Abstract
Tunica Interna endothelial cell kinase (Tie2)-expressing macrophages (TEMs) are a subgroup of tumor-associated macrophages that are associated with a poor prognosis in numerous types of cancer. The present study aimed to assess the prognostic impact of Tie2 expression in gastric cancer tissues. Between January 2009 and December 2009, 76 newly diagnosed patients with gastric cancer at the Southwest Hospital, Third Military Medical University (Chongqing, China) were enrolled. TEMs were detected using immunohistochemistry. Tie2, cluster of differentiation (CD)68 and carbonic anhydrase IX (CAIX) were analyzed using immunohistochemistry and immunofluorescent microscopy. Tie2 protein expression was analyzed using western blot analysis in hypoxic and normoxic gastric cancer tissues. The number of TEMs positively staining for Tie2 increased with the tumor-node-metastasis (TNM) stage: 0, 53.9, 75.6 and 100% in stages I, II, III and IV, respectively (P<0.001). Tumor size and lymph node involvement were significantly associated with the presence of Tie2 in the tumor stroma (P<0.001). There was no significant difference between Tie2 and CAIX, irrespective of how the patients were grouped (tumor size, lymph node involvement, TNM stage or histological grade). Tie2 protein expression was increased in the hypoxic regions of gastric tumors.Tie2 and CD68 expression colocalized in hypoxic and normoxic gastric cancer tissues. The 1-, 2- and 3-year recurrence rates of the TEM-positive group were 31.4, 56.9 and 66.7%, respectively, as compared with 8, 28 and 48%, respectively, for the TEM-negative group (P<0.05). In the TEM-negative group, 2 patients succumbed to the disease, as compared with 21 patients in the TEM-positive group (P<0.05). Therefore, high quantities of TEMs, represented by Tie2 expression, in gastric tumors may be associated with poor survival.
Keywords: Tunica Interna endothelial cell kinase, overexpression, prognosis, gastric cancer
Introduction
Gastric cancer is an important health concern with an age-adjusted incidence rate of 9.7/100,000 males and 4.8/100,000 females in the United States (1), however, this incidence rate is higher in Japan, Finland, Iceland, Brazil, Korea and China (2). Notable risk factors include diet (high salt, low animal fat, high complex carbohydrates, nitrites and red or processed meat), mucosal atrophy, chronic gastritis, smoking and Helicobacter pylori infection (3,4). At presentation, only 40% of patients with gastric cancer are curable, and the 10-year cancer-associated survival rate is 51% when the cardia is not involved (5). Treatment typically consists of a combination of surgery and chemotherapy (6,7).
Macrophages from the peripheral blood that infiltrate tumor tissues are named tumor-associated macrophages (TAMs). TAMs are an important part of solid tumors and have an essential role in tumor progression (8–10). Noy et al (11) have hypothesized that the greater the number of macrophages in the tumor, the more efficient their anti-tumor effect. However, previous studies have also identified that the presence of TAMs is associated with a poor prognosis in a number of malignancies (12–14). Certain characteristics of the TAMs may have a functional role in this effect on tumors.
Tunica Interna endothelial cell kinase (Tie2) is a receptor tyrosine kinase expressed on endothelial cells and hematopoietic stem cells (15). Tie2-expressing macrophages (TEMs) are a subgroup of TAMs, which were initially identified in a mouse breast cancer model (16) and are characterized by high expression levels of the pro-angiogenic receptor Tie2. Venneri et al (17) also identified TEMs in the peripheral blood, where they accounted for 2–7% of the blood mononuclear cells from healthy donors (17). TEMs were primarily located in the hypoxic regions of tumors and may be involved in tumor angiogenesis, thus promoting tumor progression and metastasis (18). Previous studies have demonstrated that the degree of TEM infiltration into tumor hypoxic regions may be an adverse prognostic factor for patients with cancer (14,19); however, a small number of studies (14,20) focused on the effects of Tie2 on tumor recurrence and disease-free survival.
Therefore, the aim of the present study was to assess the prognostic impact of Tie2 expression in TEMs identified in patients with gastric cancer. The results of the present study indicate Tie2 to be a novel prognostic marker for these patients or a potential target for therapy.
