Abstract
It is critically important to define disease-specific research priorities to better allocate limited resources. There is growing recognition of the value of involving patients and caregivers, as well as expert clinicians in this process. To our knowledge, this has not been done this way for kidney cancer. Using the transparent and inclusive process established by the James Lind Alliance, the Kidney Cancer Research Network of Canada (KCRNC) sponsored a collaborative consensus-based priority-setting partnership (PSP) to identify research priorities in the management of kidney cancer. The final result was identification of 10 research priorities for kidney cancer, which are discussed in the context of current initiatives and gaps in knowledge. This process provided a systematic and effective way to collaboratively establish research priorities with patients, caregivers, and clinicians, and provides a valuable resource for researchers and funding agencies.
Introduction
Priority-setting for health research that reflects the views of patients, caregivers, and the healthcare providers (expert clinicians) who treat them can improve the relevance, quality, and uptake of research.1–7 In 2010, the U.S.-based Patient-Centred Outcomes Research Institute (PCORI) was established with a mandate that includes the engagement of patients and other stakeholders in all aspects of the research process, including the development of shared research agendas to “study the issues that are most crucial to them.”8 The Canadian Institute of Health Research (CIHR) developed the Canadian Strategy for Improving Patient-Oriented Research, which recognized the important role for patients to increase the likelihood that research priorities are relevant, and recommended the creation of collaborative pan-Canadian processes for identifying and establishing research priorities.5 Despite this, the identification of research priorities in health research has not typically involved these stakeholders and many funding organizations continue to rely on researchers to submit proposals based on their own perceived priorities.9 These may not be shared by patients, caregivers, or clinicians, which can result in “costly mismatches of research-to-needs.”4,10,11 The James Lind Alliance (JLA) is an international leader in setting research priorities6 and has developed a rigorous methodology to “…enable patients, caregivers, clinicians, and the groups that represent them to ensure that research is grounded in what matters to them jointly.”6 These aims are achieved by forming priority-setting partnerships (PSPs), which bring together patients, caregivers, and clinicians, along with their representative organizations, to identify what are termed unanswered management questions and reach consensus on the top 10 shared research priorities.6 While the JLA methodology is well-established in the U.K., it has not been widely applied in North America or for the management of cancers in general.
It is estimated that there will be approximately 69 000 new cases of kidney cancer diagnosed in North America in 2016. One-third will ultimately die from the disease.12,13 As well, the development of effective targeted therapies for metastatic tumours has resulted in a growing population of patients suffering from chronic but relatively stable meta-static disease.14 There has been a vigorous renaissance of interest and research activity in kidney cancer due to these new treatments, as well as advances in genomics and the increased use of biopsy. Unfortunately, disparities and variation in clinical practice for kidney cancer remain15,16 and the research investment in kidney cancer has not been proportionate to its burden on patients, caregivers, clinicians, and healthcare resources.17,18 The development of a strategic research agenda in kidney cancer, which is informed by current evidence and the perceived priorities of stakeholders, is urgently needed and has the potential to result in research to provide solutions to fill important gaps in the management of kidney cancer and improve overall health and wellbeing of those diagnosed. In response, the Kidney Cancer Research Network of Canada (KCRNC), in collaboration with the JLA, Kidney Cancer Canada (KCC), and the Kidney Foundation of Canada (KFofC), formed a PSP to identify unanswered management questions related to kidney cancer and identify the top 10 research priorities shared by patients, caregivers, and clinicians. To our knowledge, there has never been a report of such a process for kidney cancer, nor has there been a similar consensus-based prioritization approach for any cancer type in North America.
The primary objectives of our PSP were to:
Have patients, caregivers, and expert clinicians identify unanswered questions encountered during management of kidney cancer.
Agree by consensus on a prioritized list of the top 10 shared unanswered questions and establish corresponding research priorities.
Priority-setting process and results
Step 1: Formation of steering group
A 15-person steering group was formed with seven patients/caregivers and seven expert clinicians from across Canada. The group also included an advisor from the JLA (U.K.), who provided support and advice throughout the process. The steering group’s responsibilities included defining the scope of the partnership, development of the protocol, identifying potential partners and stakeholders, and oversight of the PSP process. To fulfill this role, the steering group held at least monthly conference calls from June 2014 to March 2015.
Step 2: Identifying treatment questions
To identify unanswered questions arising during the management of kidney cancer, a broad representation of patients, caregivers, and clinicians across Canada were surveyed. The survey instrument started with the following open-ended question: “What uncertainties have you faced about the overall management of kidney cancer? Think broadly. You can include uncertainties about diagnosis, prognosis (prediction of how things may develop in the disease), treatment, and anything else. You can include as many uncertainties as you like.” The next part of the survey provided respondents with specific prompts to help them consider additional management uncertainties that they felt should be answered by research, including domains such as surgical and medical treatments, management of symptoms, lifestyle factors, and psychosocial issues. Basic demographic information was also collected to determine whether the population of interest was successfully captured.
The survey was distributed electronically between September 2014 and November, 2014 via FluidSurveys™ and paper-based copies were distributed in medical clinics, as well as at the healthcare professionals’ association meetings. The survey was advertised through the KCC and the KFofC websites and newsletters in both official languages and through social media channels. The survey was also distributed by e-blasts to the members of various clinical associations, including the Canadian Urological Association (CUA), Genitourinary Medical Oncologists of Canada (GUMOC), Canadian Association of Nurses in Oncology (CANO), and the Urology Nurses of Canada (UNC).
