Abstract
Objective
To test the hypothesis that brain pathology is associated with total daily physical activity proximate to death in older adults.
Methods
We studied brain autopsies from 428 decedents of the Rush Memory and Aging Project. The quantity of all physical activity was measured continuously for up to 10 days with actigraphy (Actical; Philips Healthcare, Bend, OR). Multiple regression analyses controlling for age and sex were used to examine the relation of brain indexes to total daily physical activity and other clinical covariates proximate to death.
Results
Average total daily activity was 1.53 × 105 counts/d (SD 1.14 × 105 counts/d), and mean age at death was 90.6 (SD 6.12) years. Nigral neuronal loss (estimate −0.232, standard error [SE] = 0.070, p = 0.001) and macroinfarcts (estimate −0.266, SE 0.112, p = 0.017) were independently associated with total daily physical activity proximate to death, accounting for an additional 2.4% of the variance of total daily activity. Other postmortem indexes (Alzheimer disease, Lewy bodies, TAR DNA-binding protein 43, hippocampal sclerosis, microinfarcts, atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy) were not associated with total daily activity. In 295 cases (70%), we derived a measure of white matter tissue integrity from postmortem brain MRI. This metric accounted for an additional 5.8% of the variance of total daily physical activity when controlling for age, sex, nigral neuronal loss, and macroinfarcts.
Conclusion
Macroinfarcts, nigral neuronal loss, and white matter pathologies are related to total daily physical activity in older adults, but further studies are needed to explain its pathologic basis more fully.
Individuals who lead a more active lifestyle manifest increased survival and fewer adverse health outcomes.1 Physical activity is a complex behavior that depends on diverse physiologic systems, including musculoskeletal structures, systemic metabolism, and cardiopulmonary systems, as well as neural systems that originate in the brain.2 Thus, degeneration and the accumulation of age-related pathologies in the brain may lead to lower levels of physical activity. The planning, initiation, and execution of physical activity are under volitional control. Thus, in addition to assessing an individual's motor capacity for example grip strength and gait speed, it is crucial to assess the quantity of how much an individual actually moves during the course of the day. Previous studies in this cohort have shown that higher levels of diverse brain pathologies are associated with lower levels of motor capacity in community-dwelling older adults proximate to death.3–5 However, it is not known to what extent brain pathologies are related to objective metrics of the quantity of total daily physical activity in community-dwelling older adults.
Unobtrusive wearable devices that record physical activity continuously for several days are now available to measure how much an older adult actually moves during normal living in the community setting. Recordings from these devices circumvent self-report biases and yield objective metrics of total daily physical activity that include both exercise and habitual physical activities.6–8 This study used data from well-characterized community-dwelling older adults to test the hypothesis that indices of brain pathology are associated with previously validated metrics of total daily physical activity and its intensity proximate to death.9
Methods
Participants
Participants were individuals from the Rush Memory and Aging Project, an ongoing cohort study in which participants agree to annual examinations and autopsy at death.9 Although the study began in 1997, actigraphy data collection was not added until 2005. All decedents with valid actigraphy testing before death and completed autopsy were included in these analyses.
Assessment of total daily physical activity
All movement was measured 24 h/d for up to 10 days with an activity monitor worn on the nondominant wrist that recorded average activity counts every 15 seconds (Actical; Mini Mitter, Bend, OR). Total daily physical activity was the average sum of all daily activity counts described more fully in the e-supplement (links.lww.com/WNL/A465). Because of the large number of counts accumulated over 24 hours, raw counts in the current study were divided by 1 × 105 (≈1 SD) to facilitate presentation and interpretation of the results. Intensity of daily activity was calculated by dividing the total daily activity counts by the total hours per day of all nonzero epochs (activity counts per hour per day).6–8 On average, the last actigraphic recordings were obtained 2 years (SD 1.91 years) before death (range 2 weeks–10 years; for 75% of cases <24 months and 90% of cases <36 months before death).
