Abstract
To date, all monotherapy clinical traumatic brain injury (TBI) trials have failed, and there are currently no Food and Drug Administration (FDA)–approved pharmacotherapies for the acute treatment of severe TBI. Due to the complex secondary injury cascade following injury, there is a need to develop multi-mechanistic combinational neuroprotective approaches for the treatment of acute TBI. As central mediators of the TBI secondary injury cascade, both mitochondria and lipid peroxidation-derived aldehydes make promising therapeutic targets. Cyclosporine A (CsA), an FDA-approved immunosuppressant capable of inhibiting the mitochondrial permeability transition pore, and phenelzine (PZ), an FDA-approved monoamine oxidase inhibitor capable of scavenging neurotoxic lipid peroxidation-derived aldehydes, have both been shown to be partially neuroprotective following experimental TBI. Therefore, it follows that the combination of PZ and CsA may enhance neuroprotection over either agent alone through the combining of distinct but complementary mechanisms of action. Additionally, as the first 72 h represents a critical time period following injury, it follows that continuous drug infusion over the first 72 h following injury may also lead to optimal neuroprotective effects. This is the first study to examine the effects of a 72 h subcutaneous continuous infusion of PZ, CsA, and the combination of these two agents on mitochondrial respiration, mitochondrial bound 4-hydroxynonenal (4-HNE), and acrolein, and α-spectrin degradation 72 h following a severe controlled cortical impact injury in rats. Our results indicate that individually, both CsA and PZ are able to attenuate mitochondrial 4-HNE and acrolein, PZ is able to maintain mitochondrial respiratory control ratio and cytoskeletal integrity but together, PZ and CsA are unable to maintain neuroprotective effects.
Keywords: : aldehyde scavenging, cyclosporine A, lipid peroxidation, mitochondria, phenelzine
Introduction
In the United States, there are more than 2.5 million documented cases of traumatic brain injury (TBI) annually,1 which resulted in direct and indirect medical costs of over $75 billion in 2010, with the most severe injuries (those resulting in hospitalization and death) accounting for 90% of these costs.2,3 Currently, there are over 5 million people in the U.S. living with a TBI-related disability.4 Further, over 50% of persons hospitalized due to TBI will continue to suffer from a TBI-associated disability more than a year following injury.5 To date, there are no Food and Drug Administration (FDA)–approved pharmacotherapies for neuroprotection following clinical TBI.6
Although numerous reasons have been cited for the failure of neuroprotective compounds in clinical TBI studies,7,8 due to the complex secondary injury cascade that occurs following injury and encompasses a multitude of neurodegenerative processes, single agent therapies may be unlikely to succeed at completely preventing the devastating neurological consequences that occur following injury.7 In fact, in 2008, the National Institutes of Health (NIH), in conjunction with the Department of Veterans Affairs, convened a workshop focusing on development of combinatorial approaches for the treatment of traumatic brain injury over the first 72 h following injury,7 underscoring the importance of developing multi-mechanistic combinational neuroprotective approaches for acute TBI.
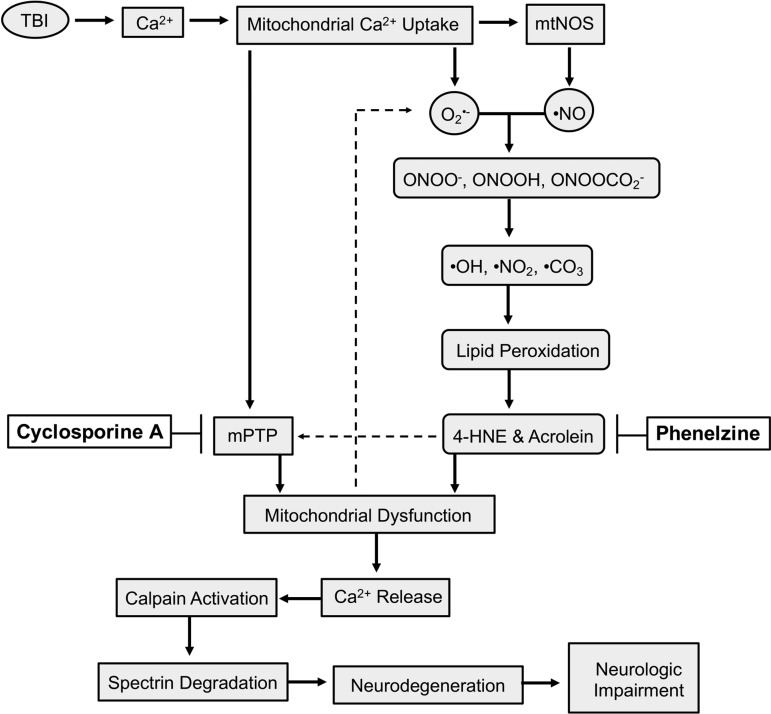
Following injury (Fig. 1), increases in intracellular calcium are taken up by mitochondria in an attempt to maintain calcium homeostasis.9,10 However, high levels of intra-mitochondrial calcium lead to opening of the mitochondrial permeability transition pore (mPTP), resulting in collapse of the mitochondrial membrane potential, cessation of adenosine triphosphate (ATP) production, and release of calcium back into the cytosol.11 Further, increases in intra-mitochondrial calcium can lead to increased generation of mitochondrial-produced reactive oxygen species and reactive nitrogen species (ROS/RNS).12,13 For example, as the levels of mitochondrial calcium increase and the mitochondrial membrane potential depolarizes, electron transport slows, allowing electrons to leak from complex-I to combine with O2 to form superoxide radical (O2•-),14 which can go on to react with nitric oxide radicals (NO•) generated by the calcium-activated mitochondrial nitric oxide synthase, forming peroxynitrite anion (ONOO-).13,15 Peroxynitrite is unique in the fact that it has quite a large diffusion radius, thus allowing it to cross membranes, expanding its area of devastation.16 Peroxynitrite is either protonated to form peroxynitrous acid (ONOOH) or reacts with CO2 to form nitrosoperoxycarbonate (ONOOCO2-), followed by decomposition into the highly reactive radicals, hydroxyl (•OH), nitrogen dioxide (•NO2) or carbonate (CO3•-).13,16,17
FIG. 1.
Simplified schematic demonstrating the role of mitochondrial dysfunction and lipid peroxidation following traumatic brain injury (TBI) and the mechanisms of action of phenelzine (PZ) and cyclosporine A (CsA). Following injury, mitochondria uptake increases in calcium (Ca2+) which leads to opening of the mitochondrial permeability transition pore (mPTP), generation of superoxide (O2•-) and activation of mitochondrial nitric oxide synthase (mtNOS), which leads to formation of nitric oxide (•NO). O2•- and •NO combine to form peroxynitrite anion (ONOO-), which can be protonated or react with CO2 to form peroxynitrous acid (ONOOH) or nitrosoperoxycarbonate (ONOOCO2-), followed by degradation into hydroxyl (•OH), nitrogen dioxide (•NO2) or carbonate (CO3•-), highly reactive radicals which initiate lipid peroxidation. Lipid peroxidation (LP) terminates with the formation of the LP-derived aldehydes 4-hydroxynonenal (4-HNE) and acrolein. 4-HNE and acrolein bind mitochondrial proteins causing mitochondrial dysfunction and can exacerbate opening of the mPTP. Mitochondrial dysfunction itself exacerbates the reactive oxygen species/nitrogen species-lipid peroxidation-aldehyde cascade. Ca2+ is released back into the cytosol upon mPTP opening, activating calpain, which degrades cytoskeletal α-spectrin, resulting in neurodegeneration and neurologic impairment. CsA inhibits mPTP. PZ scavenges the LP-derived aldehydes 4-HNE and acrolein.
These highly reactive radicals initiate lipid peroxidation (LP) of polyunsaturated fatty acids, which are highly enriched in brain cells and cellular and organelle membranes.6,17 As lipid peroxyl radicals are formed, they are able to react with neighboring polyunsaturated acids, resulting in a self-propagating process.6,17 LP terminates upon formation of LP-derived aldehydes, such as 4-hydroxynonenal (4-HNE) and acrolein.6,17 These LP-derived aldehydes have multiple reactive sites, making it possible for them to covalently bind lysine, histidine, arginine, and cysteine protein residues through both Schiff base and Michael addition reactions.6,18–20 Once covalently bound to proteins, including mitochondrial proteins, LP-derived aldehydes induce protein dysfunction and enzyme inactivation, neurotoxicity, and cellular death.21–27 In the case of mitochondria, binding of LP-derived aldehydes exacerbates impairment of bioenergetics and generation of the ROS/RNS-lipid peroxidation-aldehyde cascade, as well as opening of mPTP.28–33 Once permeability transition occurs and calcium has been released back into the cytosol, it can activate calcium-dependent proteases, such as calpain, which can cleave cytoskeletal proteins such as α-spectrin, resulting in further downstream consequences, including neurodegeneration and neurologic impairment.6,11,17,34–37
As central mediators of the TBI secondary injury cascade, both mitochondria and LP-derived aldehydes make promising neuroprotective targets. In fact, several pre-clinical studies have demonstrated the neuroprotective ability of agents that aim to prevent mitochondrial dysfunction or attenuate lipid peroxidation.29,35–48 One of the most extensively investigated mitochondrial protective agents, the FDA-approved immunosuppressant cyclosporine A (CsA), is capable of inhibiting the mPTP37,40,49,50 (Fig. 1), and has been shown to be safe for use clinically in severe TBI patients.51,52 Another drug, phenelzine (PZ; Fig. 1), which is an FDA-approved irreversible non-selective monoamine oxidase inhibitor, contains a hydrazine moiety (-NH-NH2), which is capable of scavenging LP-derived aldehydes.29,44,53–59 Importantly, scavenging of LP-derived aldehydes is likely to offer a more clinically translational approach in regards to therapeutic window, compared with conventional antioxidants, as LP-derived aldehydes are longer lived than their highly reactive free radical predecessors.19,20,60
Both CsA and PZ have individually been shown capable of attenuating pathophysiologic processes in pre-clinical models of neurotrauma.29,37,40–42,44,53,55,61–71 Therefore, it follows that the mPTP inhibitor CsA and the LP-derived aldehyde scavenger PZ may together enhance neuroprotection over either agent alone through the combining of distinct but complementary mechanisms. In fact, CsA was one of the agents identified as a candidate for combinational therapy during the NIH combinational therapy TBI workshop.7 Additionally, as the first 72 h represents a critical time period following injury,7 including the peak of mitochondrial dysfunction, lipid peroxidation, and neuronal cytoskeletal (α-spectrin) degradation,72 it follows that continuous drug infusion over the first 72 h following injury may also lead to optimal neuroprotective effects.19
This is the first study to investigate the neuroprotective effects of the combination CsA and PZ 72 h following severe controlled cortical impact (CCI) injury, a model combining focal, contusive, hemorrhagic, and diffuse injury pathologies.73,74 Additionally, this is the first study to investigate the effects of 72 h continuous infusion of CsA, PZ, and the combination on mitochondrial dysfunction, aldehydic modification of mitochondrial proteins, and α-spectrin degradation 72 h following severe CCI, using a CsA dosing paradigm that previously has been shown to improve cortical tissue sparing following CCI,63 and a PZ dosing paradigm based upon a 12 h intermittent dosing protocol that previously has been shown to be neuroprotective following CCI.29
Methods
Animals
Three-month-old male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in the Division of Laboratory Animal Resources of the University of Kentucky Medical Center. All animal husbandry was conducted in accordance with the University of Kentucky Institutional Animal Care and Use Committee. Animals were housed in a 12 h light/dark cycle and allowed food and water ad libitum.