Materials and methods
Patient characteristics
From January 2009 to December 2009, 76 newly diagnosed patients (51 males and 26 females aged 28 to 86 years) with gastric cancer who underwent surgical tumor resection at the Department of Surgery and Center of Minimally Invasive Gastrointestinal Surgery, Southwest Hospital, Third Military Medical University (Chongqing, China) by the same gastrointestinal surgery team were enrolled in the present study. Histopathological diagnosis was performed by an experienced pathologist according to the criteria of the American Joint Commission on Cancer (21). The exclusion criteria included a history of previously treated cancer and preoperative chemotherapy or radiotherapy. All patients received adjuvant oxaliplatin and capecitabine chemotherapy. The current study was approved by the Institutional Review Board of the Southwest Hospital, Third Military Medical University and written informed consent was obtained from all patients.
Data collection
Detailed clinicopathological data was collected from the medical records of each patient, including sex, age, tumor location, tumor diameter and the extent of tumor resection. Follow-up was censored in December 2013 and the collection of subsequent treatment, recurrence and survival status data was completed by this date. Progression-free survival (PFS) was defined as the time interval from diagnosis to first tumor progression, recurrence or metastasis. Overall survival (OS) was measured from diagnosis to the date of mortality or to December 2013.
Immunohistochemistry
Gastric cancer tissues were collected during surgery and fixed in 10% formalin for 24 h at 18°C, prior to paraffin embedding. Paraffin sections (4 µm thick) of gastric cancer tissues were mounted on silanized slides, dewaxed at 56°C for 30 min, deparaffinized with xylene (slices were placed in >99% xylene I and xylene II for 5 min at a time) and rehydrated using ethanol (100, 100, 95, 85, 75 and 75% ethanol, 3 min at room temperature) and washed with PBS for 3 min. Peroxidase activity was quenched with 3% hydrogen peroxidase at room temperature for 15 min and non-specific background staining was eliminated using blocking buffer (2% goat serum, 0.2% Triton X-100 and 0.1% bovine serum albumin in PBS) at room temperature for 1 h. Primary antibodies against Tie2 (dilution, 1:50; cat no. ab24859; Abcam, Cambridge, UK) or carbonic anhydrase IX (CAIX; dilution, 1:400; cat no. ab107257; Abcam) were applied overnight at 4°C. Following washing three times in PBS for 3 min, immunodetection was performed using a labeled polymer horseradish peroxidase mouse antibody (cat no. SC-51948; dilution, 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) incubated for 10 min at room temperature. Slides were subsequently washed with dH2O, visualized with 3,3′-diaminobenzidine for 10 min and counterstained with Mayers hematoxylin. The negative controls were obtained by omitting the primary antibodies.
Slides were evaluated using an Eclipse TE2000-S microscope (Nikon Corporation, Tokyo, Japan) by two independent investigators who were blinded to the clinical outcomes. Images were captured under ×400 magnification using Image-Pro Plus v5.0 software (Media Cybernetics, Inc., Rockville, MD, USA). Tie2 and CAIX expression levels in cancer cells were graded as follows: -(≤10% of positive cells), +(20%-50% of positive cells), ++(≥50% of positive cells).
Immunofluorescent staining
Paraffin sections were dewaxed by heating to 56°C for 30 min, deparaffinized using xylene I and xylene II for 5 min at a time and rehydrated using ethanol (100, 100, 95, 85, 75 and 75% ethanol, 3 min at room temperature) and washed with PBS for 3 min. Peroxidase activity was quenched using 3% hydrogen peroxidase at room temperature for 15 min and non-specific background staining was eliminated using a blocking buffer (2% goat serum, 0.2% Triton X-100 and 0.1% bovine serum albumin in PBS) at room temperature for 1 h. Primary antibodies against Tie2 (dilution, 1:50; cat no. SC-324; Santa Cruz Biotechnology, Inc.) or CD68 (dilution, 1:50; cat no. ab955; Abcam) were applied overnight at 4°C. Following washing three times with PBS for 10 min, the secondary antibodies were added; donkey anti-Rabbit IgG (Alexa Fluor 647, dilution, 1:50; cat no. A31573, or donkey anti-Mouse IgG Alexa Fluor 488, dilution, 1:50; cat no. A21202s; both Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 60 min. Then they were washed 5 times with PBS, for 10 min each time. DAPI was then added at 37°C for 5 min. This was followed by washing 3 times with PBS, for 10 min each time. The slices were then sealed using glycerin. Slides were viewed at a magnification of ×400 using a fluorescence microscope (Eclipse TE2000-S Nikon Corporation) using Image-Pro Plus v5.0 software (Media Cybernetics, Inc.).
