 was downregulated in 58 patients with gastric cancer; however, it was indicated that miR-1266 binds to the 3′-UTR of the human telomerase reverse transcriptase gene and that its expression in gastric cancer tissues was reduced in comparison with the normal group (23).
In another study, the expression of miR-185 in breast cancer tissues was reduced in comparison to the control group. The authors demonstrated that overexpression of miR-185 may inhibit the proliferation of breast cancer cells (26). The study demonstrated that miR-185 binds to the 3′-UTR region of the vascular endothelial growth factor A (VEGF-A) gene and there was also a significant inverse association between the expression of miR-185 and that of VEGF-A (26). Of note, the alignment of miR-185 with the 3′-UTR sequences of VEGF-A and BCL2 mRNAs exhibited similarity in the seed region sequence (www.microrna.org; Fig. 5). It is likely that miR-185 is also able to target the BCL2 gene. In this case, overexpression of miR-185 in cancer cells may inhibit angiogenesis while simultaneously promoting apoptosis.
Figure 5.

Prediction of miR-185 seed sequence complementary to 3′-untranslated region targets of (A) BCL2 mRNA (NM_000633), (B) VEGFA mRNA (NM_003376) and (C) SIX1 mRNA (NM_005982). miR, microRNA; VEGFA, vascular endothelial growth factor A.
Furthermore, the reduced miR-185 expression has been observed in another study performed in 2010 (27). The investigators observed downregulation of miR-185 in ovarian cancer, pediatric renal tumors and multiple breast cancer cell lines. Additionally, it was demonstrated that overexpression of miR-185 was able to reduce the expression of the SIX1 oncogene, which induces apoptosis in apoptosis-resistant cancer cells (27). Furthermore, has-miR-185 exhibits an identical binding sequence to the seed region sequences of SIX1 and BCL2 mRNAs (www.microrna.org; Fig. 5).
In another study, Zhang et al (28) revealed a significant downregulation of miR-30c in colon cancer tissue samples and in the colon cancer HCT-116 cell line (28). Additionally, overexpression of miR-30c resulted in the inhibition of cancer cell proliferation, migration and invasion (28). Although they validated targeting of the ADAM19 gene by miR-30c and proposed that ‘miR-30c inhibited cancer cells via targeting ADAM19’, this observation may be, at least in part, due to simultaneous targeting of the BCL2L1 gene by miR-30c, as the seed sequence binding sites of these genes are similar (Fig. 5). The results of the present study demonstrated that downregulation of miR-30c was paralleled with overexpression of the BCL2L1 gene. However, this assumption requires further confirmation in future studies.
In 2012, researchers revealed that miR-491 overexpression may induce apoptosis in pancreatic cancer SW1990 cells by targeting the 3′-UTR of the BCL2L1 gene. The seed match sequence region of the BCL2L1 gene was confirmed by mutagenesis tests (29). Targeting of the BCL2L1 anti-apoptotic gene by miR-491 and the subsequent reduction of cancer cell phenotype (i.e., apoptosis), supports the hypothesis of the present study, that downregulation of miR-30c may cause overexpression of its target, the BCL2L1 gene, eventually halting cell apoptosis.
In 2012, Zhao et al (30) confirmed by luciferase reporter assay that miR-125b was able to bind to the 3′-UTR of the BCL2 gene, which had previously been predicted in bioinformatics analysis. The same study also demonstrated that miR-125b suppressed hepatocellular carcinoma cell proliferation and promoted apoptosis by inhibiting the gene expression of BCL2 (30). Another study, which was performed in 2014, revealed that miR-16 was downregulated in human brain glioma tissues and that overexpression of miR-16 suppressed the expression of BCL2 and promoted cell apoptosis (31). Additionally, overexpression of miR-16 in a human glioma nude mouse model confirmed tumor growth suppression (31). A 2011 study on the human non-small cell lung cancer (NSCLC) A549 cell line indicated that miR-7 expression in the NSCLC cell line was reduced, while overexpression of miR-7 resulted in the suppression of A549 cell proliferation, as well as the induction of apoptosis (32). Furthermore, in the same study, miR-7 binding to the 3′-UTR of the BCL2 gene was been confirmed by bioinformatics prediction and luciferase reporter assay (32).
In general, downregulation of miR-1266, miR-185 and miR-30c has been previously reported in several different types of cancer cells. However, interventional studies have demonstrated that overexpression of various miRs targeting the BCL2 and BCL2L1 genes may improve cell apoptosis. Furthermore, the results of the present study demonstrated concordance between bioinformatics prediction and/or literature review with expression analysis of miR-1266, miR-185 and miR30c, as well as the BCL2 and BCL2L1 genes, in PCa tissues and PCa cell lines. It is likely that overexpression of these 3 microRNAs may suppress the proliferation of tumor cells via upregulation of the apoptosis pathway. Notably, further investigations are necessary in order to confirm whether or not selected microRNAs are bound to the 3′-UTR of the BCL2 and BCL2-XL genes and reduce their expression in PCa cell lines; and whether or not overexpression of microRNAs reduces the expression of target genes, and if the latter is co-incident with changes in cancerous phenotypes, including cell apoptosis promotion, cell proliferation reduction or cell cycle attenuation.
Acknowledgements
S. Ostadrahimi would like to thank the Pasteur Institute of Iran for the grant supporting her PhD studentship. The corresponding authors would also like to thank Dr. Hassan and Dr. Abedi for their support.