Experimental design
Two cohorts of animals were randomly assigned to the following subcutaneous continuous infusion dosing paradigm experimental groups: sham, vehicle, PZ, CsA, and phenelzine + cyclosporine A (PZ + CsA), with one cohort of animals being used for all mitochondria experiments and another cohort of animals being used for cortical α-spectrin analysis. An additional cohort of animals was randomly assigned to the following 12 h intermittent PZ dosing paradigm experimental groups: sham, vehicle, or PZ. Following injury and closing of the craniotomy site, all animals in the subcutaneous continuous infusion dosing paradigm, other than sham, were immediately implanted with two subcutaneous osmotic pumps loaded with either drug or vehicle, and additionally received two bolus doses of either drug or vehicle 15 min following impact. Animals within the subcutaneous continuous infusion dosing paradigm received the following doses (Table 1): i) sham (no impact injury, no drug administration); ii) vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline); iii) PZ (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days saline; iv) CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline); PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). Following euthanasia, one group of the subcutaneous continuous infusion dosing paradigm animals were used for mitochondria experiments while the other group was utilized for analysis of α-spectrin degradation.
Table 1.
Subcutaneous Continuous Infusion Dosing Paradigm
| Group | Loading dose (15 min post-injury) | Osmotic pump (immediate) |
|---|---|---|
| Sham | None | None |
| Vehicle | Saline s.c. | Saline |
| Cremophor i.p. | Cremphor | |
| Phenelzine | 10 mg/kg s.c. PZ | 10 mg/kg/day/3 days PZ |
| Cremophor i.p. | Cremophor | |
| Cyclosporine A | Saline s.c. | Saline |
| 20 mg/kg i.p. CsA | 10 mg/kg/day/3 days CsA | |
| Phenelzine + Cyclosporine A | 10 mg/kg s.c. PZ | 10 mg/kg/day/3 days PZ |
| 20 mg/kg i.p. CsA | 10 mg/kg/day/3 days CsA |
Demonstration of the subcutaneous continuous infusion dosing paradigm followed. Osmotic pumps were placed subcutaneously immediately following impact injury and closure of the craniotomy site. Pumps were removed 72 h later at the time of euthanasia. Loading doses were administered 15 min following impact. Cremophor (saline/650 mg cremophor/32.9% ethanol/mL) was prepared to deliver an equal amount cremophor as received by CsA animals. Saline was prepared to deliver an equal amount of saline as PZ animals.
PZ, phenelzine (in saline); CsA, cyclosporine A (in saline/650 mg cremophor/32.9% ethanol/mL); s.c., subcutaneous, i.p., intraperitoneal.
The third cohort of animals was assigned to the 12 h intermittent PZ dosing paradigm. Animals in the 12 h intermittent PZ dosing paradigm received the following dosing schedule (Table 2): sham (no impact injury, no drug administration); vehicle (15 min post-injury bolus dose of subcutaneous saline, followed by subcutaneous bolus saline every 12 h up to and including 60 h); PZ (15 min post-injury bolus dose of 10 mg/kg PZ in saline, followed by subcutaneous bolus 5 mg/kg PZ in saline every 12 h up to and including 60 h).
Table 2.
Phenelzine 12 h Intermittent Dosing Paradigm
| Group | Loading Dose (15 min post-injury) | Maintenance dosing: 12 h, 24 h, 36 h, 48 h, 60 h |
|---|---|---|
| Sham | None | None |
| Vehicle | Saline s.c. | Saline s.c. |
| Phenelzine | 10 mg/kg s.c. PZ | 5 mg/kg s.c. PZ |
Demonstration of the PZ 12 h intermittent dosing paradigm followed. Loading doses were administered 15 min following impact. Maintenance doses were administered every 12 h up to and including 60 h. Animals were euthanized 72 h post-injury. Saline was prepared to deliver an equal amount of saline as PZ animals.
PZ, phenelzine (in saline); s.c., subcutaneous.
CCI TBI
Animals were anesthetized using 4% isoflurane, shaved, and placed into a stereotaxic frame (David Kopf, Tujunga, CA) where isoflurane was maintained at 3% for the duration of the procedure. A midline incision was made to expose the skull, and a 6 mm craniotomy was created lateral to the sagittal suture and midway between lambda and bregma using a Michele Trephine (Miltex, Bethpage, NY). The exposed brain with intact dura received a severe CCI using a computer-controlled pneumatic impact device (TBI 03010; Precision Systems and Instrumentation, Fairfax Station, VA) fitted with a 5 mm beveled tip and set to impact at a depth of 2.2 mm with a 500 msec dwell time and an impact velocity of ∼3.5 m/sec, as described previously.44 After injury, Surgicel (Johnson & Johnson, Arlington, TX) was placed onto the dura and an 8 mm plastic disk was affixed to the skull using tissue adhesive (Gesswein, Bridgeport, CT) to close the craniotomy site, and the incision was closed using wound clips. Sham animals underwent the same procedure but did not receive an impact injury.
Loading dose drug administration
Fifteen minutes following injury, animals assigned to the subcutaneous continuous infusion dosing paradigm, received a loading dose of the appropriate drug. CsA (Perrigo; Minneapolis, MN; 50 mg/mL in saline/650 mg cremophor/32.9% ethanol/mL) was administered intraperitoneal at 20 mg/kg.62,63 Cremophor (CsA vehicle) was administered intraperitoneal to deliver an equivalent volume of saline/cremophor/ethanol. PZ (MP Biochemicals, Solon, OH) was administered subcutaneously at 10 mg/kg in saline.29 Saline (PZ vehicle) was administered subcutaneously to deliver an equivalent volume of saline. Sham animals did not receive injections.
Osmotic pump preparation
The day prior to implantation, osmotic pumps (2ML1, Alzet osmotic pumps; Cupertino, CA) were loaded with appropriate drug concentrations and allowed to prime overnight at 37°C, then maintained at 37°C until the time of implantation. CsA pumps were loaded to deliver 10 mg/kg/day/3 days of CsA (Perrigo; Minneapolis, MN; 50 mg/mL in saline/650 mg cremophor/32.9% ethanol/mL) based on a previously optimized CsA dosing protocol.62,63 Cremphor pumps (CsA vehicle) were loaded to deliver an equivalent volume of saline/cremophor/ethanol. Phenelzine pumps were loaded to deliver 10 mg/kg/3 days of PZ (MP Biochemicals, Solon, OH) in saline based on an approximate 12 h plasma half-life in humans and a previously optimized 12 h intermittent dosing protocol.29 Saline pumps (PZ vehicle) were loaded to deliver an equivalent amount saline.
Implantation of osmotic pumps
Immediately following impact injury and closure of the craniotomy site, a sagittal incision was made approximately half-way down the animal's back. Hemostats were used to create a subcutaneous pocket anterior and posterior to the incision site. Pre-loaded osmotic pumps (2ML1, Alzet osmotic pumps, Cupertino, CA) were inserted into the subcutaneous pockets with the flow moderator positioned away from the incision site. The incision was then closed with wound clips, and animals were allowed to recover while body temperature was maintained at 37°C using a warm water circulating heating pad. Vehicle animals received a saline pump (PZ's vehicle) and a cremophor pump (CsA's vehicle). PZ animals received a PZ pump and a cremophor pump. CsA animals received a CsA pump and a saline pump. PZ + CsA animals received a PZ pump and a CsA pump. Sham animals did not undergo pump implantation.
PZ 12-h intermittent dosing protocol
Fifteen minutes following injury, PZ animals received 10 mg/kg PZ (MP Biochemicals, Solon, OH) in saline subcutaneously, followed by 5 mg/kg PZ subcutaneously every 12 h up to including 60 h.29 Vehicle animals received an equivalent amount of saline at the appropriate time point. Sham animals did not receive injections (Table 2).
Tissue extraction and pump removal
Animals were euthanized 72 h following injury, which represents the peak of mitochondrial dysfunction, LP, and α-spectrin degradation,72 using CO2, followed by decapitation. An 8 mm cortical punch centered over the injury was collected in order to collect the epicenter of injury and the penumbral region. Cortical punches were either processed for isolation of mitochondria or cortical protein. Pumps were removed and residual volumes were measured to ensure proper drug delivery. Animals in which pumps did not run properly, as determined by the removed pumps still having most of their drug still in the pumps, were removed from the study.