Statistical analysis
Continuous data are presented as the mean ± standard deviation and were analyzed using the Student's t test. Categorical data are presented as frequencies and were analyzed using the Fisher's exact test. Pearsons chi-square test was used to compare the recurrence rate of gastric cancer up to 3 years following surgery. Survival rates were analyzed using the Kaplan-Meier method and the log-rank test. Data analysis was performed using SPSS v.13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of the patients
Table I presents the characteristics of all enrolled patients. A total of 51 male and 25 female patients were enrolled in the present study. The median age was 56 years (range, 28–86). The tumors were located in the gastric antrum in 42 patients, in the body of the stomach in 16 patients and in the cardia in 18 patients. The tumor histological grade was I in 10 patients, II in 21 patients and III in 45 patients. The tumor-node-metastasis (TNM) stage (22) was I in 9 patients, II in 13 patients, III in 41 patients and IV in 13 patients. The median follow-up was 52.3 months (range, 48–60) and no patient failed to complete follow-up.
Table I.
Clinicopathological characteristics of the patients with gastric cancer (n=76).
| Characteristic | Tie2-positive, n (%) | P-value |
|---|---|---|
| Age, years | NS | |
| <60 | 32/50 (64.0) | |
| ≥60 | 19/26 (73.1) | |
| Gender | NS | |
| Male | 34/51 (66.7) | |
| Female | 17/25 (68.0) | |
| Tumor sizeb | <0.001a | |
| T1 | 0/2 (0.0) | |
| T2 | 3/13 (23.1) | |
| T3 | 40/53 (75.5) | |
| T4 | 8/8 (100.0) | |
| Lymph node metastasesc | <0.001a | |
| N0 | 4/17 (23.5) | |
| N1 | 18/28 (64.3) | |
| N2 | 28/30 (93.3) | |
| N3 | 1/1 (100.0) | |
| TNM stage | <0.001a | |
| I | 0/9 (0.0) | |
| II | 7/13 (53.9) | |
| III | 31/41 (75.6) | |
| IV | 13/13 (100.0) | |
| Histological grade | NS | |
| I | 9/10 (90.0) | |
| II | 12/21 (57.1) | |
| III | 30/45 (66.7) | |
| Tumor location | NS | |
| Gastric antrum | 26/42 (61.9) | |
| Body of the stomach | 10/16 (62.5) | |
| Cardia | 15/18 (83.3) |
P<0.05. P-values were obtained using the Fisher's exact test. NS, not significant; Tie2, Tunica Interna endothelial cell kinase.
T1, The tumor is immersed in the basement membrane or submucosa. T2, The tumor is immersed in the muscle film or subserous membrane. T3, The tumor penetrates the serous membrane but does not infiltrate the adjacent tissue. T4, Tumor infiltration adjacent tissue.
N0, No lymph node metastasis. N1, There are 1–6 regional lymph node metastases. N2, There are 7 to 15 regional lymph node metastases. N3, There are more than 15 regional lymph node metastases.
Associations between clinicopathological characteristics and TEM recruitment in the gastric cancer stroma
The associations between certain clinicopathological characteristics and TEM recruitment in gastric cancer stroma are presented in Table I. The positive rate of TEM recruitment in gastric cancer stroma was 67.2%. TEM recruitment increased with TNM stage: 0, 53.9, 75.6 and 100% in stages I, II, III and IV, respectively (P<0.001). Tumor size and lymph node involvement were significantly associated with the presence of TEMs in the tumor stroma (P<0.001). Age, gender, histological grade and tumor location were not significantly associated with the presence of TEMs in the tumor stroma.
Association between clinicopathological characteristics and the expression of CAIX and Tie2 in gastric cancer
CAIX and Tie2 expression levels in gastric cancer cells are presented in Table II and Fig. 1. The expression of CAIX and Tie2 in gastric cancer was positive in 66/76 patients (86.8%) for CAIX and in 65/76 patients (85.5%) for Tie2. There was no significant difference between the two markers, irrespective of how the patients were grouped (tumor size, lymph node involvement, TNM stage or histological grade).
Table II.