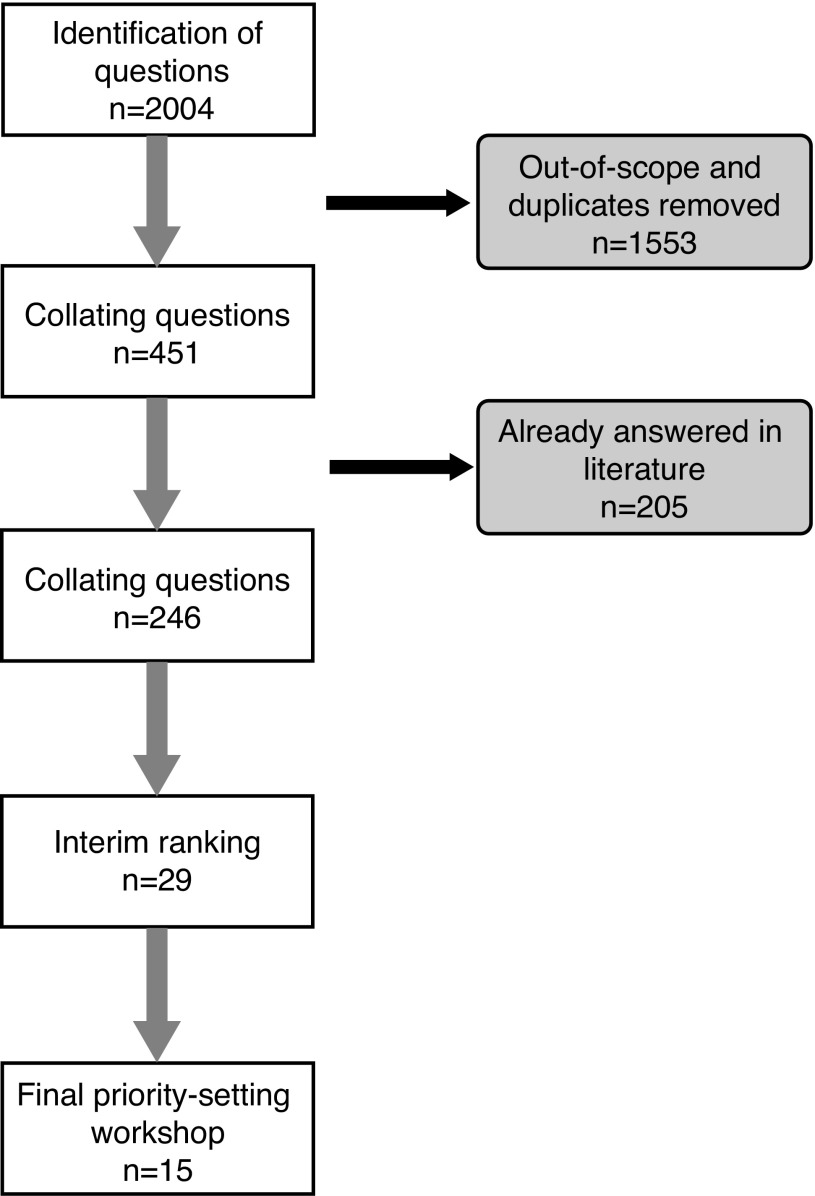
A total of 225 respondents completed the survey and 2004 treatment questions or uncertainties were identified (Fig. 1). One hundred thirty-five (59%) respondents were patients, including those who had been treated for kidney cancer in the past (n=98), those who were currently on treatment (n=34), and those who were recently diagnosed and currently waiting for surgical treatment (n=4). Sixty (27%) were clinicians, including urologists (n=25), nurses (n=18), and medical oncologists (n=7). Thirty-five (14%) were family caregivers. The large majority of surveys were completed online (92%). Most respondents expressed more than one uncertainty, with an average of nine per respondent.
Fig. 1.
Priority-setting process algorithm.
Step 3: Collating questions
During data collection, questions were concurrently organized into 10 categories using a taxonomy for kidney cancer management developed for this study by the steering group (Table 1). Questions from the survey were categorized by iteratively grouping similar ones. Duplicates were combined with notation and those deemed out-of-scope were removed. Examples of out-of-scope questions were those with a knowledge transfer issue, a personal medical condition, or if the uncertainty was felt to be unanswerable by research. Many responses included narrative texts, which were re-phrased to clarify the precise question while attempting to reflect the intent of the response. The project manager (JB) and the research assistant (JA) met weekly to analyze responses. Of the initial 2004 questions submitted, 1553 were identified as out-of-scope or duplicates and combined, leaving 451 unique questions from the survey.
Table 1.
Study-developed taxonomy
| Prevention and prediction |
| Diagnosis |
| Treatment for localized and locally advanced disease |
| Treatment for advanced/metastatic disease |
| Prognosis and followup |
| Impact of disease |
| Survivorship |
| Health economics of kidney cancer |
| Community of practice/expertise |
| Miscellaneous (including use of complementary and alternative treatments) |
The final long list was then further refined by reviewing the literature to filter out those questions already answered. The literature review involved cross-checking questions with current systematic reviews, clinical care guidelines, and individual studies. A search strategy was developed with an information specialist (RF) to identity systematic reviews and clinical care guidelines using Embase and Medline. Other databases consulted included the European Society for Medical Oncology Clinical Practice Guidelines (ESMO), the National Comprehensive Cancer Network (NCCN), the National Institute for Health and Care Excellence (NICE), the National Guideline Clearinghouse (NCG) and the Canadian Medical Association Clinical Practice Guidelines Database (CPGs). This search of the literature reduced the 451 uncertainties to 246 unique uncertainties by filtering out previously answered questions.
