Cells are dependent on their lipid composition for establishing and modulating membrane structural integrity, morphology, metabolism, and other cellular functions. For example, among the thousands of lipid species that compose eukaryotic cell membranes, the abundance and localization of polyunsaturated-fatty-acid-(PUFAs)-containing phospholipids (PUFA-PLs) is a major factor in determining the fluidity of cell membranes1. Since the cis conformation of double bonds in PUFAs hinders efficient stacking of these fatty acid tails, elevated levels of PUFA-PLs contributes to increasing membrane fluidity and thinning.
PUFAs are, however, susceptible to lipid peroxidation via reaction with molecular oxygen at bis-allylic positions, either catalyzed by lipoxygenases or through non-enzymatic mechanisms. Oxygenated PUFAs can serve as lipid signals, regulating inflammatory processes, for example. However, peroxy-PUFAs are prone to decompose into reactive species, which can damage biomolecules, such as proteins and nucleic acids. Thus, cells depend on a critical network of proteins to repair PUFA-PL peroxides; a key protein at the center of this repair network is the selenoprotein glutathione peroxidase 4 (GPX4); it is the only peroxidase in mammals capable of reducing phospholipid hydroperoxides within cell membranes.
When GPX4 activity is compromised, lipid peroxidation can cause ferroptosis, an oxidative iron-dependent form of non-apoptotic cell death. Ferroptosis has been implicated in degenerative diseases—for example, ferroptosis inhibitors are protective in models of Parkinson’s, Huntington’s and Alzheimer’s diseases2. On the other hand, ferroptosis induced by exogenous agents is selectively lethal towards tumors cells that are addicted to GPX4 repair activity, which has suggested that inducing ferroptosis may be a beneficial approach to treating some cancers2.
Cancer cells from tissues of diverse origins have been screened for their sensitivity to ferroptosis-inducing compounds3. Ferroptosis inducers, including GPX4 inhibitors, were found to selectively target cancers with a mesenchymal or otherwise drug-resistant signature. Consistent with the mesenchymal state being associated with drug resistance, another study on persister cancer cells, which are proposed to escape from conventional cytotoxic treatment through a dormant state and then revive to cause tumor relapse, revealed a similar selective dependency on the GPX4 pathway4. Indeed, GPX4 inhibitors were among the compounds most selectively lethal to such persister cells.
Examination of persister cells revealed upregulation of mesenchymal markers and downregulation of epithelial markers. This common mesenchymal character partially explained the extension of GPX4 dependency from high-mesenchymal cancers to persister cells; the underlying mechanisms of GPX4 dependency remains to be further illuminated (Figure 1).
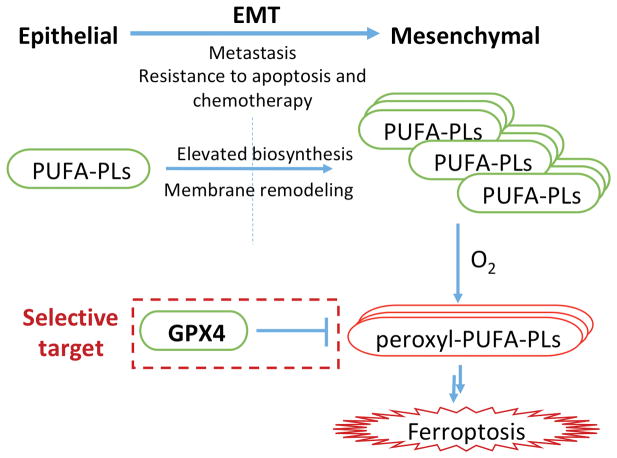
Figure 1.
Cancer cells undergo EMT to acquire resistance to apoptosis induction and chemotherapy. EMT requires elevated level of PUFA-PLs, which inevitably makes cancer cells vulnerable to lipid peroxidation and ferroptosis induced by GPX4 inhibition.