Other clinical covariates
Clinical covariates were obtained from the same cycle of the last actigraphic recording proximate to death. Age at death was computed from self-reported date of birth and date of autopsy. Sex and years of education were recorded at the study entry. Motor function summarized 10 motor performances.10 Global cognition was based on 19 cognitive tests.9 Body mass index was based on measured weight and height.9 Self-reported assessments included physical activity, social and cognitive activities,9 the number of 4 vascular diseases (myocardial infarction, congestive heart failure, claudication, and stroke), and 3 vascular risk factors (hypertension, diabetes mellitus, and smoking).9 Depressive symptoms included a clinical diagnosis of depression, neuroticism, extraversion, social isolation, apathy, and sleep fragmentation.9 APOE genotype was dichotomized into those with ≥1 copy of the ε4 allele (e-supplement, links.lww.com/WNL/A465).9
Assessment of postmortem histopathology and brain imaging indexes
At death, the brain was extracted and hemisected. One cerebral hemisphere was placed in 4% formaldehyde solution and refrigerated in preparation for histopathologic studies and postmortem MRI.9 A uniform gross and microscopic examination collected indexes including a summary measure for Alzheimer disease (AD) pathology, TAR DNA-binding protein 43 (TDP-43), Lewy bodies, hippocampal sclerosis, nigral neuronal loss, chronic macroinfarcts and microinfarcts, cerebral amyloid angiopathy, atherosclerosis, and arteriolosclerosis.9 We also quantified the transverse relaxation rate constant (R2) of tissue in each voxel using postmortem brain MRI spin-echo images (e-supplement, links.lww.com/WNL/A465).11
Analyses
Bivariate correlations or t tests were used to compare total daily physical activity and its intensity with other covariates. A series of linear regression models examined indexes of brain pathologies and total daily physical activity and its intensity, controlling for age and sex in all models. We also added terms for covariates that might attenuate the association of postmortem indexes with total daily physical activity. In a final model, we added interaction terms to examine whether postmortem indexes and total daily activity varied with motor or cognitive function, dementia, or APOE ε4 gene status proximate to death.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all study participants.
Data availability