Step 4: Interim ranking of questions
The list of 246 questions, including the source and frequency of each (number of times the question was identified by patients, caregivers, and clinicians) was circulated to a subsample of 10 respondents who had participated in the first survey and expressed an interest in further participating in an interim ranking exercise, and also to the 15-member steering group. Over the course of two weeks, these individuals ranked the relative importance of each question, commented on the wording of the question, and indicated their own top-ranked 20. The rankings were entered in a spreadsheet and scored. The steering group then considered the ratings and agreed on a short list of 29 questions that were taken forward for consideration at a final priority-setting workshop.
Step 5: Final priority-setting workshop
The final face-to-face priority-setting workshop took place in February 2015 with the aim of reaching consensus on the top 10 research priorities. There were twenty-three participants from across Canada (10 patients; two caregivers; seven physicians; two nurses; one psychologist, and one dietitian) plus three facilitators with experience in priority-setting processes who facilitated the discussions and encouraged equitable participation of all attendees.
In preparation for the workshop, participants were provided with the short list of 29 questions and were asked to review and rank their own top 10. The one-day workshop followed the JLA protocol and used a nominal group technique to reach consensus. Participants were divided into three groups (with a near equal mix of patients, care providers, and clinicians) who met separately for the remainder of the morning. Each participant stated his/her own views on the questions he/she felt most and least strongly about. Groups were provided with a set of cards with the questions printed on one side and the examples of original uncertainties on the other side. Using a “diamond nine” ranking approach,19,20 each group ranked the 29 questions. During the break, the rankings from the three groups were combined. All workshop participants then reconvened and were provided with group and overall rankings. Clear areas of consensus/disparity between the groups were highlighted and discussed and some items were reworded and combined. Following this, participants were re-allocated to three different small groups to reconsider the aggregate list of 29 ranked questions. During this second round, there was specific focus on the top 15, with the goal of agreeing on the top 10. In the final step, the rankings from all three groups were again combined and the aggregate top 15 were presented in order on individual cards and were discussed. The final 10 were agreed to by the whole group.
The resultant 10 priorities recommended for future research studies in kidney cancer were:
-
(three-way tie)
Development and evaluation of new effective treatment for patients with advanced kidney cancer of the non-clear-cell varieties/subtypes.
Identification and validation of biomarkers that may be used to predict the response to a treatment for kidney cancer.
Identification and validation of biomarkers that may be used for the detection of kidney cancer.
Development and evaluation of new immunotherapies for the treatment of kidney cancer, including immune biomarkers of patient and tumour characteristics and response.
Identification and validation of novel indicators or biomarkers that can be used to predict the development and progression of metastatic kidney cancer.
Assessment of supportive care needs and appropriate supportive care interventions for patients with kidney cancer and their families.
Development of decision-making tools for patients and healthcare providers to help guide treatment decisions in all stages of kidney cancer
Defining the role and criteria for using biopsy in the management of kidney cancer.
Evaluation of the impact of differences in regional funding and access to treatment on patient outcomes for kidney cancer.
Identification of risk factors and cause(s) of kidney cancer.
Table 2 shows examples of the unanswered management questions related to each research priority.
Table 2.
Examples of unanswered management questions
| Development and evaluation of new, effective treatment for patients with advanced kidney cancer of the non-clear-cell varieties/subtypes |
|
| Identification and validation of biomarkers that may be used to predict the response to a treatment for kidney cancer |
|
| Identification and validation of biomarkers that may be used for the detection of kidney cancer |
|
| Development and evaluation of new immunotherapies for the treatment of kidney cancer, including immune biomarkers of patient and tumour characteristics and response |
|
| Identification and validation of novel indicators or biomarkers that can be used to predict the development and progression of metastatic kidney cancer |
|
| Assessment of supportive care needs and appropriate supportive care interventions for patients with kidney cancer and their families |
|
| Development of decision-making tools for patients and healthcare providers to help guide treatment decisions in all stages of kidney cancer |
|
| Defining the role and criteria for using biopsy in the management of kidney cancer |
|
| Evaluation of the impact of differences in regional funding and access to treatment on patient outcomes for kidney cancer |
|
| Identification of risk factors and cause(s) of kidney cancer |
|
Discussion and future directions
Using a rigorous and transparent process that included the perspectives of patients, caregivers, and the clinicians who care for them, the kidney cancer PSP reached consensus on the top 10 unanswered management questions and resultant research priorities. They encompass the full range of care, from prevention to diagnosis to treatment of kidney cancer. Each priority can lead to the development of research hypotheses that would be actionable by a research project or program.