Isolation of Ficoll-purified cortical mitochondria
Mitochondria were isolated as described previously.12,44 Cortical punch tissue was immediately homogenized in ice-cold isolation buffer (215 mmol/L mannitol, 75 mmol/L sucrose, 0.1% bovine serum albumin, 20 mmol/L HEPES, 1 mmol/L EGTA, pH 7.2) using Potter-Elvejhem homogenizers. Samples were then centrifuged twice at 1300 g for 3 min at 4°C. Supernatants were additionally centrifuged at 13,000 g for 10 min at 4°C. The crude mitochondrial pellet was resuspended in isolation buffer, placed into a nitrogen bomb at 1200 psi for 10 min at 4°C to release synaptic mitochondria, and then layered onto a discontinuous 7.5% and 10% Ficoll gradient and centrifuged for 100,000 g for 30 min at 4°C. The mitochondrial pellet was resuspended in isolation buffer without EGTA, centrifuged at 10,000 g for 10 min at 4°C to remove Ficoll, and then resuspended in isolation buffer without EGTA to a final concentration of ∼10 mg/kg. Protein concentrations were determined with a BCA protein assay kit (ThermoFisher, Cleveland, OH) and measured at absorbance 562 nm with a BioTek Synergy HT plate reader (Winooski, VT).
Mitochondrial bioenergetics analysis
Mitochondrial respiratory function was measured using a Clark-type electrode in a continuously stirred, sealed, and thermostatically controlled chamber maintained at 37°C (Oxytherm System; Hansatech Instruments, Norfolk, UK). Mitochondria (60 μg-80 μg) were placed into the chamber containing 250 μL of KCl respiration buffer (125 mmol/L KCl, 2 mmol/L MgCl2, 2.5 mmol/L KH2PO4, 0.1% bovine serum albumin, 20 mmol/L HEPES, pH 7.2). Following a 1 min equilibration, complex-I respiration was initiated by addition of 5 mmol/L pyruvate and 2.5 mmol/L malate. Two boluses of 150 μmol/L ADP were added and state III respiration was monitored. The ATP synthase inhibitor oligomycin (2 μmol/L) was added and state IV respiration was monitored. The protonophore FCCP (2 μmol/L) was added and maximal state V(I) respiration was monitored. Complex-I was inhibited by addition of 100 nmol/L rotenone. Complex-II maximal respiration was initiated by the addition of succinate (10 mmol/L) and state V(II) was monitored. Respiratory rates for individual states were calculated as nmol O2 per mg of protein per min. Respiratory control ratio (RCR) was calculated by dividing state III respiration rate (second bolus of ADP) by state IV respiration rate.12,75 Following bioenergetics analysis, remaining mitochondrial protein was immediately frozen at −80°C for use in Western blot.
Western blot analysis: cortical α-spectrin degradation and lipid peroxidation-induced cortical mitochondrial oxidative damage
Cortical punch tissue collected from the cohorts of animals designated for analysis of α-spectrin degradation was immediately placed into ice-cold Triton-lysis buffer (1% Triton, 20 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L EGTA, 10 mmol/L EDTA, 20 mmol/L HEPES, pH 7.4) containing protease inhibitors (Complete Mini™ Protease Inhibitor Cocktail tablet, Roche). Similarly, mitochondria protein being stored at −80°C following bioenergetics analysis was thawed and resuspended in lysis buffer. All samples were sonicated, vortexed, and then centrifuged at 14,000 rpm for 30 min at 4°C. Protein concentrations were determined with a BCA protein assay kit (ThermoFisher, Cleveland, OH) and measured at absorbance 562 nm with a BioTek Synergy HT plate reader (Winooski, VT). Due to the limited yield of mitochondrial protein following Ficoll-purified isolation, bioenergetics analysis and processing for Western blot, only samples containing greater than 100 μg of protein, allowing for analysis of both 4-HNE and acrolein, were utilized for Western blot. For analysis of α-spectrin, 10 μg of protein was run on a 3–8% Tris-Acetate Criterion™ XT polyacrylamide gel (Bio-Rad, Hercules, CA) with XT Tricine Running Buffer (Bio-Rad). For analysis of 4-HNE or acrolein, 50 μg of protein was run on a 4–12% Tris-Acetate Criterion™ XT polyacrylamide gel (Bio-Rad) with MOPS buffer (Bio-Rad). Following separation of proteins by polyacrylamide gel, protein was transferred to a nitrocellulose membrane using a semi-dry electro-transferring unit for 15 min (α-spectrin) or 1 h (4-HNE, acrolein) at 15V. Following transfer, membranes were blocked at room temperature for 1 h in Tris-buffered saline (TBS) containing 5% milk. Membranes were incubated with the following primary antibodies in TBS with Tween (TBST) containing 5% milk overnight at 4°C: mouse monoclonal anti-αII-spectrin (Enzo, Farmingdale, NY, 1:5,000), rabbit polyclonal anti-β-tubulin (Abcam, Cambridge, CA, 1:5000-1:10,000), rabbit polyclonal anti-4HNE (Alpha Diagnostics, San Antonio, TX; 1:1000), rabbit polyclonal anti-acrolein (Abcam, Cambridge, CA, 1:2000), and rabbit polyclonal anti-VDAC (EMD Millipore, Billerica, MA, 1:30,000). Membranes were washed in TBST following incubation with primary antibody.
Membranes were incubated with the following secondary antibodies in TBST with 5% milk for 1 h at room temperature: goat anti-rabbit IgG IRdye800CW (Rockland, Limerick, PA, 1:5000), goat anti-rabbit IgG Alexa Fluor 680 (ThermoFisher, Waltham, MA1:10,000), and goat anti-mouse IgG IRDye800CW (Rockland, Limerick, PA, 1:5000) Membranes were washed in TBST, and then imaged and quantified using the Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). For analysis of α-spectrin degradation, α-spectrin breakdown products were normalized to tubulin. For analysis of mitochondrial protein, 4-HNE and acrolein were normalized to VDAC. A protein loading control was loaded onto each gel to control for inter-gel variability. For α-spectrin, bands at 145 kD and 150 kD were analyzed. Due to broad binding of 4-HNE and acrolein to lysine, histidine, arginine, cysteine protein residues, as well as differential banding patterns between 4-HNE and acrolein,72 mitochondrial 4-HNE was analyzed between 80 kD-150 kD and mitochondrial acrolein was analyzed between 60 kD-100 kD.
Statistical analysis
Statistical analysis was conducted using Prism version 7.0 (Graph Pad, San Diego, CA). Results are reported as mean ± standard deviation. Analysis was done by one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls (SNK) post hoc analysis when appropriate. A p value <0.05 was considered significant. Grubb's test was used to remove statistical outliers.
Results
CCI TBI–induced mitochondrial bioenergetics dysfunction
State III
ADP activation of ATP synthase and coupling of electron transport with oxidative phosphorylation. A one-way ANOVA revealed a statistically significant effect across all groups (F [4,35] = 19.45, p < 0.0001). Post hoc testing (SNK) revealed that compared with sham, state III respiration was significantly impaired for vehicle (p < 0.0001), PZ (p < 0.0001), CsA (p < 0.0001), and PZ + CsA (p < 0.0001), thus indicating that severe CCI significantly decreased state III respiration compared with sham, and that 72 h subcutaneous continuous infusion of neither PZ, CsA, nor PZ + CsA was able to significantly attenuate CCI-induced decreases in state III respiration (Fig. 2A).
FIG. 2.
Effect of the 15 min post-injury loading dose +72 h subcutaneous continuous infusion dosing paradigm on mitochondrial oxygen consumption 72 h following severe cortical impact injury for (A) state III (pyruvate/malate/ADP), (B) state IV (oligomycin), (C) state V(I) (FCCP), and (D) state V(II) (rotenone/succinate). Sham (no impact injury, no drug administration). Vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ = phenelzine (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). CsA = cyclosporine A (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; Subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). One-way analysis of variance followed by Student-Newman-Keuls post hoc test. ***p < 0.001; ****p < 0.0001, compared with sham. Error bars represent mean ± standard deviation. n = 8 rats per group.
State IV
oligomycin inhibition of ATP synthase. A one-way ANOVA revealed a statistically significant effect across all groups (F [4,35] = 11.44, p < 0.0001). Post hoc testing (SNK) revealed that compared with sham, state IV respiration was significantly impaired for vehicle (p < 0.0001), PZ (p < 0.0001), CsA (p < 0.001), and PZ + CsA (p < 0.0001), thus indicating that severe CCI significantly decreased state IV respiration compared with sham, and that 72 h subcutaneous continuous infusion of neither PZ, CsA, nor PZ + CsA was able to significantly attenuate CCI-induced decreases in state IV respiration (Fig. 2B).
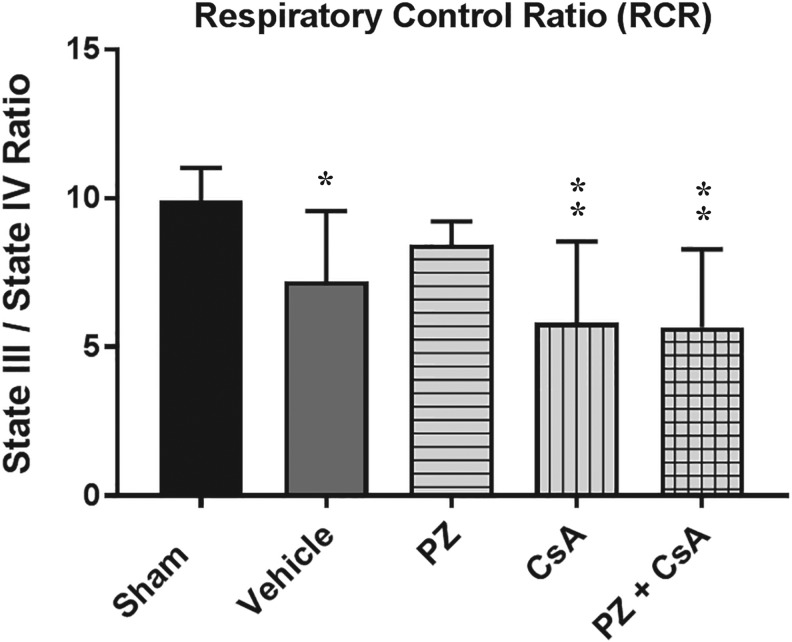
RCR: State III respiration/state IV respiration
A one-way ANOVA revealed a statistically significant effect across all groups (F [4,35] = 5.961, p = 0.0009). Post hoc testing (SNK) revealed that compared with sham, RCR was significantly impaired for vehicle (p < 0.05), CsA (p < 0.01), and PZ + CsA (p < 0.01), whereas PZ was not significantly different from either vehicle (p > 0.05) or sham (p > 0.05), thus indicating that severe CCI significantly decreased RCR compared with sham, and that 72 h subcutaneous continuous infusion of neither CsA nor PZ + CsA was able to significantly attenuate CCI-induced decreases in RCR. However, PZ was able to maintain RCR, compared with sham (Fig. 3).