CAIX and Tie2 expression in gastric cancer (n=76).
| CAIX, n (%) | Tie2, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | − | + | ++ | − | + | ++ | P-value |
| Tumor sizea | NS | ||||||
| T1 | 2 (100.0) | 0 | 0 | 2 (100.0) | 0 | 0 | |
| T2 | 3 (25.0) | 8 (66.7) | 1 (8.3) | 3 (25.0) | 9 (75.0) | 0 | |
| T3 | 5 (9.4) | 13 (24.5) | 35 (66.0) | 5 (9.4) | 24 (45.3) | 24 (45.3) | |
| T4 | 0 | 0 | 9 (100.0) | 0 | 2 (22.2) | 7 (77.8) | |
| Lymph nodesb | NS | ||||||
| N0 | 9 (50.0) | 6 (33.3) | 3 (16.7) | 10 (55.6) | 6 (33.3) | 2 (11.1) | |
| N1 | 1 (3.6) | 15 (53.6) | 12 (42.9) | 1 (3.6) | 21 (75.0) | 6 (21.4) | |
| N2 | 0 | 2 (8.0) | 23 (92.0) | 0 | 8 (32.0) | 17 (68.0) | |
| N3 | 0 | 0 | 5 (100.0) | 0 | 0 | 5 (100.0) | |
| TNM stage | NS | ||||||
| I | 5 (55.5) | 4 (44.4) | 0 | 5 (55.5) | 4 (44.4) | 0 | |
| II | 4 (33.3) | 6 (50.0) | 2 (16.7) | 4 (33.3) | 6 (50.0) | 2 (16.7) | |
| III | 1 (2.4) | 11 (26.2) | 30 (71.4) | 1 (2.4) | 24 (57.1) | 17 (40.5) | |
| IV | 0 | 0 | 13 (100.0) | 0 | 1 (7.7) | 12 (92.3) | |
| Histological grade | NS | ||||||
| I | 2 (20.0) | 2 (20.0) | 6 (60.0) | 2 (20.0) | 2 (20.0) | 6 (60.0) | |
| II | 3 (14.3) | 9 (42.9) | 9 (42.9) | 4 (19.1) | 11 (52.4) | 6 (28.6) | |
| III | 5 (11.1) | 14 (31.1) | 26 (57.8) | 5 (11.1) | 19 (42.2) | 21 (46.7) | |
P-values were obtained using the Fisher's exact test. NS, not significant; Tie2, Tunica Interna endothelial cell kinase; CAIX, carbonic anhydrase IX.
T1, The tumor is immersed in the basement membrane or submucosa. T2, The tumor is immersed in the muscle film or subserous membrane. T3, The tumor penetrates the serous membrane but does not infiltrate the adjacent tissue. T4, Tumor infiltration adjacent tissue.
N0, No lymph node metastasis. N1, There are 1–6 regional lymph node metastases. N2, There are 7 to 15 regional lymph node metastases. N3, There are more than 15 regional lymph node metastases.
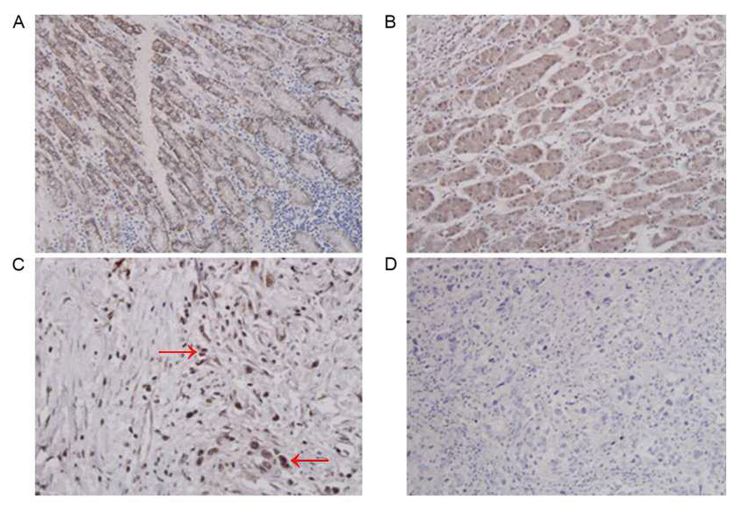
Figure 1.