Development and evaluation of new effective treatment for patients with advanced kidney cancer of the non-clear-cell varieties/subtypes (Priority 1a)
Despite advances on the outcome of patients with advanced kidney cancer using new targeted therapies, optimal treatment for non-clear-cell varieties, such as papillary, chromophobe, and collecting-duct cancers, still remains uncertain. This is due to the fact that patients with non-clear-cell histology are typically excluded from trials of targeted agents. While there is evidence to suggest that targeted agents currently approved for kidney cancer may be somewhat effective in non-clear-cell histologies in retrospective literature and some small subgroup analyses of clinical trials, more research is required. Clinical trials such as ASPEN21 and ESPN22 have set benchmarks for tyrosine kinase inhibitor outcomes; however; the trials are small and conclusions are difficult to reach about superiority of one treatment vs. another. Future clinical trials evaluating other systemic agents or combined therapies in this population are needed. For example, molecular research that compares subtypes may aid in the understanding of why some patients with non-clear-cell kidney cancer have extremely good response to currently available targeted therapy and can also be used to inform the development of new targeted agents for the various non-clear-cell subgroups.23 The Cancer Genome Atlas has studied papillary renal cell carcinomas and characterized type I cancers with MET mutations and divided type II papillary renal cell carcinomas into at least three subgroups with activation of the NRF2-ARE pathway, CDKN2A loss, and CIMP, the latter two conveying a poor prognosis.24 Further studies need to further characterize and target these changes and elucidate their role in the era of immune-oncology. Finally, clinical trials should be specifically designed to evaluate current targeted agents in non-clear-cell varieties. Of note, there is a SWOG-led intergroup trial of metastatic papillary renal cell carcinoma randomizing patients between standard-of-care sunitinib vs. volitinib, cabozantinib or crizotinib, the latter three of which have activity against MET (NCT02761057). These types of clinical trials should be prioritized.
The role of biomarkers and other novel indicators in both the detection of kidney cancer and its progression (Priorities 1b, 1c, 5)
Early detection of small, asymptomatic kidney cancers probably increases survival, but also reduces the need for more invasive treatments, such as total nephrectomy, resulting in fewer complications, faster recovery, and lower costs, as well as preservation of renal function and minimization of future kidney disease.25 The identification and validation specific tumour biomarker(s) may provide a cost-effective, safe, and more reliable tool for kidney cancer screening. While tumour biomarkers are not yet available for application,26 there is some emerging and encouraging research in this area. For example, recent evidence suggests that urine aquaporin-1 (AQP1) and perilipin-2 (PLIN2) concentrations are elevated in patients with kidney cancer, but not in benign kidney diseases, and that these may be sensitive and specific biomarkers for the early non-invasive detection of clear-cell or non-clear -cell subtypes of kidney cancer.27,28 Other potential novel biomarkers that are currently being investigated include urinary exosomes (EX), which have some potential to become additional prognostic disease markers for kidney cancer.29 The identification of biomarkers predicting response to therapy with targeted agents or immunotherapy remains a priority. Only clinically validated and reliable predictive biomarkers will allow us to optimally use the current armamentarium of available treatment options and integrate novel active agents. No clinically validated biomarkers ready for clinical use have yet been identified. Intra-tumour heterogeneity, with the majority of genetic aberrations being sub-clonal, is a formidable challenge.30,31 A number of candidate biomarkers have been identified for targeted agents, including loss of function mutations in VHL or TSC1; differences in host genetics, such as single nucleotide polymorphisms in VEGF, HIV-1α, or Il-8 or mutations in the tumour suppressor genes BAP-1, SETD-2; or a composite score of circulating biomarkers, e.g., Il-18.32–35 Since only approximately 20% of patients have no benefit at all from targeted agents, identifying markers of primary resistance may also be crucial. Lack of standardization in assessing biomarkers is another challenge, particularly in immunotherapy. The assessment of PD-L1 immunohistochemistry as a predictive biomarker is impacted by multiple unresolved issues, including variable detection antibodies, differing immunohistochemistry (IHC) cutoffs, tissue preparation, processing variability, primary vs. metastatic biopsies, and staining of tumour vs. immune cells.25–29,36–41
Development and evaluation of new immunotherapies for the treatment of kidney cancer, including immune biomarkers of patient and tumour characteristics and response (Priority 4)
Immune-oncology (IO) is an exciting area of treatment of metastatic renal cell carcinoma that is experiencing a renaissance. Novel therapies used in combination and sequentially with targeted therapies have the potential to improve outcomes in kidney cancer and results from ongoing and future research trials will help inform future treatment strategies.34 A number of strategies are currently under investigation, including checkpoint inhibitors and immune modulators, cancer vaccines, adoptive cell therapy, monoclonal antibodies, and cytokines.26,32 The most developed IO agent in metastatic renal cell carcinoma is nivolumab, a newly approved and expensive immune checkpoint inhibitor that has demonstrated significant improvements in overall survival33,34 and is also now being studied in combination with cabozantinib, a new small-molecule inhibitor of tyrosine kinases (ClinicalTrials.gov number, NCT02496208), and ipilimumab, a CTLA4 inhibitor (ClinicalTrials. gov number, NCT02231749). Despite this renaissance, patient selection with biomarkers has not been successful. Several attempts to use PDL1 expression as a biomarker of efficacy for these immuno-oncology agents have not been successful. Because at least 30–40% of patients have no benefit from PD1-directed therapy, it is important to identify a biomarker so that we do not waste the valuable time the patient has on therapy that is not effective.