All data included in these analyses are available via the Rush Alzheimer's Disease Center Research Resource Sharing Hub, which can be found at radc.rush.edu. It has descriptions of the studies and variables and a dynamic query function to aid searches for data and biospecimens for selected data. There is a login, after which any qualified investigator can submit requests for deidentified data.
Results
Total daily physical activity
There were 428 participants. Their clinical and postmortem characteristics are included in table 1. Participants wore the Actical for an average of 9.5 days (SD 1.77 days). Total daily physical activity levels ranged from 0.06 × 105 to 6.56 × 105 counts/d (mean 1.53 ×105 counts/d, SD = 1.14 × 105 counts/d). Prior structured testing of sitting and writing for 3 minutes (43 activity counts), floor sweeping for 3 minutes (1; 721 activity counts), and walking for 5 minutes at 2.5 mph (2; 355 activity counts) may help contextualize these daily counts.12 On average, the intensity of daily physical activity was 0.19 × 105 activity counts/h/d (SD 0.09 × 105 activity counts/h/d).
Table 1.
Clinical and postmortem characteristics (n = 428)a
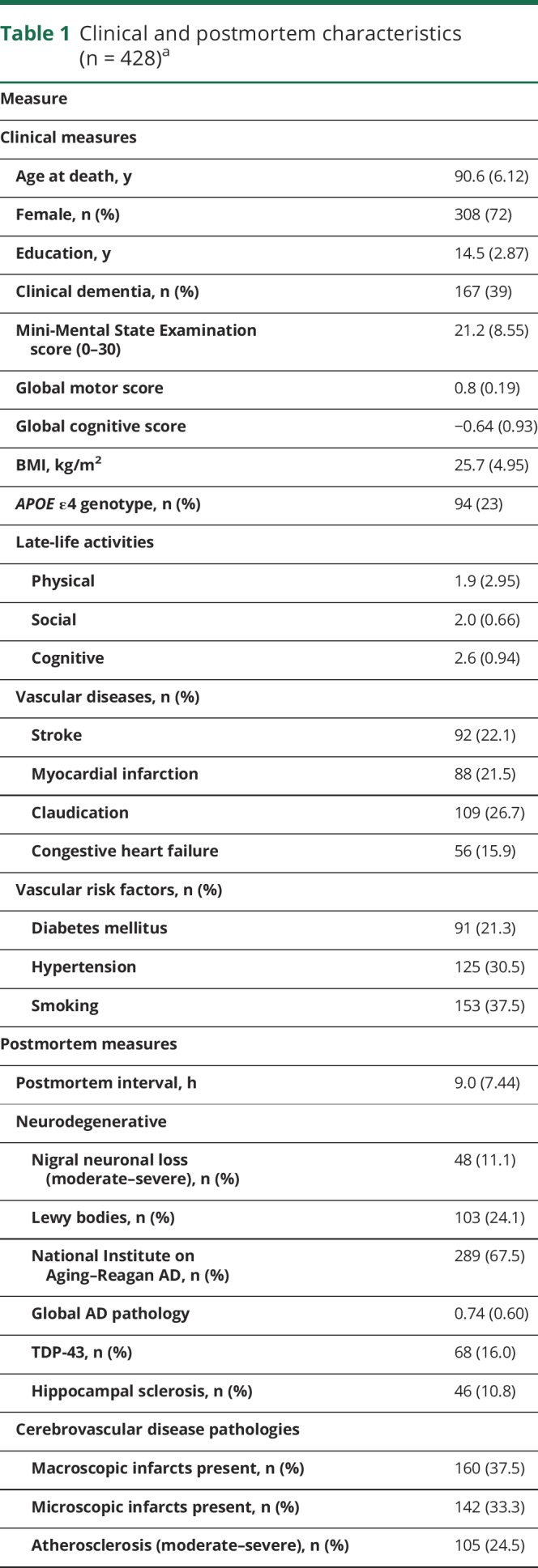
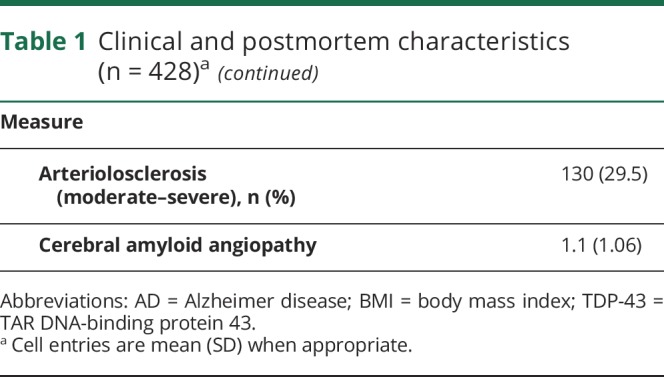
Both physical activity measures were highly related (r = 0.88, p < 0.001) and showed similar relationships to demographics. Total daily physical activity was related to age (r −0.17, p < 0.001) and education (r −0.11, p = 0.022). Total daily physical activity was similar in women and men (women 1.55 × 105 counts/d [SD 1.17 × 105 counts/d] vs men 1.47 × 105 counts/d [SD 1.06 × 105 counts/d], t(426) = 1.22, p = 0.521). Both measures were modestly associated with other clinical covariates (table e-1, links.lww.com/WNL/A464).
Brain pathology and total daily physical activity
Age at death ranged from 66 to 108 years. On average, participants showed 3 different brain pathologies. One or more of the 10 pathologies summarized in table 1 were observed in nearly all cases (95.6%); 0, n = 19 (4.4%); 1, n = 58 (13.6%); 2, n = 91 (21.3%); 3, n = 93 (21.7%); 4, n = 88 (20.6%); 5, n = 42 (9.8%); and 6 to 8, n = 37 (8.6%).
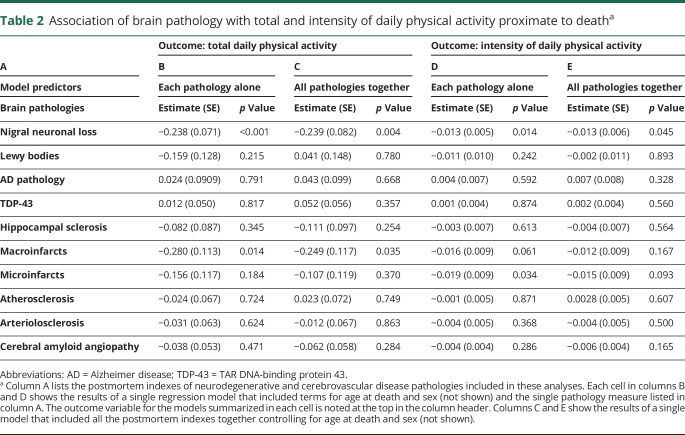
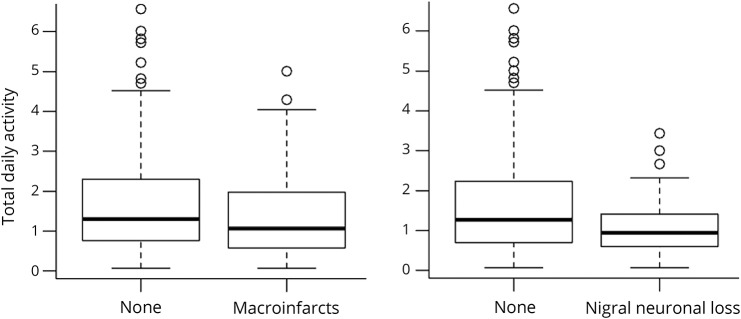
With the use of regression models that controlled for age and sex, nigral neuronal loss and macroinfarcts were associated with total daily physical activity (table 2, column B). Figure 1 illustrates that the presence of macroinfarcts or nigral neuronal loss was associated with lower levels of total daily physical activity. However, indexes of other neurodegenerative and cerebrovascular disease pathologies were not associated with total daily physical activity (table 2, column B).
Table 2.