Immunohistochemical examination of human gastric cancer tissues for Tie2 and CAIX. (A) Gastric cancer tissue with positive Tie2 expression. Tie2 was primarily expressed in the gastric cancer cells. Tie2 is mainly expressed in the cytoplasm of macrophages, and the cytoplasm is stained brown and yellow. (B) CAIX positive expression in gastric cancer tissue. CAIX is mainly expressed in the cytoplasm of gastric cancer cells, and the cytoplasm is stained brown and yellow. (C) Numerous Tie2-expressing macrophages were located in the tumor stroma (marked with red arrows). (D) Gastric cancer tissue that is negative for Tie2 expression. Magnification, ×400. Tie2, Tunica Interna endothelial cell kinase; CAIX, carbonic anhydrase IX.
Tie2 expression level and its association with hypoxia
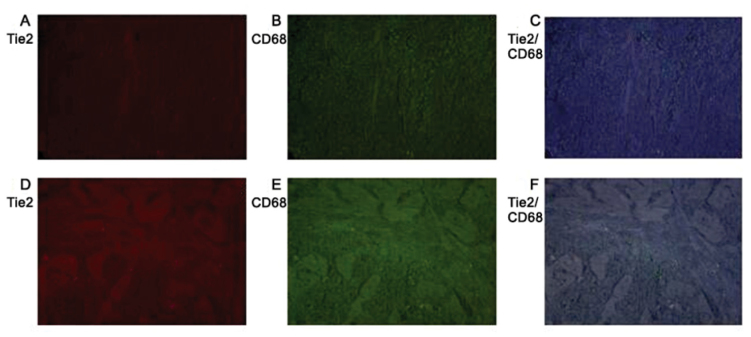
Table II demonstrates that Tie2 protein expression was increased in the hypoxic regions of gastric tumors. Comparing the expression of Tie2 and CAIX in gastric cancer tissues, it was identified that the expression of Tie2 in gastric cancer tissues was consistent with the expression of hypoxia marker CAIX in gastric cancer tissues. Fig. 2 identifies that Tie2 and CD68 (a macrophage-specific marker) expression colocalizes in gastric cancer tissues. TEMs are a subset of mononuclear macrophages that infiltrate tumor tissues.
Figure 2.
Immunofluorescence analysis for (A) CD68, (B) Tie2 and (C) merged images in hypoxic gastric cancer tissues, and (D) CD68, (E) Tie2 and (F) merged images in normoxic cancer tissues. Magnification, ×400. CD68, cluster of differentiation 68; Tie2, Tunica Interna endothelial cell kinase.
Recurrence of gastric cancer
The 1-, 2- and 3-year recurrence rates are presented in Table III. The recurrence rates of the TEM-positive group were 31.4, 56.9 and 66.7%, respectively, compared with 8, 28 and 48%, respectively, for the TEM-negative group (P<0.05). Tie2 patients with positive gastric cancer experienced a significantly lower incidence of tumor recurrence within three years after surgery than those with negative Tie2 expression. (P<0.05; Fig. 3).
Table III.
Tumor recurrence rate of gastric cancer patients within three years of surgery.
| Recurrence date | TEM-negative, n (%) | TEM-positive, n (%) | P-value |
|---|---|---|---|
| 1-year | 2/25 (8.0) | 16/51 (31.4) | 0.024a |
| 2-year | 7/25 (28.0) | 29/51 (56.9) | 0.018a |
| 3-year | 12/25 (48.0) | 34/51 (66.7) | 0.118 |
P<0.05. P-values were estimated using the Pearson χ2 method. TEM, TEM, Tunica Interna endothelial cell kinase-expressing macrophages.
Figure 3.
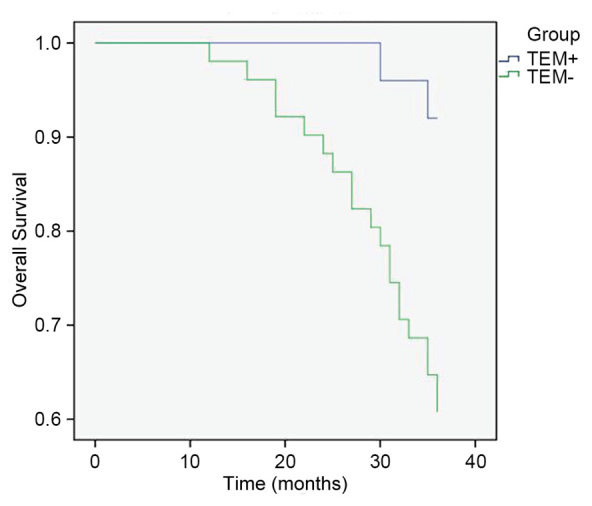
Kaplan-Meier analysis of patient survival rates according to TEM status. Patients with TEM-gastric cancer had a reduced survival rate compared with those with TEM+ cancer. P<0.05, TEM+ vs. TEM-. TEM, Tunica Interna endothelial cell kinase-expressing macrophages.