Funding
The present study was supported by a grant from The Pasteur Institute of Iran (grant no. 93/0110/7967).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Experimental design was performed by PFE, RM and SO. The experiments were carried out by SO, SF and MP. Data were analyzed by SO and PFE. Samples were gathered by MoA, HS and MaA. Discussions, comments and clarification of the subject matter were provided by MAV, MH, MK and LTT. MAV and MH contributed reagents, materials and analytical tools. SO and PFE wrote the manuscript.
Ethics approval and consent to participate
The present study was approved by the Pasteur Institute of Iran Ethical Review Board. Written informed consent was obtained from all participants prior to the clinical samples being obtained.
Consent for publication
Written informed consent was obtained from all participants prior to publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tabayoyong W, Abouassaly R. Prostate cancer screening and the associated controversy. Surg Clin North Am. 2015;95:1023–1039. doi: 10.1016/j.suc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Medical history and etiology of prostate cancer. Epidemiol Rev. 2001;23:159–162. doi: 10.1093/oxfordjournals.epirev.a000783. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, St Onge J, Dhillon K, Chorneyko A. PSA density improves prediction of prostate cancer. Can J Urol. 2014;21:7312–7321. [PubMed] [Google Scholar]
- 5.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350:207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- 7.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 9.Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology. 2009;50:113–121. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 10.Fujita Y, Kojima T, Kawakami K, Mizutani K, Kato T, Deguchi T, Ito M. miR-130a activates apoptotic signaling through activation of caspase-8 in taxane-resistant prostate cancer cells. Prostate. 2015;75:1568–1578. doi: 10.1002/pros.23031. [DOI] [PubMed] [Google Scholar]
- 11.Liu XD, Zhang LY, Zhu TC, Zhang RF, Wang SL, Bao Y. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol. 2015;8:4525–4534. [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Qiu W, Zhang G, Xu S, Gao Q, Yang Z. MicroRNA-204 targets JAK2 in breast cancer and induces cell apoptosis through the STAT3/BCl-2/survivin pathway. Int J Clin Exp Pathol. 2015;8:5017–5025. [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X, Yin H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem Biophys Res Commun. 2010;399:1–6. doi: 10.1016/j.bbrc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Hossini AM, Eberle J. Apoptosis induction by Bcl-2 proteins independent of the BH3 domain. Biochem Pharmacol. 2008;76:1612–1619. doi: 10.1016/j.bcp.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Li CY, Chu JY, Yu JK, Huang XQ, Liu XJ, Shi L, Che YC, Xie JY. Regulation of alternative splicing of Bcl-x by IL-6, GM-CSF and TPA. Cell Res. 2004;14:473–479. doi: 10.1038/sj.cr.7290250. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z, Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 19.Revil T, Toutant J, Shkreta L, Garneau D, Cloutier P, Chabot B. Protein kinase C-dependent control of Bcl-x alternative splicing. Mol Cell Biol. 2007;27:8431–8441. doi: 10.1128/MCB.00565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-N. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 22.Organization WH. National cancer control programmes: Policies and managerial guidelines. World Health Organization; 2002. [Google Scholar]
- 23.Chen L, Lü MH, Zhang D, Hao NB, Fan YH, Wu YY, Wang SM, Xie R, Fang DC, Zhang H, et al. miR-1207-5p and miR-1266 suppress gastric cancer growth and invasion by targeting telomerase reverse transcriptase. Cell Death Dis. 2014;5:e1034. doi: 10.1038/cddis.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsham JL, Kalinowski FC, Epis MR, Ganda C, Brown RA, Leedman PJ. Clinical potential of microRNA-7 in cancer. J Clin Med. 2015;4:1668–1687. doi: 10.3390/jcm4091668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Rana TM. Therapeutic targeting of microRNAs: Current status and future challenges. Nat Rev Drug Discov. 2014;13:622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Tian S, Wang HB, Chu DP, Cao JL, Xia HF, Ma X. MiR-185 is involved in human breast carcinogenesis by targeting Vegfa. FEBS Lett. 2014;588:4438–4447. doi: 10.1016/j.febslet.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, Rao MK. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene. 2010;29:4971–4979. doi: 10.1038/onc.2010.233. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Q, Yu L, Qin D, Huang R, Jiang X, Zou C, Tang Q, Chen Y, Wang G, Wang X, Gao X. Role of microRNA-30c targeting ADAM19 in colorectal cancer. PLoS One. 2015;10:e0120698. doi: 10.1371/journal.pone.0120698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo R, Wang Y, Shi WY, Liu B, Hou SQ, Liu L. MicroRNA miR-491-5p targeting both TP53 and Bcl-XL induces cell apoptosis in SW1990 pancreatic cancer cells through mitochondria mediated pathway. Molecules. 2012;17:14733–14747. doi: 10.3390/molecules171214733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao A, Zeng Q, Xie X, Zhou J, Yue W, Li Y, Pei X. MicroRNA-125b induces cancer cell apoptosis through suppression of Bcl-2 expression. J Genet Genomics. 2012;39:29–35. doi: 10.1016/j.jgg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al. MicroRNA-16 inhibits glioma cell growth and invasion through suppression of BCL2 and the nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 2014;105:265–271. doi: 10.1111/cas.12351. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7:805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.