Association of brain pathology with total and intensity of daily physical activity proximate to deatha
Figure 1. Brain pathology and level of total daily physical activity proximate to death.
Boxplot scores of the presence or absence of macroinfarcts and nigral neuronal loss with levels of total daily physical activity proximate to death.
In further analyses, we replaced the summary measure for AD pathology with measures of paired helical filaments tau tangles and β-amyloid. Neither density of paired helical filaments tau tangles (estimate −0.432, SE 0.348, p = 0.214) nor the burden of β-amyloid (estimate −0.005, SE 0.381, p = 0.989) was associated with total daily physical activity. Models examining high vs low levels of AD and TDP-43 also were not associated with total daily physical activity (results not shown).
Because nigral neuronal loss can be associated with Parkinson disease, we repeated these analyses excluding persons with clinically diagnosed Parkinson disease (n = 14, 3.4%), and the results were unchanged (table e-2, links.lww.com/WNL/A464).
Because many older adults have evidence of >1 neuropathology, we examined a single model that included terms for all 10 indexes together to determine which were independently associated with total daily physical activity. Nigral neuronal loss and macroinfarcts were independently associated with total daily physical activity proximate to death (table 2, column B). Together, these pathologies accounted for 2.4% of the variance of total daily physical activity in addition to age and sex (table 2, column C). Individuals without nigral neuronal loss or macroinfarcts had higher levels of total daily physical activity compared to those participants with either 1 or both pathologies present.
To ensure that our results are not limited to activity profiles close to death, we examined the associations of postmortem indexes with the next-to-last measure of total daily physical activity obtained on average 3 years proximate to death. Nigral neuronal loss and macroinfarcts remained associated with total daily physical activity. The associations of postmortem indexes and total daily physical activity were also unchanged when we adjusted for whether actigraphic recordings were obtained in the fall/winter vs spring/summer (results not shown).
Total daily physical activity may include different types of physical activities. We repeated the models described above with a surrogate for aerobic activity, the intensity of daily activity. Nigral neuronal loss but not macroinfarcts was associated with total daily activity proximate to death (table 2, columns D and E).
Brain pathology, other clinical covariates, and total daily physical activity
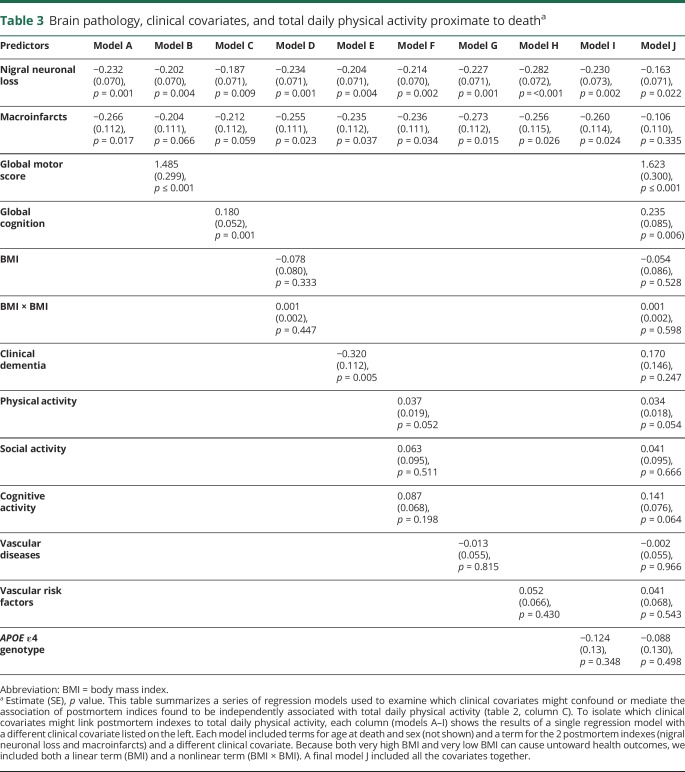
Higher levels of neuropathology can degrade health, thereby causing lower physical activity. In further analyses, we sought to isolate which conditions mediate the association of neuropathology with total daily physical activity. Including terms for motor and cognitive function showed partial attenuation of the association of macroinfarcts with physical activity, suggesting partial mediation via motor and cognitive function (table 3, models A and B). In contrast, the associations of nigral neuronal loss and macroinfarcts with total daily physical activity were not attenuated when controlling for other common health covariates (table 3, models C–I). The association of microinfarcts was attenuated when all the clinical covariates were included in a single model. In contrast, the association of nigral neuronal loss was only partially attenuated and remained associated with total daily physical activity (table 3, model J).
Table 3.