Defining the role and criteria for using biopsy in the management of kidney cancer (Priority 8)
With the development of targeted drugs for metastatic kidney cancer, there has been a renewed interest in the use of renal mass biopsy (RMB) to assess pretreatment tumour histology in order to individualize and personalize therapy.42 Likewise, due to concerns regarding overtreatment of small renal masses (SRMs) (and kidney cancers), RMB has been proposed as triage tool to identify pretreatment histology of incidentally diagnosed SRMs, with the objective of decreasing unnecessary surgical procedures and associated adverse events for benign masses, and also potentially for low-metastatic-potential kidney cancers.43
Despite the growing evidence supporting the safety, reliability, and accuracy of RMBs in the management of SRMs,43–45 there remains skepticism among urologists as to their clinical usefulness, as many believe they rarely provide actionable information.46 Consequently, in spite of their promising role in both the diagnosis and treatment of SRMs/kidney cancer, RMBs have not been widely adopted by the urological community. Thus, future work is needed to debunk the current concerns of urologists regarding the use of RMBs in the management of SRMs42 and to explore how RMBs can be integrated with treatment algorithms to inform risk stratification of patients with SRMs and aid in decision-making.47,48
Recent advances in genetics and epigenetics has improved our understanding of kidney cancers and their clinical outcomes. Studies have shown that gene expression profiling is feasible on tissues49,50 obtained through RBM. As a result, the role of RMB seems promising and may expand even more in the next decade.
Given the current evidence, it seems plausible that the future of SRM management will combine pathological, molecular, and genetic information obtained via RMB, which will improve our ability to predict the behaviour of these lesions, guide management, and ultimately, facilitate personalization of care.
Assessment of supportive care needs and appropriate supportive care interventions for patients with kidney cancer and their families (Priority 7)
Studies to date have documented unmet informational and supportive care (psychological, emotional, and social) needs in patients with kidney cancer and suggest that the treatment of kidney cancer can impact negatively on physical and psychosocial functioning;51–53 however, as with other cancer types, there remains a need for rigorous research regarding the optimal approach to supportive care needs assessment.54 The provision of supportive care interventions (i.e., patient education, psychosocial support, palliative care, rehabilitation) have the potential to improve patient outcomes,55 but have not yet been developed or tested in this population.56 Future research, including qualitative studies to determine the types of interventions kidney cancer patients feel would meet their psychosocial needs, along with prospective and well-designed randomized, controlled trials should focus on the development of practical supportive care interventions. KCC is currently collaborating with members of KCRNC on a cross-sectional survey study to identify needs and barriers to access to treatment, information, and support for patients with kidney cancer in Canada.57 In addition, a proposed project through the KRCNC is the development of a prospective research database of patient-reported outcomes for patients diagnosed with kidney cancer in Canada.58 This data could be used to further identify supportive care needs and assist in the development of and evaluation of supportive care interventions.
The development of decision-making tools for patients and healthcare providers to help guide treatment decisions in all stages of kidney cancer (Priority 8)
Shared decision-making is particularly relevant for patients diagnosed with kidney cancer who face a range of decisions regarding surgical alternatives for early-stage cancer or second-/third-line treatments for metastatic disease. Shared decision-making is the process by which decisions are made between clinicians and patients based on the best available evidence and patients’ informed values.59 These decisions are complex, given that patients must understand and weigh the benefits and harms across treatment options, and this information is frequently presented in inaccessible, academic formats. Still, many patients and family members wish to be active participants in making decisions regarding their medical care. Patients are often faced with circumstances where there is no one “best” treatment choice, but rather a personal decision that incorporates their own values and preferences.
There is high-quality evidence that decision aids compared to usual care improve people’s engagement in decision-making, knowledge of options, accurate risk perceptions of outcomes, and reduce their decisional conflict related to feeling uninformed and unclear about their personal values;60 however, decision tools have not been developed or evaluated in kidney cancer61,62 and there are many challenges to integrating them into the process or care that need to be addressed through research.63,64
Some examples of proposed decision aids that could be developed for patients diagnosed with kidney cancer include the use of cytoreductive nephrectomy for newly diagnosed metastatic disease and options for management of small renal masses.52,59–64
Evaluate the impact of differences in regional funding and access to treatment on patient outcomes for kidney cancer (Priority 9)
Given the current Canadian healthcare system limitations with provincial planning and budgeting, patients, families, and clinicians are concerned about timely access to quality care and treatments. For example, access to oral targeted therapies for kidney cancer differs by province and not all patients are eligible for coverage. In addition to funding differences, there is also documented variation in practice patterns, with evidence showing that rural residents are less likely to receive partial nephrectomies compared to their urban counterparts.65 Further, patients who must travel to receive treatment have higher mortality rates compared to those who have urological care available in their immediate communities.66 Odisho et al found direct access to a urologist resulted in an 8–14% reduction in kidney cancer mortality.66 More research is needed to determine the disparities and differences in kidney cancer management and outcomes; ongoing surveillance will provide critical evidence to help delineate the current magnitude and pattern of health delivery gaps over time in relation to different and changing healthcare policies so that improvement strategies can be implemented.67 Based on this priority, the KCRNC membership is actively looking at regional differences in kidney cancer care through the pan-Canadian Kidney Cancer information system, which collects data on kidney cancer patients from 15 academic centres across Canada.68
The identification of risk factors and cause(s) of kidney cancer (Priority 10)
Patients and family were particularly interested in research to identify risk factors for kidney cancer, and certainly more work is needed in this area, as it is ideal to prevent rather than just better treat kidney cancer. Like other cancers, the etiology and risk (and protective) factors of kidney cancer are not completely understood. There is some research suggesting that the increased incidence of renal cell carcinoma and other kidney tumours may be, in part, due to the rise in hypertension and obesity.69 Lifestyle and health behaviours, such as physical activity, smoking, and alcohol consumption, may also play an etiological role, although more research is needed to establish causal relationships.70 There is also interest in genetics and its role in the pathogenesis of renal cell cancer. There are a number of single genes associated with the development of familial and inherited forms of renal cell cancer. These include highly penetrant genes, such as VHL, MET, BAP1, and FLCN, where individuals develop RCC at young ages and have a family history suggestive of an inherited etiology.71 While rare, personalized approaches to the care of mutation carriers can be implemented based on the gene and mutation.72 Other more common genetic variants and their interactions with environmental exposures have been proposed to influence renal cell cancer risk in non-heritable forms of renal cell carcinoma. To date, the majority of studies have been based on genome-wide association studies, but results have been mixed.73,74 The advancement in in next-generation sequencing for genome scale studies will enable researchers to conduct more comprehensive evaluations of common genetic variations and have the potential to lead to novel discoveries into genetic determinants and how their interactions with environment may influence renal cell cancer etiology.70
Conclusion
While these top 10 uncertainties and resulting research priorities should not be the sole driver of the research agenda, we believe they should receive careful consideration by funders and researchers alike. Each of the priorities could be explored with carefully designed research. Given the CIHR’s mandate to include patients in determining what type of research should be funded, this study presents an effective way to collaboratively establish research priorities with patients, caregivers, and healthcare providers.