Discussion
The aim of the present study was to assess the prognostic impact of Tie2 expression in TEMs identified in patients with gastric cancer. These results indicated that Tie2 and CD68 expression colocalizes in gastric cancer tissues. Tie2 positivity increased with TNM stage. Tumor size and lymph node involvement were significantly associated with the presence of TEM recruitment in the tumor stroma. It was identified that there was no significant difference in tumor stage between Tie2 and CAIX expression, irrespective of how the patients were grouped (tumor size, lymph node involvement, TNM stage or histological grade). Tie2 protein expression was increased in the hypoxic regions of the gastric tumors. The 1-, 2- and 3-year recurrence rates were increased in the TEM-positive group, as compared with in the TEM-negative group. In the TEM-negative group, the recurrence rate of tumor recurrence within 3 years after surgery was significantly higher than the TEM-positive group.
CAIX expression levels reflect the state of tumor hypoxia, and its expression in solid tumors is an established marker of poor prognosis (23–26). In the present study, CAIX was used to locate the hypoxic areas in gastric cancer tissues. However, there was no significant difference in the association between CAIX and prognostic markers compared with Tie2 and prognostic markers, suggesting that CAIX and Tie2 are associated with tumor hypoxia. In addition, western blot analysis demonstrated that Tie2 protein levels are increased in hypoxic tumor tissues.
In the present study, Tie2 immunohistochemistry was used to reflect the infiltration of tumors by TEMs, as TEMs are the only cells known to express Tie2 (17). Positive Tie2 expression was associated with tumor size, lymph node involvement and TNM stage, which are established prognosis markers for gastric cancer (5). Indeed, previous studies have identified that the degree of TEM infiltration of tumor hypoxic regions may be an adverse prognostic factor in patients with breast cancer (14,20); the results of the present study suggest that this may also apply to gastric cancer.
In a study by Reed et al (27), high rates of disease recurrence have been associated with the abundance of lymphatic channels and vascularization within the gastric wall, which may provide opportunity for cancer cells to migrate. Tie2 is a receptor involved in angiogenesis (19). The results of the present study demonstrated that the number of patients with Tie2-positive gastric cancer was increased alongside an increase in the rate of regional lymph node invasion, which is concordant with the observations of Reed et al (27).
Following surgery, the gastric cancer recurrence rate was increased in the Tie2-positive group and Tie2 protein expression was higher in hypoxic gastric cancer tissues compared with in normoxic gastric cancer tissues. The hypoxic microenvironment of a tumor serves two roles. Firstly, hypoxia may lead to tumor necrosis but the microenvironment surrounding the tumor necrosis also induces angiogenesis, thus promoting tumor progression and metastasis (28–33). A number of previous studies have established that TEM infiltration in the tumor tissue promotes tumor angiogenesis in the microenvironment and, therefore, contribute to tumor progression and the metastasis of tumor cells (10,30,31,33,34). Furthermore, with the development of the tumor, the hypoxic state of the tumor microenvironment induces the macrophage phenotype to change from M1 to M2, resulting in an modification from immune surveillance and cytotoxicity to immune escape, which promotes tumor progression and metastasis (35). These previous studies support the results of the present study, demonstrating that Tie2-positive TAMs located in the stroma of gastric cancer have an affect on tumor recurrence and patient survival.
The present study has limitations. The sample size was small and from a single institution. In addition, a comprehensive panel of prognostic markers was not assessed. Further investigation is required to fully assess the role of Tie2 in the prognosis of gastric cancer, including in vitro experiments using TEMs in hypoxic and normoxic environments.
In conclusion, Tie2 positive expression (representing infiltrated TEMs) in gastric tumors may be associated with poorer survival. Tie2 has the potential to be used as a prognostic marker for gastric cancer and may be a novel therapeutic target.