Brain pathology, clinical covariates, and total daily physical activity proximate to deatha
Next, we added interaction terms to investigate whether health covariates modified the association of postmortem indexes with total daily physical activity. While total daily physical activity is lower in individuals with dementia, on the basis of the negative estimate for the clinical dementia term (table 3, model E), the association of postmortem indexes and total daily physical activity did not vary between cases with and without dementia (table e-3, links.lww.com/WNL/A464). In addition, the association of postmortem indexes with total daily physical activity did not vary with the level of cognitive or motor function proximate to death or with the APOE ε4 genotype (table e-3).
Psychosocial traits or symptoms and impaired sleep that are related to total daily physical activity might link (mediate) brain pathology to total daily physical activity. We found that apathy, extraversion, and sleep fragmentation were associated with total daily physical activity but not depressive symptoms, a clinical diagnosis of depression, social isolation, or neuroticism (table e-4, links.lww.com/WNL/A464).
We repeated the regression models from table 2, adding terms for each of the 3 covariates that were related to total daily physical activity. The associations of all 10 brain pathologies with total daily physical activity were unchanged when we added terms for apathy (table e-5, links.lww.com/WNL/A464), extraversion (table e-6), and sleep fragmentation (table e-7). Thus, there was no evidence that these covariates link (mediate) postmortem indexes with total daily physical activity.
Next, we added interaction terms for each of the 3 covariates with the 10 brain pathologies to examine whether these covariates modified the association of brain pathologies and total daily physical activity. Apathy, extraversion, and sleep fragmentation did not show consistent modification of the associations of brain pathologies with total daily physical activity (table e-8, links.lww.com/WNL/A464).
Postmortem brain white matter tissue integrity, brain pathology, and total daily physical activity proximate to death
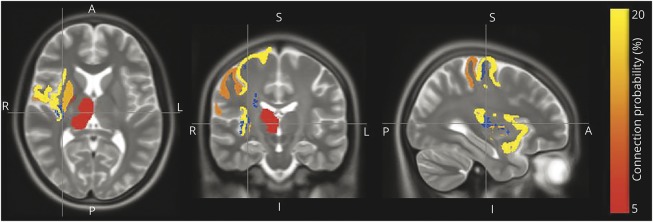
Quantifying the condition of brain white matter with conventional histopathology is difficult. In prior work, we developed a metric of brain tissue integrity, R2, derived from postmortem brain MRIs. R2 is the reciprocal of T2, which is done to improve its metric properties. This postmortem metric is independently associated with cognitive phenotypes when controlling for brain histopathology in the same model.13 In the current study, ≈70% (295 of 428) of decedents with indexes of histopathology also had postmortem brain MRI. Participants with and without postmortem brain imaging showed similar age at death, sex, years of education, total daily physical activity proximate to death, and levels of each of the 10 pathologies assessed in this study (results not shown). In these cases, a voxel-by-voxel analysis showed that the composite R2 metrics from 4 brain regions were associated with total daily physical activity. These regions included the precentral and postcentral gyrus, insular cortex, and putamen and are illustrated in blue. The most common gray matter terminals of white matter fibers passing through these regions are shown in other colors (figure 2).
Figure 2. Postmortem brain R2 and total daily physical activity proximate to death.
Axial, coronal, and sagittal postmortem brain MRI sections depicting the regions (insular cortex, precentral and postcentral gyrus, and putamen) in which white matter tissue integrity (R2) is most strongly associated with total daily physical activity proximate to death (blue) and the most common gray matter terminals of white matter fiber passing through those regions (red/orange/yellow). The red-to-yellow coloring of each gray matter region corresponds to the probability that a white matter fiber passing through the blue R2 region has one of its terminals at that region. A = anterior; I = inferior; P= posterior; S = superior.
In a regression model controlling for age and sex, these 4 regions were independently associated with total daily physical activity: region 1, estimate −0.1936 (SE 0.061, p = 0.002); region 2, estimate 0.162 (SE 0.059, p = 0.007); region 3, estimate −0.180 (SE 0.064, p = 0.005); and region 4, estimate −0.192 (SE 0.063, p = 0.003). The mean R2 across these 4 regions accounted for an additional 5.8% of the variance of total daily physical activity when controlling for age and sex. In a final model, adding nigral neuronal loss and macroinfarcts to the previous model with terms for the average R2, age, and sex accounted for an additional 1.6% of the variance of total daily physical activity.