Acknowledgement/funding
Members of the Steering Committee who are not authors: Eric Hyndman, MD; Stephen Andrew; Christine Collins; Marion Cooper; Mary Mackinnon; Karen Ross; Andrew Weller; and Wim Wolfs. The work was supported by a grant from the Canadian Institutes of Health Research (Grant #138851).
Footnotes
Competing interests: Dr. Bhatt has received honoraria from Astellas U.K. Dr. Laupacis has been an advisor for Novartis. Dr. Basappa has been an advisor for Astellas, AstraZeneca, BI, BMS, Janssen, Novartis, and Pfizer; and has received honoraria from Astellas, BMS, Janssen, Novartis, and Pfizer. Dr. Canil has attended advisory boards for Bayer, BMS, Esai, Merck, Pfizer and Roche; has been a speaker for Bayer and Sanofi; and has received sponsorship for an international preceptorship from Pfizer. Dr. Al-Asaaed has been an advisor for Janssen and Pfizer; has received grants/honoraria from Amgen, Astellas, Celgene, Novartis, and Pfizer; and has participated in clinical trials supported by Janssen. Dr. Heng has been an advisor for BMS, Novartis, and Pfizer. Dr. Wood has been an advisor for Astellas, Novartis, and Pfizer, but received no financial compensation; and has participated in clinical trials supported by AstraZeneca, BMS, Exelixis, Merck, Pfizer, and Roche. Dr. Kollmannsberger has been an advisor for Astellas, BMS, Novartis, Pfizer, and Roche; has received presentation honoraria from BMS, Pfizer, and Novartis; and has participated in clinical trials supported by Astellas, AstraZeneca, BMS, Janssen, Merck, Novartis, Pfizer, and Sanofi. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Caron-Flinterman JF, Broerse JE, Bunders JE. The experiential knowledge of patients: A new resource for biomedical research? Soc Sci Med. 2005;60:2575–84. doi: 10.1016/j.socscimed.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Entwistle VA, Renfrew MJ, Yearley S, et al. Lay perspectives: Advantages for health research. BMJ. 1998;316:463–6. doi: 10.1136/bmj.316.7129.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodare H, Lockwood S. Involving patients in clinical research improves the quality of research. BMJ. 1999;319:724–5. doi: 10.1136/bmj.319.7212.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CIHR. International review panel report 2011. [Accessed October 31, 2017]. [cited 2014 August 10]. Available from: http://www.cihr-irsc.gc.ca/e/43993.html.
- 5.CIHR. Canada’s strategy for patient-oriented research: Improving health outcomes through evidence-informed care. 2013. [Accessed October 31, 2017]. [cited 2013 September 27]. Available from: http://www.cihr-irsc.gc.ca/e/44000.html.
- 6.The James Lind Alliance J. [Accessed October 31, 2017]. [cited 2014. August 10]. Available from: http://www.lindalliance.org/index.asp.
- 7.Cowan K, Oliver S. The James Lind Alliance Guidebook: version 5. United Kingdom: The James Lind Alliance; 2013. p. 96. [Google Scholar]
- 8.Patient-Centred Outcomes Research Institute. Easier treatment for serious infection. [Accessed October 31, 2017]. Available from: http://www.pcori.org/
- 9.Stewart R, Oliver S. A systematic map of studies of patients’ and clinicians’ research. London: The James Lind Alliance; 1998. p. 55. [Google Scholar]
- 10.Oliver S, Armes DG, Gyte G. Public involvement in setting a national research agenda: A mixed methods evaluation. Patient. 2009;2:179–90. doi: 10.2165/11314860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Tallon D, Chard J, Dieppe P. Relation between agendas of the research community and the research consumer. Lancet. 2000;355:2037–40. doi: 10.1016/S0140-6736(00)02351-5. [DOI] [PubMed] [Google Scholar]
- 12.De P, Otterstatter MC, Semenciw R, et al. Trends in incidence, mortality, and survival for kidney cancer in Canada, 1986–2007. Cancer Causes Control. 2014;25:1271–81. doi: 10.1007/s10552-014-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. A snapshot of kidney cancer: Incidence and mortality. 2014. [Accessed October 31, 2017]. Available from: http://www.cancer.gov/research/progress/snapshots/kidney.