FIG. 3.
Effect of the 15 min post-injury loading dose +72 h subcutaneous continuous infusion dosing paradigm on mitochondrial respiratory control ratio (state III respiration/state IV respiration) 72 h following severe cortical impact injury. Sham (no impact injury, no drug administration). Vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ = phenelzine (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). CsA = cyclosporine A (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). One-way analysis of variance followed by Student-Newman-Keuls post hoc test. *p < 0.05; **p < 0.01, compared with sham. Error bars represent mean ± standard deviation. n = 8 rats per group.
State V(I): FCCP protonophore addition and initiation of maximal complex-I respiration
A one-way ANOVA revealed a statistically significant effect across all groups (F [4,35] = 33.55, p < 0.0001). Post hoc testing (SNK) revealed that compared with sham, state V(I) respiration was significantly impaired for vehicle (p < 0.0001), PZ (p < 0.0001), CsA (p < 0.0001), and PZ + CsA (p < 0.0001), thus indicating that severe CCI significantly decreased state V(I) respiration compared with sham, and that 72 h subcutaneous continuous infusion of neither PZ, CsA, nor PZ + CsA was able to significantly attenuate CCI-induced decreases in state V(I) respiration (Fig. 2C).
State V(II): rotenone inhibition of complex-I and succinate activation of maximal complex-II respiration
A one-way ANOVA revealed a statistically significant effect across all groups (F [4,35] = 19.55, p < 0.0001). Post hoc testing (SNK) revealed that compared with sham, state V(II) respiration was significantly impaired for vehicle (p < 0.0001), PZ (p < 0.0001), CsA (p < 0.0001), and PZ + CsA (p < 0.0001), thus indicating that severe CCI significantly decreased state V(II) respiration compared with sham, and that 72 h subcutaneous continuous infusion of neither PZ, CsA, nor PZ + CsA was able to significantly attenuate CCI-induced decreases in state V(II) respiration (Fig. 2D).
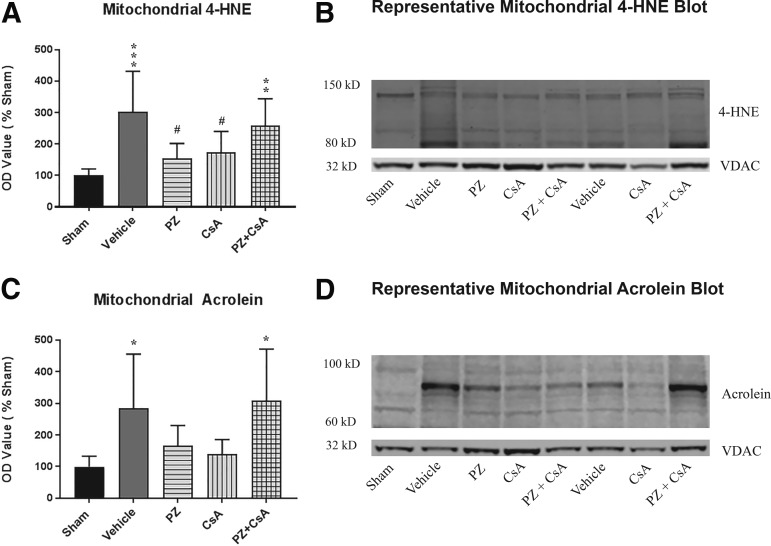
CCI TBI modification of mitochondrial proteins by 4-HNE and acrolein
4-HNE
A one-way ANOVA revealed a statistically significant effect across all groups (F [4,30] = 6.765, p = 0.0005). Post hoc testing (SNK) revealed that compared with sham, binding of 4-HNE to mitochondrial proteins was significantly increased for vehicle (p < 0.001) and PZ + CsA (p < 0.01), and that binding of 4-HNE to mitochondrial proteins was significantly decreased in the PZ group (p < 0.05) and the CsA group (p < 0.05) compared with vehicle, with neither the PZ group or the CsA group being significantly different from sham (p > 0.05), thus indicating that severe CCI significantly increases formation of 4-HNE bound mitochondrial proteins compared with sham, and that 72 h subcutaneous continuous infusion of either PZ or CsA, but not PZ + CsA, significantly decreases CCI-induced formation of 4-HNE bound mitochondrial proteins (Fig. 4A).
FIG. 4.
Effect of the 15 min post-injury loading dose +72 h subcutaneous continuous infusion dosing paradigm on binding of (A) 4-hydroxynonenal (4-HNE) or (C) acrolein to mitochondrial protein 72 h following severe cortical impact injury as assessed by Western blot. Representative Western blot images demonstrating analysis of (B) 4-HNE between 150 kD and 80 kD and internal loading control voltage-dependent anion channel (VDAC) and analysis of (D) Acrolein between 100 kD and 60 kD and internal loading control VDAC. Sham (no impact injury, no drug administration). Vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ = phenelzine (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). CsA = cyclosporine A (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). One-way analysis of variance followed by Student-Newman-Keuls post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, compared with sham; #p < 0.05, compared with vehicle. Error bars represent mean ± standard deviation. n = 6-8 rats per group.
Acrolein
A one-way ANOVA revealed a statistically significant effect across all groups (F [4,32] = 4.607, p = 0.0047). Post hoc testing (SNK) revealed that compared with sham, binding of acrolein to mitochondrial proteins was significantly increased for vehicle (p < 0.05) and PZ + CsA (p < 0.05). The PZ + CsA group also was significantly increased compared with the CsA group (p < 0.05). Although the PZ group and the CsA group were not significantly different from vehicle (p > 0.05), there was a 42% and 51% decrease in mean acrolein binding of mitochondrial proteins compared with vehicle for the PZ and CsA groups, respectively. Further, the PZ group and the CsA group were not significantly different from sham (p > 0.05), thus indicating that severe CCI significantly increases formation of acrolein bound mitochondrial proteins compared with sham, and that 72 h subcutaneous continuous infusion of either PZ or CsA, but not PZ + CsA, decreases CCI-induced formation of acrolein bound mitochondrial proteins (Fig. 4C).
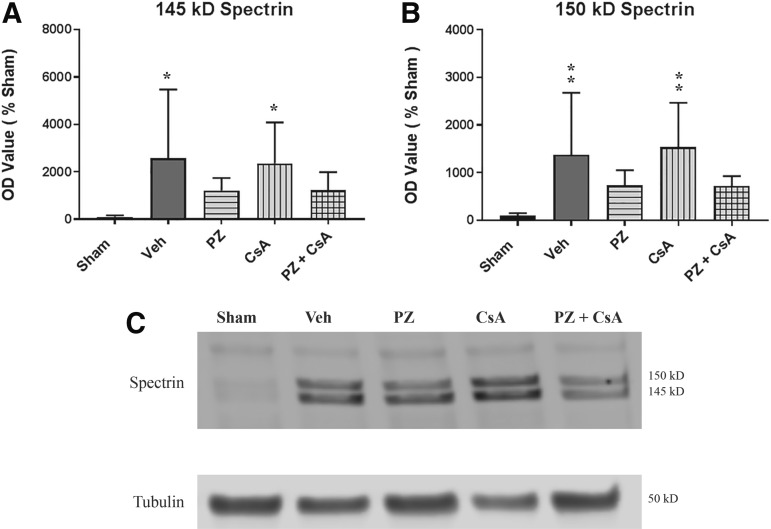
CCI TBI–induced α-spectrin degradation: continuous infusion dosing paradigm
145 kD
Calpain-dependent α-spectrin breakdown product. A one-way ANOVA revealed a statistically significant effect across all groups (F [4,34] = 3.032, p = 0.0306). Post hoc testing (SNK) revealed that compared with sham, 145 kD α-spectrin breakdown products were significantly increased for vehicle (p < 0.05) and CsA (p < 0.05). Although the PZ group and the PZ + CsA group were not significantly different from vehicle (p > 0.05), there was a 53% and a 53% decrease in mean absorbance compared with vehicle for the PZ and PZ + CsA groups, respectively. Further, the PZ group and the PZ + CsA group were not significantly different from sham (p > 0.05) or from each other (p > 0.05), thus indicating that severe CCI significantly increases formation of the 145 kD calpain dependent α-spectrin breakdown product, and that 72 h subcutaneous continuous infusion of either PZ or PZ + CsA maintains cytoskeletal α-spectrin integrity to a similar degree, as compared with sham (Fig. 5A).
FIG. 5.
Effect of the 15 min post-injury loading dose +72 h subcutaneous continuous infusion dosing paradigm on degradation of cytoskeletal protein α-spectrin into (A) 145 kD (calpain dependent) and (B) 150 kD (calpain and caspase-3 dependent) α-spectrin breakdown products 72 h following severe cortical impact injury as assessed by Western blot. (C) Representative Western blot image demonstrating analysis of 145 kD and 150 kD α-spectrin bands and the internal loading control tubulin. Sham (no impact injury, no drug administration). Veh = Vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ = phenelzine (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). CsA = cyclosporine A (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). One-way analysis of variance followed by Student-Newman-Keuls post hoc test. *p < 0.05, **p < 0.01, compared with sham. Error bars represent mean ± standard deviation. n = 7-9 rats per group.
150 kD
Calpain/caspase 3-dependent α-spectrin breakdown product. A one-way ANOVA revealed a statistically significant effect across all groups (F [4,34] = 4.49, p = 0.0051). Post hoc testing (SNK) revealed that compared with sham, 150 kD α-spectrin breakdown products were significantly increased for vehicle (p < 0.01) and CsA (p < 0.01). Although the PZ group and the PZ + CsA group were not significantly different from vehicle (p > 0.05), there was a 47% and 47% decrease in mean absorbance compared with vehicle for the PZ and PZ + CsA groups, respectively. Further, the PZ group and the PZ + CsA group were not significantly different from sham (p > 0.05) or from each other (p > 0.05), thus indicating that severe CCI significantly increases formation of the 150 kD calpain/caspase 3-dependent α-spectrin breakdown product, and that 72 h subcutaneous continuous infusion of either PZ or PZ + CsA maintain cytoskeletal α-spectrin integrity to a similar degree, as compared with sham (Fig. 5B).