Discussion
In a group of >400 older community-dwelling older adults, we examined the extent to which postmortem indexes of brain pathology were associated with objective metrics of total daily physical activity. These associations were robust and not linked to total daily physical activity through a wide range of health and psychosocial traits or symptoms. While nigral neuronal loss and macroinfarcts were associated with total daily physical activity proximate to death, these indexes accounted for <3% of its variance. Novel postmortem MRI brain metrics of brain white matter tissue integrity accounted for almost 6% of the variance of total daily physical activity controlling for age, sex, and indexes of conventional histopathology. These cross-sectional data suggest that the accumulation of age-related brain pathologies, in particular white matter tissue integrity, may contribute to lower levels of physical activity in older adults. However, the majority of the variance of total daily physical activity was unexplained in these analyses, underscoring the necessity for further studies to more fully elucidate the biology underlying total daily physical activity in older adults.
Accumulating health deficits in older adults impair diverse physiologic systems, which can degrade an individual's ability or capacity to maintain prior levels of physical activity. For example, antemortem brain imaging studies in older adults suggest that infarcts and loss to brain white matter integrity are associated with lower levels physical activity.14 In addition, several clinical studies using PET of cerebral β-amyloid deposition suggest that higher levels of β-amyloid burden are associated with poorer motor function.15,16 Prior clinical-autopsy studies in this cohort have reported the association of brain pathology with several motor constructs that assess motor capacity, but these reports did not examine metrics of the actual quantity of daily motor function.3–5,17,18 The current study leveraged available technology that provides prolonged continuous multiday recordings of how much older adults actually moved for up to 10 days in the community setting. Analyses in the current study show that some but not all brain pathologies are associated with total daily physical activity in community-dwelling older adults when controlling for motor capacity.
Prior reports in this cohort showed that nigral neuronal loss and macroinfarcts were also related to the levels of several other motor capacity constructs proximate to death.3–5,17,18 A prior in vivo brain imaging study reported that higher levels of physical activity in community-dwelling older adults were associated with less evidence of silent brain infarctions.14 In a prior study, total daily physical activity was associated with incident AD and the rate of cognitive decline of several cognitive domains.7 The association of total daily physical activity with macroinfarcts but not AD pathology in the current study raises the question of whether the potential cognitive benefits of a more active lifestyle might derive from effects on the non-AD pathologies such as macroinfarcts, which can also affect cognitive function. While nigral neuronal loss is an important component of the pathology of Parkinson disease, in the current study, we did not find an association between Lewy bodies and the level of total daily physical activity. This finding contrasts with other motor constructs that have shown an association with both Lewy bodies and nigral neuronal loss.18 Nigral neuronal loss may be a sensitive indicator of not only Lewy bodies but also other neurodegenerative processes and vascular processes.19 Indexes of other microvascular disease and neurodegenerative pathologies have been associated with some but not all of the motor phenotypes examined in this cohort. Given that <10% of the variance of motor phenotypes can be attributed to brain pathology, some of the differences between these studies may reflect limited power for some of the indexes examined.3–5,17,18 The metrics for total daily activity used in this study quantified all physical activities together, while prior studies examined different motor constructs. Thus, the differences in the associations of neuropathology observed with varied motor phenotypes could also derive from distinctions in their underlying neurobiology, which might be amenable to targeted interventions. Further studies with newer devices and algorithms that can quantify specific physical activities may help resolve these issues.
Our findings that traditional indexes of histopathology explain only a small proportion of the variance of total daily physical activity suggest that other brain pathologies remain to be identified. White matter tracts, composed of myelin, are crucial for connecting neurons in motor-related brain regions to neural systems.20–22 However, it is difficult to quantify myelin with conventional histopathology techniques. To circumvent this difficulty, we used a previously developed metric for white matter microvascular tissue alterations derived from postmortem brain MRI of the cases analyzed in this study. We found that the postmortem R2 metric is independently associated with and accounts for 2 to 3 times more of the variance of total daily physical activity than conventional indexes of histopathology.11,13,23–27 These findings are consistent with antemortem imaging studies suggesting that higher levels of physical activity are associated with better white matter integrity.14 However, not all prior studies have shown an association between physical activity metrics in older adults and brain imaging white matter indexes.28,29 R2 indexes in 4 specific brain regions were most strongly associated with total daily physical activity in the current study.
While the findings in the current study are significant, it is unclear why postmortem brain indexes accounted for a minority of the variance of total daily physical activity compared to up to 50% for cognitive function. While neural networks underlying cognition are limited to the brain,30 neural systems controlling movement extend beyond the brain to regulate skeletal muscle activity.31–33 Thus, to determine the full extent to which age-related CNS pathologies underlie total daily physical activity, it is likely to be necessary to examine postmortem indexes in motor regions outside the brain.34–36 It is also possible that the construct of total daily physical activity may not be related to indexes of brain pathologies, as we have reported for other constructs of volitional late-life activities such as the frequency of cognitive activities.37,38 Prior studies suggest that the improved brain health manifested by individuals with higher levels of physical activity may derive from better structural brain integrity, neuroplasticity, and angiogenesis, but the mechanisms underlying these changes are unknown.39 Further work is needed to identify molecular systems and pathways within the distributed motor neural systems that may underlie total daily physical activity without leaving a pathologic footprint.39 These data are crucial for developing effective strategies to facilitate more active lifestyles to ensure improved brain health in old age.