Overexpression of mesenchymal state genes is associated with epithelial-mesenchymal transition (EMT). Since EMT increases motility of tumor cells and enables the invasion of a primary tumor to distant sites, EMT is part of the process of metastasis. EMT also renders cancer cells resistance to apoptosis and chemotherapy5. EMT requires plasma membrane remodeling to increase fluidity, which is associated with elevated biosynthesis of PUFA-PLs. Given that PUFAs are more susceptible to peroxidation than saturated or mono-unsaturated fatty acid PLs, cells in an EMT state should have increased dependency on GPX4 to control lipid peroxides. Therefore, cancer cells undergoing EMT that acquire resistance to apoptosis induction may inevitably become vulnerable to lipid peroxidation and ferroptosis induced by GPX4 inhibition. The necessity of the EMT regulator and lipogenic factor ZEB1 for GPX4 dependency and sensitivity to fatty acid desaturase-2 (FADS2) activation support this theory3. The disabled antioxidant program in persister cells might be a result of this overwhelming lipid oxidation, considering that recycling of GPX4 consumes reduced glutathione (GSH) and NADPH4. Additionally, therapy-resistant cancer cells with a high-mesenchymal state due to a mesenchymal origin may exhibit similar genetic alterations and protein expression, explaining their vulnerability to ferroptosis.
Therapeutic possibilities
As cancer cells can evolve into a high-mesenchymal or related drug-resistant state and become resistant to apoptosis induction, one may be able to selectively target such resistant cancer cells through ferroptosis induction. The most effective compounds in this context might be direct GPX4 inhibitors. For example, in-vivo xenografts of GPX4-knockout high-mesenchymal therapy-resistant melanoma regressed after cessation of ferrostatin-1 (a lipophilic antioxidant that suppresses the effect of loss of GPX4) and did not relapse after ceasing dabrafenib and trametinib treatment, while GPX4-wild-type xenografts continued to grow in both experiments3.
However, since the gpx4 gene is essential for embryonic development and some adult tissue homeostasis in mice, there is a concern about potential toxicity of therapeutic use of GPX4 inhibitors. However, GPX4 inhibitors were selectively lethal to persister cancer cells, with minimal effect on parental cells and non-transformed cells, suggesting that the addiction to GPX4 may create a suitable therapeutic window. Despite this, the therapeutic window of GPX4 inhibitor remains to be examined, such as its potential adverse impact on the nervous system and kidney in particular.
In addition to GPX4 direct inhibitors, persister cells might also be vulnerable to other ferroptosis inducers. For example, erastin and its derivatives, which inhibit system xc- and deplete the intracellular GPX4 cofactor GSH, is also selectively lethal to persister cancer cells, with minimal effects on parental cells. Additionally, high-mesenchymal state cancers are sensitive to statins, which can inhibit biosynthesis of GPX4 and deplete mevalonate-derived coenzyme Q10. Although tumors might develop other resistance mechanisms for compounds such as erastin and statins, the high potential therapeutic index may be useful: for example, knockout of the system xc- gene slc7A11 has minimal effects in mice, suggesting that normal tissues don’t require system xc- activity, at least in mice.
Dependency on GPX4 may thus derive from elevated synthesis and usage of PUFA-PLs by some cancer cells, particularly the most drug-resistant and aggressive types. Further experiments examining this lipid metabolism pathway may reveal additional therapeutic targets with different profiles. The GPX4 pathway thus represents a new frontier in targeting some of the most intractable cancers.
Acknowledgments
Funding: The research of the authors is supported by NCI/NIH (to B. Stockwell, R35CA209896 and P01CA087497; and to S. Schreiber, GM038627 and U01CA176152).
Footnotes
Notes: The authors declare no competing financial interest.
References
- 1.Agmon E, Stockwell BR. Lipid homeostasis and regulated cell death. Curr Opin Chem Biol. 2017;39:83–89. doi: 10.1016/j.cbpa.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viswanathan VS, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hangauer MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]