CCI TBI–induced α-spectrin degradation: PZ 12 h intermittent dosing paradigm
145 kD
Calpain dependent α-spectrin breakdown products. A one-way ANOVA revealed a statistically significant effect across all groups (F [2,21] = 6.128, p = 0.008). Post hoc testing (SNK) revealed that compared with sham, 145 kD α-spectrin breakdown products were significantly increased for vehicle (p < 0.05) and PZ (p < 0.01), thus indicating that severe CCI significantly increases formation of the 145 kD calpain-dependent α-spectrin breakdown product, and that a subcutaneous bolus dose of PZ (10 mg/kg) 15 min post-injury followed by subcutaneous maintenance doses (5 mg/kg) every 12 h up to and including 60 h is unable to attenuate degradation (Fig. 6A).
FIG. 6.
Effect of the 15 min post-injury loading dose +12 h intermittent phenelzine (PZ) dosing paradigm on degradation of cytoskeletal protein α-spectrin into (A) 145 kD (calpain dependent) and (B) 150 kD (calpain and caspase-3 dependent) α-spectrin breakdown products 72 h following severe cortical impact injury as assessed by Western blot. (C) Representative Western blot image demonstrating analysis of 145 kD and 150 kD α-spectrin bands and the internal loading control tubulin. Sham (no impact injury, no drug administration). Vehicle (15 min post-injury bolus dose subcutaneous saline, followed by subcutaneous bolus saline every 12 h up to and including 60h). PZ = phenelzine (15 min post-injury bolus dose of 10 mg/kg PZ in saline, followed by subcutaneous bolus 5 mg/kg PZ in saline every 12 h up to and including 60 h). One-way analysis of variance followed by Student-Newman-Keuls post hoc test. *p < 0.05, **p < 0.01, compared with sham. Error bars represent mean ± standard deviation. n = 7-9 rats per group.
150 kD
Calpain/caspase 3-dependent α-spectrin breakdown products. A one-way ANOVA revealed a statistically significant effect across all groups (F [2,21] = 5.426, p = 0.0126). Post hoc testing (SNK) revealed that compared with sham, 150 kD α-spectrin breakdown products were significantly increased for vehicle (p < 0.05) and PZ (p < 0.05), thus indicating that severe CCI significantly increases formation of the 150 kD calpain/caspase 3-dependent α-spectrin breakdown product, and that a subcutaneous bolus dose of PZ (10 mg/kg) 15 min post-injury followed by subcutaneous maintenance doses (5 mg/kg) every 12 h up to and including 60 h is unable to attenuate degradation (Fig. 6B).
Discussion
TBI induction of mitochondrial dysfunction and lipid peroxidation-derived aldehyde generation
It is has been firmly established that mitochondrial dysfunction, lipid peroxidation, and production of LP-derived aldehydes occurs following TBI, and that each contributes to TBI pathology.6,12,13,17,72,76,77 For example, mitochondrial dysfunction can contribute to induction of the ROS/RNS-lipid peroxidation-aldehyde cascade, energy failure, and downstream consequences such cytoskeletal and neuronal degeneration and neurologic impairment,6,11–13,17,34,36,37 while the LP-derived aldehydes, including 4-HNE and acrolein, can have directly toxic effects on cellular function,21,23–27,29–33,48,57 with a notably devastating effect on the mitochondria.
Cyclosporine A and inhibition of the mitochondrial permeability transition pore
Inhibition of the mitochondrial permeability transition pore utilizing the FDA-approved immunosuppressant CsA as a neuroprotective strategy following TBI has remained a focus among the neurotrauma community for the past 20 years. Overall, in experimental TBI, CsA or its non-immunosuppressive analog NIM811 have been shown to prevent mitochondrial dysfunction, decrease oxidative damage, attenuate axonal pathology and neurodegeneration, and improve behavioral function.37,40–42,61,65–67,69,71,78
The continuous CsA dosing paradigm utilized here—20 mg/kg CsA delivered intraperitoneal 15 min post-injury combined with subcutaneous continuous infusion of 10 mg/kg/day/3 day CsA—is based on previously optimized dosing paradigms demonstrating that continuous infusion of CsA is more neuroprotective than bolus dosing.62,63 Additionally, a 72 h continuous infusion CsA dosing protocol has proven safe for use in severe TBI and was suggestive of improved outcomes.51 However, previous to our study, the effects of a continuous infusion of CsA following experimental TBI had not been assessed using outcome measures distinct from cortical tissue sparing. Our results indicate (Table 3) that while CsA was able to significantly decrease TBI-induced increases in aldehydic-modified mitochondrial proteins (Fig. 4), it was unable to prevent mitochondrial respiratory dysfunction (Fig. 2 and Fig. 3) or cytoskeletal α-spectrin degradation (Fig. 5).
Table 3.
Summary of Results: Subcutaneous Continuous Infusion Dosing Paradigm
| Vehicle | PZ | CsA | PZ + CsA | |
|---|---|---|---|---|
| Mitochondrial RCR | ↓* | ↔^ | ↓* | ↓* |
| Mitochondrial individual respiratory states | ↓* | ↓* | ↓* | ↓* |
| Mitochondrial 4-HNE | ↑* | ↓# | ↓# | ↑* |
| Mitochondrial acrolein | ↑* | ↔^ | ↔^ | ↑* |
| Spectrin degradation | ↑* | ↔^ | ↑* | ↔^ |
Effect of the 15 min post-injury loading dose +72 h subcutaneous continuous infusion dosing paradigm on the above listed outcome measures 72 h following severe cortical impact injury. Vehicle (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ (15 min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline). CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: saline). PZ + CsA (15 min post-injury bolus dose: intraperitoneal 20 mg/kg CsA in cremophor/ethanol/saline; 15 min post-injury bolus dose: subcutaneous 10 mg/kg PZ in saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days CsA in cremophor/ethanol/saline; subcutaneous osmotic pump: 10 mg/kg/day/3 days PZ in saline).
PZ, phenelzine; CsA, cyclosporine A; RCR, respiratory control ratio (state III/state IV); 4-HNE, 4-hydroxynonenal; ↓* = significantly decreased from sham, ↑* = significantly increased from sham, ↓# = significantly decreased from vehicle, ↔^ = not significantly different from sham.
The fact that continuous infusion of CsA is able to decrease TBI-induced increases in mitochondrial bound 4-HNE and acrolein, is consistent with the fact that inhibition of the permeability transition pore in brain mitochondria is able to prevent calcium-induced increases in ROS,79 which would in turn decrease the ROS/RNS-lipid peroxidation-aldehyde cascade (Fig. 1). However, this does not seem to be enough to maintain mitochondrial respiratory function or prevent downstream calpain-mediated cytoskeletal degradation, at least at the 72 h post-injury time point examined. This finding is not entirely unprecedented, as there are known limitations to the ability of CsA to prevent permeability transition.72,80–83 However, it also is possible that continuous infusion of CsA did have a protective effect on mitochondrial respiration that was masked in our total mitochondrial preparations (synaptic and non-synaptic mitochondria) due to the fact that CsA has a greater neuroprotective effect on synaptic mitochondria than non-synaptic mitochondria,41 but at 72 h post-injury synaptic mitochondria make up a minority of the mitochondrial population.74,84,85 However, we consider this explanation unlikely when taken in conjunction with the fact that continuous infusion of CsA also was unable to prevent cytoskeletal α-spectrin degradation (Fig. 5).
An additional caveat to consider is the fact that the CsA dosing paradigm utilized in this study was originally optimized using cortical tissue sparing.62,63 It is known that CsA preservation of cortical tissue following experimental TBI has a U-shaped dose–response curve62 and it is possible that that the dose–response curve for CsA in relation to cortical tissue sparing is not identical to the dose–response curve for CsA in relation to mitochondrial respiratory protection. This speculation, along with the fact that CsA has multiple mechanisms of action,82,86 may explain some of the disparities seen across CsA TBI studies, because although there have been a preponderance of studies supporting CsA's neuroprotective capabilities, this is not the first study to demonstrate that CsA neuroprotection can vary across outcome measures.87
Phenelzine scavenging of lipid peroxidation-derived aldehydes 4-HNE and acrolein
Several approaches have been utilized in an attempt to attenuate the lipid peroxidation cascade following neurotraumatic injury ranging from the use of antioxidants to the use of iron chelators.6,17,35,88 However, many of these approaches are limited by a variety of factors, including limited therapeutic window, blood–brain barrier penetrability and elimination rate.6,17 Fortunately, scavenging of LP-derived aldehydes, such as 4-HNE and acrolein (the stable final breakdown products of lipid peroxidation) represents a practical therapeutic approach.6,19
Phenelzine, a monoamine oxidase inhibitor clinically used for intractable depression contains a hydrazine moiety (-NH-NH2) making it capable of binding and scavenging both free LP-derived neurotoxic aldehydes, such as 4-HNE and acrolein, as well as binding aldehyde-protein conjugates, including mitochondrial protein conjutates, preventing further cross-linking.19,56,57,59,89,90
Both in vitro and in vivo studies have shown hydrazine-containing compounds, including PZ, to be neuroprotective in models of brain and spinal cord injury, decreasing generation of LP-derived aldehydes, improving mitochondrial bioenergetics, attenuating tissue damage, and improving behavioral outcomes.29,44,47,48,53,55 Importantly, ex vivo studies have confirmed that the ability of PZ to protect mitochondria against exogenously administered 4-HNE or acrolein is due to the presence of the hydrazine moiety.29
The continuous PZ dosing paradigm utilized here, 10 mg/kg PZ delivered subcutaneously 15 min post-injury combined with subcutaneous continuous infusion of 10 mg/kg/day/3 days PZ, was based on previous work showing that 12 h intermittent administration of PZ (10 mg/kg PZ delivered subcutaneously 15 min post-injury followed by 5 mg/kg PZ maintenance dosing every 12 h) was neuroprotective following severe CCI,29 with the hypothesis being that continuous infusion of PZ should further enhance neuroprotection over the 12 h intermittent dosing paradigm.