There are limitations to this study. This was a cross-sectional observational study that used linear models to analyze clinical and postmortem measures from decedents. Brain pathology may accumulate over decades before death, and its clinical manifestations across the aging lifespan may be nonlinear. Adding gray matter indexes from postmortem brain imaging would clarify whether the R2 metric is an independent imaging correlate of physical activity. Moreover, adding antemortem structural and pathology imaging, i.e., amyloid, to postmortem brain imaging may help clarify the causal direction of these cross-sectional associations. Because of the age of this cohort, there were few men, so these results may not be typical for the full aging spectrum. The activity monitors used did not differentiate between different types of movements, underscoring the need for studies with newer devices that provide more detailed activity metrics.40,41
This study has several strengths. Objective continuous physical activity metrics were obtained from a relatively large number of older adults in their own homes. Thus, these data may be more representative of the full health spectrum observed in the community setting. Moreover, unique postmortem brain histopathology and postmortem brain imaging indexes were combined with these activity metrics and analyses adjusted for a wide range of motor, cognitive, and health covariates.
Acknowledgment
The authors thank all the participants in the Rush Memory and Aging Project. They also thank the staff of the Rush Alzheimer's Disease Center. More information on obtaining Rush Memory and Aging Project data for research use can be found at the RADC Research Resource Sharing Hub (radc.rush.edu).
Glossary
- AD
Alzheimer disease
- TDP-43
TAR DNA-binding protein 43
Author contributions
Drafting/revising manuscript for content: A.S.B., L.Y., R.J.D., R.S.W., A.L., J.A.S., D.A.B. Study concept or design: A.S.B., L.Y., D.A.B. Analyses or interpretation of the data: A.S.B., B.D.J., R.S.W., A.L., D.A.B. Acquisition of data: A.S.B., D.A.B., R.J.D., J.A.S. Statistical analysis: A.S.B., L.Y. Study supervision or coordination: A.S.B., D.A.B. Obtaining funding: A.S.B., D.A.B.
Study funding
This work was supported by the NIH (R01AG17917, R01NS78009, R01AG052488), the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
Disclosure
A. Buchman receives support from NIH grants (R01AG043379, R01NS078009, R01AG47679, R01AG017917, P30AG10161, P20 MD0068860, R01 AG040039, and R01 AG022018). R. Dawe receives support from NIH grants (R01AG053446, P30AG010161, RF1AG015819, R01AG034374, R01AG052488). L. Yu receives support from NIH grants (R01AG053446, RF1AG015819, R01AG017917, RF1AG036042, U01AG046152, R01AG054058, R01AG034374, R01AG033678, R01DK099269, R01AG052488, R01AG050631, RF1AG048056). A. Lim receives support from NIH grant R01AG052488; Canadian Institutes of Health Research grants MOP125934, CPG140200, and MSH136642; and Ontario Ministry of Innovation grant ER16-12-034. R. Wilson receives support from NIH grants (RF1AG022018, P30AG010161, RF1AG015819, R01AG017917, U01AG046152-02S1, R01AG054058, R01AG034374, R01AG033678, 3U01AG029824-06S1, R01AG051635, R01NS093870). J. Schneider reports serving on the Scientific Advisory Board for Grifols, Lilly, and Genentech; serving as a consultant to the Michael J. Fox Foundation and National Hockey League; and receiving support from NIH grants (R01AG042210, UH2NS100599, R01NS084965, RF1AG022018, P30AG010161, RF1AG015819, R01AG017917, U01AG046152, R01AG054058, R01AG034374, R01AG033678, R01NS089674, R01AG043379,R01AG047976, R01DK099269, R01AG048108, R01AG033570, U01AG046161, RF1AG054057, P01AG014449). D. Bennett receives support from NIH grants (P30AG010161, RF1AG015819, R01AG017917, RF1AG036042, U01AG046152, U01AG046152-02S1, R01AG054058, R01AG052200, R01AG053446, RF1AG051641, R01DK099269, R01AG048108, R01AG033570, U01AG046161, R01AG052488, R01NS086736, RF1AG054057, P01AG014449, R01AG050431, R01NS093870, R01AG050631, UM1HG009443, RF1AG048056, R01AG046174, RF1AG052476). Go to Neurology.org/N for full disclosures.