- 14.Harrison MR, Hirsch BR, George D, et al. Real-world outcomes in metastatic renal cell carcinoma: Insights from a joint community-academic registry. J Oncol Pract. 2014;10:e63–e72. doi: 10.1200/JOP.2013.001180. [DOI] [PubMed] [Google Scholar]
- 15.Miller DC, Saigal C, Banerjee, et al. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112:1708–17. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan HJ, Meyer AM, Kuo TM, et al. Provider-based research networks and diffusion of surgical technologies among patients with early-stage kidney cancer. Cancer. 2015;121:836–43. doi: 10.1002/cncr.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Cancer Research Alliance. Cancer research investment in Canada, 2008–2012: The Canadian Cancer Research Alliance’s survey of government and voluntary sector investment in cancer research in 2012. Toronto: CCRA; 2015. [Google Scholar]
- 18.National Cancer Institute. NCI-funded research portfolio (NFRP) 2013. [Google Scholar]
- 19.US Department of Health and Human Services. Healthy people 2010 toolkit: Setting health priorities and objectives. Washington DC: US Department of Health and Human Services; 2010. p. 28. [Google Scholar]
- 20.Rockett M, Percival S. Thinking for learning. Stafford: Network Educational Press; 2002. [Google Scholar]
- 21.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus vs. sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomized, phase 2 trial. Lancet Oncol. 2016;17:378–88. doi: 10.1016/S1470-2045(15)00515-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannira NM, Jonascha E, Albigesb L, et al. Everolimus vs. sunitinib prospective evaluation in metastatic non-clear-cell renal cell carcinoma (ESPN): A randomized, multicentre, phase 2 trial. Eur Eurol. 2016;69:866–74. doi: 10.1016/j.eururo.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network. Linehan WM, Spellman PT, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374:135–45. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive molecular characterization of papillary renal cell carcinoma. N Engl J Med. 2016;374:135–45. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 26.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11:517–25. doi: 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 27.Morrissey JJM, Mobley J, Figenshau RS, et al. Urine aquaporin-1 and perilipin-2 differentiate renal carcinomas from other imaged renal masses and bladder and prostate cancer. Mayo Clin Proc. 2015;90:35–42. doi: 10.1016/j.mayocp.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrissey JJ, Mellnick VM, Luo J, et al. Evaluation of urine aquaporin-1 and perilipin-2 concentrations as biomarkers to screen for renal cell carcinoma: A prospective cohort study. JAMA Oncol. 2015;1:204–12. doi: 10.1001/jamaoncol.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzen CA, Blackwell RH, Foreman KE, et al. Urinary exosomes: The potential for biomarker utility, intercellular signaling, and therapeutics in urologic malignancy. J Urol. 2015 doi: 10.1016/j.juro.2015.08.115. S0022-5347:05509-3. [DOI] [PubMed] [Google Scholar]
- 30.Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: A meta-analysis. BMJ Open. 2015;5:e006404. doi: 10.1136/bmjopen-2014-006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat Rev. 2014;40:533–47. doi: 10.1016/j.ctrv.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Escudier B. Emerging immunotherapies for renal cell carcinoma. Ann Oncol. 2012;23:viii35–40. doi: 10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- 33.Thomas JS, Kabbinavar F. Metastatic clear-cell renal cell carcinoma: A review of current therapies and novel immunotherapies. Crit Rev Oncol Hematol. 2015;96:527–33. doi: 10.1016/j.crit-revonc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab vs. everolimus in advanced renal cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomaszewski JJ, Uzzo RG, Smaldone MC. Heterogeneity and renal mass biopsy: A review of its role and reliability. Cancer Biol Med. 2014;11:162–72. doi: 10.7497/j.issn.2095-3941.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sankin A, Hakimi AA, Mikkilineni N, et al. The impact of genetic heterogeneity on biomarker development in kidney cancer assessed by multiregional sampling. Cancer Med. 2014;3:1485–92. doi: 10.1002/cam4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear-cell renal cell carcinomas defined by multi-region sequencing. Nat Genet. 2014;46:225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwiatkowski DJ, Choueiri TK, Fay AP, et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:2445–52. doi: 10.1158/1078-0432.CCR-15-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonpavde G, Choueiri TK. Precision medicine for metastatic renal cell carcinoma. Urol Oncol. 2014;32:5–15. doi: 10.1016/j.urolonc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Voss MH, Chen D, Marker M, et al. Circulating biomarkers and outcomes from a randomized, phase 3 trial of sunitinib vs. everolimus for patients with metastatic renal cell carcinoma. Br J Cancer. 2016;114:642–9. doi: 10.1038/bjc.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Molec Cancer Therapy. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 42.Leppert JT, Hanley J, Wagner TH, et al. Utilization of renal mass biopsy in patients with renal cell carcinoma. J Urol. 2014;83:774–80. doi: 10.1016/j.urology.2013.