Our results indicate (Table 3) that continuous infusion of PZ was able to maintain RCR (Fig. 3), decrease TBI-induced increases in aldehydic-modified mitochondrial proteins (Fig. 4), and maintain cytoskeletal α-spectrin integrity (Fig. 5), although it was unable to prevent decreases in individual states of mitochondrial respiration (Fig. 2). Encouragingly, this data parallels the results obtained in our 12 h intermittent PZ dosing studies, in which PZ also demonstrated maintenance of RCR and decreases in 4-HNE and acrolein modification of mitochondrial proteins29 without being able to attenuate decreases in individual mitochondrial respiratory states (unpublished data). However, one important improvement was seen with the continuous infusion PZ dosing paradigm (Table 4) because whereas continuous infusion of PZ was able to maintain cytoskeletal α-spectrin integrity (Fig. 5), 12 h intermittent dosing of PZ was not (Fig. 6). Although both of these dosing paradigms were designed based on an approximate 12 h plasma half-life of PZ in humans, the fact that the continuous infusion dosing paradigm represented an improvement over intermittent dosing is unsurprising given recent findings that suggest the half-life of PZ in rats may be much shorter than 12 h.53
Table 4.
Spectrin Degradation: Phenelzine Continuous Infusion Vs. Phenelzine Intermittent Dosing
| PZCI | PZID | |
|---|---|---|
| Spectrin degradation | ↔^ | ↑* |
Comparison of the effect of the PZ continuous infusion dosing paradigm versus the PZ intermittent dosing paradigm on α-spectrin degradation 72h following severe cortical impact injury. PZCI = phenelzine continuous infusion (15min post-injury bolus dose: intraperitoneal cremophor/ethanol/saline, 15min post-injury bolus dose: subcutaneous 10mg/kg PZ in saline, Subcutaneous osmotic pump: cremophor/ethanol/saline, Subcutaneous osmotic pump: 10mg/kg/day/3d PZ in saline). PZID = phenelzine intermittent dosing (15min post-injury subcutaneous bolus dose of 10mg/kg PZ in saline followed by subcutaneous bolus dose of 5mg/kg PZ in saline every 12h up to and including 60h). ↑* = significantly increased from sham,
PZCI, continuous infusion; PZID, intermittent dosing.
Multiple possibilities may explain the ability of continuous infusion of PZ to decrease TBI-induced increases in mitochondrial bound 4-HNE and acrolein and maintain RCR and cytoskeletal α-spectrin integrity without being able to improve individual mitochondrial respiratory states. First, the ability of PZ to scavenge aldehydes19,59 would have a direct effect on decreasing aldehyde binding of mitochondrial proteins, as well as lead to attenuation of the mitochondrial dysfunction-ROS/RNS-lipid peroxidation-aldehyde cascade (Fig. 1), further decreasing binding of 4-HNE and acrolein to mitochondrial proteins. This decrease in mitochondrial bound 4-HNE and acrolein would then prevent mitochondrial permeability transition,91 decreasing the release of calcium back into the cytosol and attenuating calpain-dependent α-spectrin degradation. Further, the decrease in 4-HNE and acrolein mitochondrial binding also may result in a slight improvement in mitochondrial bioenergetics, resulting in maintenance of RCR, a general measure of mitochondrial health, but one that does not have a significant enough effect on mitochondrial bioenergetics to impact individual states of respiration.
Second, it is possible that we have again masked a significant mitochondrial protective effect. As stated previously, PZ can bind mitochondrial aldehyde-protein conjugates.19,59 It is unknown what effect this has on post-injury mitochondrial dynamics and mitophagy. In fact, in general, the long-term consequences of drug-aldehyde adduct formation are unknown.57 It is possible that the binding of PZ to mitochondria which are beyond saving is preventing these mitochondria from being degraded by normal post-injury processes while also significantly improving the bioenergetics of less damaged mitochondria. The unusual presence of these considerably more damaged mitochondria in our isolated mitochondria preps could mask improvements to individual states of mitochondrial respiration. Such an explanation would be consistent with an overall mitochondrial protective effect suggested by the α-spectrin data, as well as the improvements in tissue sparing seen in our previous 12 h intermittent PZ dosing studies,29 despite a lack of improvement to individual mitochondrial respiratory states (unpublished data).
Last, although in vitro experiments comparing PZ with the non-aldehyde scavenging monoamine oxidase inhibitor pargyline have confirmed that phenelzine's aldehyde scavenging properties are due to the presence of a hydrazine moiety,29,56 it is unknown what effect monoamine oxidase inhibition has on the bioenergetics of injured mitochondria or in the broader context of TBI pathophysiology. The function of catecholamines and monoamine oxidase following brain injury remains controversial.92–95 In fact, for PZ to remain a clinically translation pharmacotherapy, its role as a monoamine oxidase inhibitor in the context of brain injury must be elucidated. As such, we have planned future experiments aimed at determining the effect of monoamine oxidase inhibition following TBI.
Phenelzine + CsA: a multi-mechanistic combinatory pharmacotherapy approach
As pointed out earlier, all monotherapy clinical trials for TBI have failed, and although there have been numerous reasons cited for these failures, due to the heterogeneity of injury and the complexity of the secondary injury cascade, there has been a call for pre-clinical studies to focus on the development of combinational therapies for the acute (first 72 h) treatment of TBI.7 Several strategies have been proposed to aid in the selection of combinatorial agents, including but not limited to therapies that affect multiple targets and therapies that target convergent pathways.7 This study was aimed at achieving both these goals by combining two drugs that have separate targets, the mPTP inhibitor CsA and the LP-derived aldehyde scavenger PZ both of which should also be capable of attenuating the convergent mitochondria dysfunction-ROS/RNS-lipid peroxidation-aldehyde cascade (Fig. 1). In fact, CsA was one of the drugs identified by the combinational therapy TBI working group as being a promising combinational candidate.7
Our results indicate (Table 3) that continuous infusion of PZ (10 mg/kg PZ subcutaneous 15 min post-injury +10 mg/kg/day subcutaneous infusion of PZ for 3 days) combined with continuous infusion of CsA (20 mg/kg CsA intraperitoneal 15 min post-injury +10 mg/kg/day subcutaneous infusion of CsA for 3 days) is unable to improve mitochondrial bioenergetics (either RCR or individual respiratory states; Fig. 2 and Fig. 3) or attenuate 4-HNE or acrolein bound to mitochondrial proteins (Fig. 4). Interestingly, it seems that the combining of PZ and CsA, negates the protective effects that individual administration of PZ or CsA had on formation of 4-HNE and acrolein bound mitochondrial proteins (Fig. 4). Although the combination of PZ and CsA was able to maintain cytoskeletal α-spectrin integrity (Fig. 5), it did so to a degree similar to PZ alone, suggesting that this is most likely an effect mainly attributable to PZ.
While these combinatorial results are disappointing, they are not entirely surprising given the fact that individually the continuous infusion CsA dosing paradigm utilized, which had been previously optimized based on cortical tissue sparing,62,63 did not have a protective effect on all of the neurochemical outcome measures studied.
Moreover, this study combined two dosing paradigms that had been previously optimized for each drug individually.29,63 In fact, this is not the first study to find that utilization of doses optimized for monotherapy did not result in neuroprotection once combined.96 Indeed, the complex pharmacokinetics and pharmacodynamics that results from the combining of multiple drugs has been identified as one of the most challenging hurdles in the development of combinational therapies for the treatment of TBI.7 Although utilization of an alternate dosing paradigm following dose–response studies of the combined drugs together could lead to improved results, upon further consideration, the complex pharmacokinetics of each individual drug may make the clinical translation of the combination unfeasible.
For example, CsA is known to be metabolized by cyp3A4,97 and although PZ is not technically classified as a cyp3A4 inhibitor, there is data to suggest that PZ is capable of inhibiting cyp3A4.98,99 Further, CsA has a U-shaped dose–response curve64 and has limited blood–brain barrier penetrability,100 which increases following injury due to blood–brain barrier disruption101 and decreases in mitochondrial production of ATP,10,11 leading to failure of ATP-dependent p-glycoprotein drug efflux pumps, of which CsA is both a substrate and an inhibitor.100 Further, PZ itself has a complicated pharmacology being both a monoamine oxidase inhibitor and a monoamine oxidase substrate, and has several active metabolites.102
Along similar lines, although the choice of the combination PZ and CsA was aimed at targeting distinct mechanisms that converge on overlapping pathways (Fig. 1), consistent with stated combinational TBI therapy design goals,7 both of these drugs are capable of physically binding the mitochondria CsA through binding of the matrix protein cyclophilin D,49,50,82,103 and PZ both through binding of 4-HNE and acrolein bound mitochondrial conjugates19,57 and through irreversible binding of the outer mitochondrial membrane protein monoamine oxidase,94,104 which in hindsight may have resulted in too many non-physiologic interactions for the mitochondria to handle.
It is possible that assessment of additional outcome measures such as cortical tissue sparing or behavior could have yielded improved results. Indeed, achieving behavioral improvements is an utmost goal in TBI research. However, it must be noted that although a recent summary of NIH-funded pre-clinical combinatorial TBI drug studies reported improvements in non-behavioral outcomes, these positive effects were not maintained in long-term behavioral studies.96 Therefore, it is unlikely that utilization of our current PZ + CsA combinational dosing paradigm would lead to improvements in more difficult-to-achieve behavioral outcomes.