Acknowledgements
The authors would like to thank Dr. Ariel Yang from the Abramson Family Cancer Research Institute, University of Pennsylvania (Philadelphia, USA) for his valuable help proofreading the present study and Dr. Dong Yi from the Department of Statistics, The Third Military Medical University (Chongquing, China) for assistance with the statistical methods.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 30901426).
Availability of data and materials
The datasets generated and analyzed in the present study are included in this published article.
Authors' contributions
WJY, YXH, XY, and XLF performed the research. YXH and PWY designed the research. WJY, YS, HLY, PY and HLD analyzed the data. WJY and YXH wrote the paper.
Ethics approval and consent to participate
The current study was approved by the Institutional Review Board of the Southwest Hospital, Third Military Medical University and written informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: A global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, Tang S, Shu Z, Cai J, Dai S, et al. Red and processed meat intake is associated with higher gastric cancer risk: A meta-analysis of epidemiological observational studies. PLoS One. 2013;8:e70955. doi: 10.1371/journal.pone.0070955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doll R, Peto R, Boreham J, Sutherland I. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer. 2005;92:426–429. doi: 10.1038/sj.bjc.6602359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F. Italian Research Group for Gastric C (IRGGC): Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: Analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486–491. doi: 10.1097/SLA.0b013e3182389b1a. [DOI] [PubMed] [Google Scholar]
- 6.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev: CD004064. 2010 doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Gertler R, Rosenberg R, Feith M, Schuster T, Friess H. Pouch vs. no pouch following total gastrectomy: Meta-analysis and systematic review. Am J Gastroenterol. 2009;104:2838–2851. doi: 10.1038/ajg.2009.456. [DOI] [PubMed] [Google Scholar]
- 8.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 11.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Netten JP, George EJ, Ashmead BJ, Fletcher C, Thornton IG, Coy P. Macrophage-tumour cell associations in breast cancer. Lancet. 1993;342:872–873. doi: 10.1016/0140-6736(93)92734-B. [DOI] [PubMed] [Google Scholar]
- 13.Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: Association with angiogenesis and poor prognosis. Oncology. 1999;57:138–142. doi: 10.1159/000012021. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–431. [PubMed] [Google Scholar]
- 15.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 16.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 18.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 19.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kübler K, Ayub TH, Weber SK, Zivanovic O, Abramian A, Keyver-Paik MD, Mallmann MR, Kaiser C, Serçe NB, Kuhn W, Rudlowski C. Prognostic significance of tumor-associated macrophages in endometrial adenocarcinoma. Gynecol Oncol. 2014;135:176–183. doi: 10.1016/j.ygyno.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging handbook. New York: Springer; 2001. [Google Scholar]
- 22.Ajani JA, Beaii-saab T, Yang G, et al. NCCN clinical practice guidelines in oncology[M]: gastric cancer. 2009 [Google Scholar]
- 23.Kaluz S, Kaluzova M, Stanbridge EJ. The role of extracellular signal-regulated protein kinase in transcriptional regulation of the hypoxia marker carbonic anhydrase IX. J Cell Biochem. 2006;97:207–216. doi: 10.1002/jcb.20633. [DOI] [PubMed] [Google Scholar]
- 24.Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouysségur J, Berra E. HIF-1alpha and CA IX staining in invasive breast carcinomas: Prognosis and treatment outcome. Int J Cancer. 2007;120:1451–1458. doi: 10.1002/ijc.22436. [DOI] [PubMed] [Google Scholar]
- 25.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahimi-Horn MC, Pouyssegur J. The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Int Rev Cytol. 2005;242:157–213. doi: 10.1016/S0074-7696(04)42004-X. [DOI] [PubMed] [Google Scholar]
- 27.Reed VK, Krishnan S, Mansfield PF, Bhosale PR, Kim M, Das P, Janjan NA, Delclos ME, Lowy AM, Feig BW, et al. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:741–747. doi: 10.1016/j.ijrobp.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu B, Cheng SY. Angiopoietin-2: Development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11:111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- 30.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 31.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: Implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94:101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 34.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: Regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 35.Manzur M, Hamzah J, Ganss R. Modulation of the ‘blood-tumor’ barrier improves immunotherapy. Cell Cycle. 2008;7:2452–2455. doi: 10.4161/cc.7.16.6451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in the present study are included in this published article.