References
- 1.Warburton D, Charlesworth S, Ivey A, Nettlefold L, Bredin S. A systematic review of the evidence for Canada's physical activity guidelines for adults. Int J Behav Nutr Phys Act 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 3.Buchman AS, Yu L, Wison RS, et al. Post-mortem brain pathology is related to declining respiratory function in community-dwelling older adults. Front Aging Neurosci 2015;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci 2014;69:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology 2013;80:712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchman AS, Yu L, Boyle PA, Shah RC, Bennett DA. Total daily physical activity and longevity in old age. Arch Intern Med 2012;172:444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah R, Buchman A, Leurgans S, Boyle P, Bennett D. Association of total daily physical activity with disability in community-dwelling older persons: a prospective cohort study. BMC Geriatr 2012;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchman AS, Leurgans SE, Yu L, et al. Incident parkinsonism in older adults without Parkinson disease. Neurology 2016;87:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawe RJ, Yu L, Leurgans SE, et al. Postmortem MRI: a novel window into the neurobiology of late life cognitive decline. Neurobiol Aging 2016;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport 2006;77:64–80. [DOI] [PubMed] [Google Scholar]
- 13.Dawe RJ, Bennett DA, Schneider JA, et al. Ex vivo T2 relaxation: associations with age-related neuropathology and cognition. Neurobiol Aging 2014;35:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willey JZ, Moon YP, Paik MC, et al. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology 2011;76:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian Q, Resnick SM, Bilgel M, Wong DF, Ferrucci L, Studenski SA. Beta-amyloid burden predicts lower extremity performance decline in cognitively unimpaired older adults. J Gerontol A Biol Sci Med Sci 2017;72:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wennberg AM, Savica R, Hagen CE, et al. Cerebral amyloid deposition is associated with gait parameters in the mayo clinic study of aging. J Am Geriatr Soc 2016;65:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in older adults and its association with adverse health outcomes and neuropathology. J Gerontol A Biol Sci Med Sci 2016;71:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol 2012;71:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol 2006;59:166–173. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 21.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr, Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005;53:649–654. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Dawe RJ, Buchman AS, et al. Ex vivo MRI transverse relaxation in community based older persons with and without Alzheimer's dementia. Behav Brain Res 2017;322:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotrotsou A, Schneider JA, Bennett DA, et al. Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol Aging 2015;36:2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotrotsou A, Bennett DA, Schneider JA, et al. Ex vivo MR volumetry of human brain hemispheres. Magn Reson Med 2014;71:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One 2011;6:e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawe RJ, Bennett DA, Schneider JA, Vasireddi SK, Arfanakis K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn Reson Med 2009;61:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 2016;131:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jochem C, Baumeister SE, Wittfeld K, et al. Domains of physical activity and brain volumes: a population-based study. Neuroimage 2017;156:101–108. [DOI] [PubMed] [Google Scholar]
- 30.Boyle PA, Wilson RS, Yu L, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Ann Neurol 2013;74:478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drew T, Marigold DS. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol 2015;33:25–33. [DOI] [PubMed] [Google Scholar]
- 32.Levine AJ, Lewallen KA, Pfaff SL. Spatial organization of cortical and spinal neurons controlling motor behavior. Curr Opin Neurobiol 2012;22:812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brownstone RM, Bui TV, Stifani N. Spinal circuits for motor learning. Curr Opin Neurobiol 2015;33:166–173. [DOI] [PubMed] [Google Scholar]
- 34.Buchman AS, Nag S, Shulman JM, et al. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson's disease. Mov Disord 2012;27:1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchman AS, Leurgans SE, Nag S, et al. Spinal arteriolosclerosis is common in older adults and associated with parkinsonism. Stroke 2017;48:2792–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchman AS, Nag S, Leurgans SE, et al. Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson's disease. Brain Pathol Epub 2017 Sep 28. [DOI] [PMC free article] [PubMed]
- 37.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 2013;81:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vemuri P, Mormino EC. Cognitively stimulating activities to keep dementia at bay. Neurology 2013;81:308–309. [DOI] [PubMed] [Google Scholar]
- 39.Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol (1985) 2011;111:1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wijndaele K, Westgate K, Stephens SK, et al. Utilization and harmonization of adult accelerometry data: review and expert consensus. Med Sci Sports Exerc 2015;47:2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedson P, Bowles HR, Troiano R, Haskell W. Assessment of physical activity using wearable monitors: recommendations for monitor calibration and use in the field. Med Sci Sports Exerc 2012;44:S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in these analyses are available via the Rush Alzheimer's Disease Center Research Resource Sharing Hub, which can be found at radc.rush.edu. It has descriptions of the studies and variables and a dynamic query function to aid searches for data and biospecimens for selected data. There is a login, after which any qualified investigator can submit requests for deidentified data.