Conclusion
In summary, we found that subcutaneous continuous infusion of PZ (10 mg/kg PZ subcutaneous 15 min post-injury +10 mg/kg/day subcutaneous infusion of PZ for 3 days) maintained mitochondrial RCR and neuronal cytoskeletal integrity, and decreased binding of 4-HNE and acrolein to mitochondrial proteins 72 h following severe controlled cortical impact injury. In fact, continuous infusion of PZ was able to maintain cytoskeletal integrity, whereas 12 h intermittent dosing of PZ was not (10 mg/kg PZ subcutaneous 15 min post-injury +5 mg/kg PZ subcutaneous every 12 h up to and including 60 h). Additionally, continuous infusion of CsA (20 mg/kg CsA intraperitoneal 15 min post-injury +10 mg/kg/day subcutaneous infusion of CsA for 3 days) decreased binding of 4-HNE and acrolein to mitochondrial proteins. Although the combination of PZ + CsA was able to maintain cytoskeletal integrity, it did so to a similar degree as PZ alone, suggestive of a purely PZ effect, and was unable to attenuate other outcome measures. In fact, once combined, the protective effect PZ and CsA individually had on formation of mitochondrial bound 4-HNE and acrolein was lost.
We concede that this study was limited to a single-dosing paradigm and time-point and a limited number of outcome measures, making it difficult to conclude that the combinational therapy PZ + CsA is unable to enhance neuroprotection under any circumstances. However, upon contemplation of the complicated pharmacokinetics and pharmacodynamics of each drug individually, coupled with the fact that both drugs can physically interact with the mitochondria, in hindsight we believe the combination of CsA and PZ may lack feasible clinical translation and that any further animal studies utilizing the combination may prove unethical. Therefore, for the time being, we feel that our future directions are better spent further investigating the less well-characterized drug, PZ, including long-term behavioral studies as well as the effect the non-aldehyde scavenging PZ mechanism of action, monoamine oxidase inhibition, has on mitochondrial function and neuroprotection following TBI. Importantly, we also believe this study highlights some of the challenges in developing combinational therapies for traumatic brain injury including, optimization of combined dosing paradigms, consideration of altered pharmacokinetics and pharmacodynamics, choice of outcome measure and time-points, as well as variations in neuroprotective efficacy across distinct outcome measures.
Acknowledgments
This work was supported by NIH-NINDS 5R01 NS083405 & 5R01 NS084857. Jacqueline R. Kulbe is currently supported by NIH-NINDS NRSA NS096876.
References
- 1.Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Serveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronado V.G., McGuire L.C., Faul M.F., Sugerman D.E., and Pearson W.S. (2012). Traumatic brain injury epidemiology and public health issues, in: Brain Injury Medicine: Principles and Practice. Zasler N.D., Katz D.I., Zafonte R.D. (eds). Demos Medical Publishing: New York, pps. 84–100 [Google Scholar]
- 3.Finkelstein E.A., Corso P.S., and Miller T.R. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford University Press: New York [Google Scholar]
- 4.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 5.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 6.Hall E.D., Vaishnav R.A., and Mustafa A.G. (2010). Antioxidant therapies for traumatic brain injury. Neurotherapeutics 7, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margulies S. andHicks R.; Combination Therapies for Traumatic Brain Injury Workshop Leaders. (2009). Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma 26, 925–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., and Manley G.T.; Workshop Scientific Team and Advisory Panel Members. (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y., Gu Q., Peterson P.L., Muizelaar J.P., and Lee C.P. (1997). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23–34 [DOI] [PubMed] [Google Scholar]
- 10.Fiskum G. (2000). Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma 17, 843–855 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., and Springer J.E. (2005). Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 79, 231–239 [DOI] [PubMed] [Google Scholar]
- 12.Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., and Hall E.D. (2006). Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 26, 1407–1418 [DOI] [PubMed] [Google Scholar]
- 13.Hall E.D., Wang J.A., Bosken J.M., and Singh I.N. (2015). Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomembr. 48, 169–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand M.D., Affourtit C., Esteves T.C., Green K., Lambert A.J., Miwa S., Pakay J.L., and Parker N. (2004). Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 37, 755–767 [DOI] [PubMed] [Google Scholar]
- 15.Bringold U., Ghafourifar P., and Richter C. (2000). Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic. Biol. Med. 29, 343–348 [DOI] [PubMed] [Google Scholar]
- 16.Radi R. (1998). Peroxynitrite reactions and diffusion in biology. Chem. Res. Toxicol. 11, 720–721 [DOI] [PubMed] [Google Scholar]
- 17.Bains M. and Hall E.D. (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 1822, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz K.S. and Petersen D.R. (2013). An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med. 59, 85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldini G., Dalle-Donne I., Facino R.M., Milzani A., and Carini M. (2007). Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 27, 817–868 [DOI] [PubMed] [Google Scholar]
- 20.LoPachin R.M., Barber D.S., and Gavin T. (2008). Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 104, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R., Rickett T., and Sun W. (2011). Acrolein-mediated injury in nervous system trauma and diseases. Mol. Nutr. Food Res. 55, 1320–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pocernich C.B. and Butterfield D.A. (2003). Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox. Res. 5, 515–520 [DOI] [PubMed] [Google Scholar]
- 23.Moghe A., Ghare S., Lamoreau B., Mohammad M., Barve S., McClain C., and Joshi-Barve S. (2015). Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol. Sci. 143, 242–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo J., Robinson J.P., and Shi R. (2005). Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem. Int. 47, 449–457 [DOI] [PubMed] [Google Scholar]
- 25.Lovell M.A., Xie C., and Markesbery W.R. (2001). Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 22, 187–194 [DOI] [PubMed] [Google Scholar]
- 26.Hamann K., Durkes A., Ouyang H., Uchida K., Pond A., and Shi R. (2008). Critical role of acrolein in secondary injury following ex vivo spinal cord trauma. J. Neurochem. 107, 712–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruman I., Bruce-Keller A.J., Bredesen D., Waeg G., and Mattson M.P. (1997). Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J. Neurosci. 17, 5089–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packer M.A. and Murphy M.P. (1995). Peroxynitrite formed by simultaneous nitric oxide and superoxide generation causes cyclosporin-A-sensitive mitochondrial calcium efflux and depolarisation. Eur. J. Biochem. 234, 231–239 [DOI] [PubMed] [Google Scholar]
- 29.Cebak J.E., Singh I.N., Hill R.L., Wang J., and Hall E.D. (2016). Phenelzine protects brain mitochondrial function in vitro and in vivo following traumatic brain injury by scavenging the reactive carbonyls 4-hydroxynonenal and acrolein leading to cortical histological neuroprotection. J. Neurotrauma 34, 1302–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaishnav R.A., Singh I.N., Miller D.M., and Hall E.D. (2010). Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. J. Neurotrauma 27, 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo J. and Shi R. (2005). Acrolein induces oxidative stress in brain mitochondria. Neurochem. Int. 46, 243–252 [DOI] [PubMed] [Google Scholar]
- 32.Luo J. and Shi R. (2004). Acrolein induces axolemmal disruption, oxidative stress, and mitochondrial impairment in spinal cord tissue. Neurochem. Int. 44, 475–486 [DOI] [PubMed] [Google Scholar]
- 33.Keller J.N., Mark R.J., Bruce A.J., Blanc E., Rothstein J.D., Uchida K., Waeg G., and Mattson M.P. (1997). 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 80, 685–696 [DOI] [PubMed] [Google Scholar]
- 34.Deng Y., Thompson B.M., Gao X., and Hall E.D. (2007). Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Exp. Neurol. 205, 154–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng-Bryant Y., Singh I.N., Carrico K.M., and Hall E.D. (2008). Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 28, 1114–1126 [DOI] [PubMed] [Google Scholar]
- 36.Mustafa A.G., Wang J.A., Carrico K.M., and Hall E.D. (2011). Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J. Neurochem. 117, 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mbye L.H., Singh I.N., Carrico K.M., Saatman K.E., and Hall E.D. (2009). Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J. Cereb. Blood Flow Metab. 29, 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandya J.D., Pauly J.R., Nukala V.N., Sebastian A.H., Day K.M., Korde A.S., Maragos W.F., Hall E.D., and Sullivan P.G. (2007). Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic Brain injury in rodents. J. Neurotrauma 24, 798–811 [DOI] [PubMed] [Google Scholar]
- 39.Greco T., Glenn T.C., Hovda D.A., and Prins M.L. (2015). Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 36, 1603–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mbye L.H., Singh I.N., Sullivan P.G., Springer J.E., and Hall E.D. (2008). Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp. Neurol. 209, 243–253 [DOI] [PubMed] [Google Scholar]
- 41.Kulbe J.R., Hill R.L., Singh I.N., Wang J., and Hall E.D. (2017). Synaptic mitochondria sustain more damage than non-synaptic mitochondria following traumatic brain injury and are protected by cyclosporine A. J. Neurotrauma. 34, 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Readnower R.D., Pandya J.D., McEwen M.L., Pauly J.R., Springer J.E., and Sullivan P.G. (2011). Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J. Neurotrauma 28, 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mustafa A.G., Singh I.N., Wang J., Carrico K.M., and Hall E.D. (2010). Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. J. Neurochem.114, 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., and Hall E.D. (2013). Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 33, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh I.N., Sullivan P.G., and Hall E.D. (2007). Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J. Neurosci. Res. 85, 2216–2223 [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y., Singh I.N., and Hall E.D. (2009). Tempol protection of spinal cord mitochondria from peroxynitrite-induced oxidative damage. Free Radic. Res. 43, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J., Zheng L., Marquis A., Walls M., Duerstock B., Pond A., Vega-Alvarez S., Wang H., Ouyang Z., and Shi R. (2014). Neuroprotective role of hydralazine in rat spinal cord injury-attenuation of acrolein-mediated damage. J. Neurochem. 129, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamann K., Nehrt G., Ouyang H., Duerstock B., and Shi R. (2008). Hydralazine inhibits compression and acrolein-mediated injuries in ex vivo spinal cord. J. Neurochem. 104, 708–718 [DOI] [PubMed] [Google Scholar]
- 49.Nicolli A., Basso E., Petronilli V., Wenger R.M., and Bernardi P. (1996). Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J. Biol. Chem. 271, 2185–192 [DOI] [PubMed] [Google Scholar]
- 50.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., Robbins J., and Molkentin J.D. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 [DOI] [PubMed] [Google Scholar]
- 51.Hatton J., Rosbolt B., Empey P., Kryscio R., and Young B. (2008). Dosing and safety of cyclosporine in patients with severe brain injury. J. Neurosurg. 109, 699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazzeo A.T., Brophy G.M., Gilman C.B., Alves O.L., Robles J.R., Hayes R.L., Povlishock J.T., and Bullock M.R. (2009). Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J. Neurotrauma 26, 2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z., Park J., Butler B., Acosta G., Vega-Alvarez S., Zheng L., Tang J., McCain R., Zhang W., Ouyang Z., Cao P., and Shi R. (2016). Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J. Neurochem. 138, 328–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song M.S., Baker G.B., Dursun S.M., and Todd K.G. (2010). The antidepressant phenelzine protects neurons and astrocytes against formaldehyde-induced toxicity. J. Neurochem. 114, 1405–1413 [DOI] [PubMed] [Google Scholar]
- 55.Wood P.L., Khan M.A., Moskal J.R., Todd K.G., Tanay V.A., and Baker G. (2006). Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Res. 1122, 184–190 [DOI] [PubMed] [Google Scholar]
- 56.Galvani S., Coatrieux C., Elbaz M., Grazide M.H., Thiers J.C., Parini A., Uchida K., Kamar N., Rostaing L., Baltas M., Salvayre R., and Negre-Salvayre A. (2008). Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 45, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 57.Burcham P.C., Fontaine F.R., Kaminskas L.M., Petersen D.R., and Pyke S.M. (2004). Protein adduct-trapping by hydrazinophthalazine drugs: mechanisms of cytoprotection against acrolein-mediated toxicity. Molec. Pharmacol. 65, 655–664 [DOI] [PubMed] [Google Scholar]
- 58.Burcham P.C., Kerr P.G., and Fontaine F. (2000). The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox. Rep. 5, 47–49 [DOI] [PubMed] [Google Scholar]
- 59.Burcham P.C. and Pyke S.M. (2006). Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Molec. Pharmacol. 69, 1056–1065 [DOI] [PubMed] [Google Scholar]
- 60.LoPachin R.M., Gavin T., Petersen D.R., and Barber D.S. (2009). Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem. Res. Toxicol. 22, 1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan P.G., Thompson M.B., and Scheff S.W. (1999). Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 62.Sullivan P.G., Thompson M., and Scheff S.W. (2000). Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161, 631–637 [DOI] [PubMed] [Google Scholar]
- 63.Sullivan P.G., Sebastian A.H., and Hall E.D. (2011). Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J. Neurotrauma 28, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan P.G., Rabchevsky A.G., Hicks R.R., Gibson T.R., Fletcher-Turner A., and Scheff S.W. (2000). Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295 [DOI] [PubMed] [Google Scholar]
- 65.Scheff S.W. and Sullivan P.G. (1999). Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma 16, 783–792 [DOI] [PubMed] [Google Scholar]
- 66.Riess P., Bareyre F.M., Saatman K.E., Cheney J.A., Lifshitz J., Raghupathi R., Grady M.S., Neugebauer E., and McIntosh T.K. (2001). Effects of chronic, post-injury cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor. Neurol. Neurosci. 18, 1–8 [PubMed] [Google Scholar]
- 67.Okonkwo D.O. and Povlishock J.T. (1999). An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J. Cereb. Blood Flow Metab. 19, 443–451 [DOI] [PubMed] [Google Scholar]
- 68.Okonkwo D.O., Melon D.E., Pellicane A.J., Mutlu L.K., Rubin D.G., Stone J.R., and Helm G.A. (2003). Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport 14, 463–466 [DOI] [PubMed] [Google Scholar]
- 69.Okonkwo D.O., Buki A., Siman R., and Povlishock J.T. (1999). Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport 10, 353–358 [DOI] [PubMed] [Google Scholar]
- 70.McEwen M.L., Sullivan P.G., and Springer J.E. (2007). Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J. Neurotrauma 24, 613–624 [DOI] [PubMed] [Google Scholar]
- 71.Kilbaugh T.J., Bhandare S., Lorom D.H., Saraswati M., Robertson C.L., and Margulies S.S. (2011). Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 28, 763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill R.L., Singh I.N., Wang J.A., and Hall E.D. (2017). Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem. Int. 111, 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osier N.D. and Dixon C.E. (2016). The controlled cortical impact model: applications, considerations for researchers, and future directions. Front. Neurol. 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 75.Pandya J.D., Pauly J.R., and Sullivan P.G. (2009). The optimal dosage and window of opportunity to maintain mitochondrial homeostasis following traumatic brain injury using the uncoupler FCCP. Exp. Neurol. 218, 381–389 [DOI] [PubMed] [Google Scholar]
- 76.Gilmer L.K., Roberts K.N., Joy K., Sullivan P.G., and Scheff S.W. (2009). Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 26, 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall E.D., Detloff M.R., Johnson K., and Kupina N.C. (2004). Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J. Neurotrauma 21, 9–20 [DOI] [PubMed] [Google Scholar]
- 78.Alessandri B., Rice A.C., Levasseur J., DeFord M., Hamm R.J., and Bullock M.R. (2002). Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma 19, 829–841 [DOI] [PubMed] [Google Scholar]
- 79.Hansson M.J., Mansson R., Morota S., Uchino H., Kallur T., Sumi T., Ishii N., Shimazu M., Keep M.F., Jegorov A., and Elmer E. (2008). Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic. Biol. Med. 45, 284–294 [DOI] [PubMed] [Google Scholar]
- 80.Brustovetsky N. and Dubinsky J.M. (2000). Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J. Neurosci. 20, 8229–8237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chinopoulos C., Starkov A.A., and Fiskum G. (2003). Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J. Biol. Chem. 278, 27382–27389 [DOI] [PubMed] [Google Scholar]
- 82.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., and Bernardi P. (2005). Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J. Biol. Chem. 280, 18558–18561 [DOI] [PubMed] [Google Scholar]
- 83.Garcia N., Martinez-Abundis E., Pavon N., and Chavez E. (2007). On the opening of an insensitive cyclosporin A non-specific pore by phenylarsine plus mersalyl. Cell. Biochem. Biophys. 49, 84–90 [DOI] [PubMed] [Google Scholar]
- 84.Hall E.D., Bryant Y.D., Cho W., and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 85.McKee C.A. and Lukens J.R. (2016). Emerging roles for the immune system in traumatic brain injury. Front. Immunol. 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu J., Farmer J.D., Jr., Lane W.S., Friedman J., Weissman I., and Schreiber S.L. (1991). Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 [DOI] [PubMed] [Google Scholar]
- 87.Dixon C.E., Bramlett H.M., Dietrich W.D., Shear D.A., Yan H.Q., Deng-Bryant Y., Mondello S., Wang K.K., Hayes R.L., Empey P., Povlishock J., Tortella F.C., and Kochanek P.M. (2015). Cyclosporine treatment in traumatic brain injury: operation brain trauma therapy. J. Neurotrauma. 33, 553–566 [DOI] [PubMed] [Google Scholar]
- 88.Panter S.S., Braughler J.M., and Hall E.D. (1992). Dextran-coupled deferoxamine improves outcome in a murine model of head injury. J. Neurotrauma 9, 47–53 [DOI] [PubMed] [Google Scholar]
- 89.Lopachin R.M., Gavin T., Decaprio A., and Barber D.S. (2012). Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant–target interactions. Chem. Res. Toxicol. 25, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burcham P.C., Kaminskas L.M., Fontaine F.R., Petersen D.R., and Pyke S.M. (2002). Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology 181–182, 229–236 [DOI] [PubMed] [Google Scholar]
- 91.Kristal B.S., Park B.K., and Yu B.P. (1996). 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. J. Biol. Chem. 271, 6033–6038 [DOI] [PubMed] [Google Scholar]
- 92.Cohen G. and Kesler N. (1999). Monoamine oxidase and mitochondrial respiration. J. Neurochem. 73, 2310–2315 [DOI] [PubMed] [Google Scholar]
- 93.Osier N.D. and Dixon C.E. (2016). Catecholaminergic based therapies for functional recovery after TBI. Brain Res. 1640, 15–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song M.S., Matveychuk D., MacKenzie E.M., Duchcherer M., Mousseau D.D., and Baker G.B. (2013). An update on amine oxidase inhibitors: multifaceted drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 44, 118–124 [DOI] [PubMed] [Google Scholar]
- 95.Kobori N., Hu B., and Dash P.K. (2011). Altered adrenergic receptor signaling following traumatic brain injury contributes to working memory dysfunction. Neuroscience 172, 293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Margulies S., Anderson G., Atif F., Badaut J., Clark R., Empey P., Guseva M., Hoane M., Huh J., Pauly J., Raghupathi R., Scheff S., Stein D., Tang H., and Hicks M. (2016). Combination therapies for traumatic brain injury: fetrospective considerations. J. Neurotrauma 33, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanhove T., Annaert P., and Kuypers D.R. (2016). Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab. Rev. 48, 88–112 [DOI] [PubMed] [Google Scholar]
- 98.Gillman P.K. (2011). Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J. Clin. Psychopharmacol. 31, 66–74 [DOI] [PubMed] [Google Scholar]
- 99.Polasek T.M., Elliot D.J., Somogyi A.A., Gillam E.M., Lewis B.C., and Miners J.O. (2006). An evaluation of potential mechanism-based inactivation of human drug metabolizing cytochromes P450 by monoamine oxidase inhibitors, including isoniazid. Br. J. Clin. Pharmacol. 61, 570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsuji A., Tamai I., Sakata A., Tenda Y., and Terasaki T. (1993). Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter, P-glycoprotein. Biochem. Pharmacol. 46, 1096–1099 [DOI] [PubMed] [Google Scholar]
- 101.Prakash R. and Carmichael S.T. (2015). Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr. Opin. Neurol. 28, 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matveychuk D., Nunes E., Ullah N., Aldawsari F.S., Velazquez-Martinez C.A., and Baker G.B. (2014). Elevation of rat brain tyrosine levels by phenelzine is mediated by its active metabolite beta-phenylethylidenehydrazine. Prog. Neuropsychopharmacol. Biol. Psychiatry 53, 67–73 [DOI] [PubMed] [Google Scholar]
- 103.Halestrap A.P. and Davidson A.M. (1990). Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 268, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edmondson D.E., Binda C., Wang J., Upadhyay A.K., and Mattevi A. (2009). Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry 48, 4220–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]