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard PO, Jewett MA, Bhatt JR, et al. Renal tumour biopsy for small renal masses: A single-centre, 13-year experience. Eur Urol. 2015;68:1007–13. doi: 10.1016/j.eururo.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Eurol. 2016;69:660–73. doi: 10.1016/j.euru-ro.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 45.Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: Systematic review of the literature. J Urol. 2016;195:1340–7. doi: 10.1016/j.juro.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutikov A, Smaldone MC, Uzzo RG, et al. Renal mass biopsy: Always, sometimes, or never? Eur Eurol. 2016;70:403–6. doi: 10.1016/j.eururo.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Blute ML, Drewry A, Abel EJ. Percutaneous biopsy for risk stratification of renal masses. Ther Adv Urol. 2015;7:265–74. doi: 10.1177/1756287215585273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halverson SJ, Kunju LP, Bhalla R, et al. Accuracy of determining small renal mass management with risk stratified biopsies: Confirmation by final pathology. J Urol. 2013;189:441–6. doi: 10.1016/j.juro.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Rogers CG, Ditlev JA, Tan M, et al. Microarray gene expression profiling using core biopsies of renal neoplasia. Am J Transl Res. 2009;1:55–61. [PMC free article] [PubMed] [Google Scholar]
- 50.Lane BR, Li J, Zhou M, et al. Differential expression in clear-cell renal cell carcinoma identified by gene expression profiling. J Urol. 2009;181:849–60. doi: 10.1016/j.juro.2008.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ficarra V, Novella G, Sarti A, et al. Psychosocial well-being and general health status after surgical treatment for localized renal cell carcinoma. Int Urol Nephrol. 2002;34:441–6. doi: 10.1023/A:1025683306449. [DOI] [PubMed] [Google Scholar]
- 52.Anastasiadis AG, Davis AR, Sawczuk IS, et al. Quality of life aspects in kidney cancer patients: Data from a national registry. Support Care Cancer. 2003;11:700–6. doi: 10.1007/s00520-003-0484-2. [DOI] [PubMed] [Google Scholar]
- 53.Moretto P, Jewett MAS, Basiuk J, et al. Kidney cancer survivorship survey of urologists and survivors: The gap in perceptions of care, but agreement on need. Can Urol Assoc J. 2014;8:190–4. doi: 10.5489/cuaj.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howell D, Mayo S, Currie S, et al. Psychosocial health care needs assessment of adult cancer patients: A consensus-based guideline. Support Care Cancer. 2012;20:3343–54. doi: 10.1007/s00520-012-1468-x. [DOI] [PubMed] [Google Scholar]
- 55.CJSE Panel. Howell D, Hack TF, et al. Survivorship services for adult cancer populations: A pan-Canadian guideline. Curr Oncol. 2011;18:e265–81. doi: 10.3747/co.v18i6.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong ST, Butow PN, Tong A, et al. Patients’ experiences and perspectives of multiple concurrent symptoms in advanced cancer: A semi-structured interview study. Support Care Cancer. 2016;24:1373–86. doi: 10.1007/s00520-015-2913-4. [DOI] [PubMed] [Google Scholar]
- 57.Kidney Cancer Canada. [Accessed October 31, 2017]. Available from: http://www.kidneycancercanada.ca/
- 58.KCRNo Canda Patient and Caregiver Forum: Live it! Survive it! Cure it! From living with kidney cancer to a cure. 2016. [Accessed October 31, 2017]. Available from: http://www.kidneycancercanada.ca/news-plus-events/watch-past-meetings/2016-patient-caregiver-forum/
- 59.Makoul G, Clayman ML. An integrative model of shared decision-making in medical encounters. Patient Educ Couns. 2006;60:301–12. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Stacey D, Légaré F, Col NF, et al. John Wiley & Sons, Ltd, editor. Reviews TCCCDoS. 2014. Decision aids for people facing health treatment or screening decisions (Review) [DOI] [PubMed] [Google Scholar]
- 61.Patient Decision Aids Research Group OHRI. A to Z inventory of decision aids. 2016. [Accessed October 31, 2017]. Available from: https://decisionaid.ohri.ca/azlist.html.
- 62.Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: A systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S14. doi: 10.1186/1472-6947-13-S2-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Légaré F, Stacey D, Turcotte S, et al. Interventions for improving the adoption of shared decision-making by healthcare professionals. Cochrane Database Syst Rev. 2014;15:CD006732. doi: 10.1002/14651858.CD006732.pub3. [DOI] [PubMed] [Google Scholar]
- 64.Stacey D, Samant R, Bennett C. Decision-making in oncology: A review of patient decision aids to support patient participation. CA: Cancer J Clin. 2008;58:293–304. doi: 10.3322/CA.2008.0006. [DOI] [PubMed] [Google Scholar]
- 65.Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the United States: Results from a population-based cohort. Int Braz J Urol. 2011;37:663–70. doi: 10.1590/S1677-55382011000500018. [DOI] [PubMed] [Google Scholar]
- 66.Odisho AY, Cooperberg MR, Fradet V, et al. Urologist density and county-level urologic cancer mortality. J Clin Oncol. 2010;28:2499–504. doi: 10.1200/JCO.2009.26.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed S, Shahid RK. Disparity in cancer care: A Canadian perspective. Curr Oncol. 2012;19:e376–82. doi: 10.3747/co.19.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kidney Cancer Canada. Accessing Treatment for Kidney Cancer. 2016. [Accessed October 31, 2017]. Available from: http://www.kidneycancercanada.ca/for-patients-and-caregivers/treatment-access-information/treatment-access-information-by-provinceterritory/access-to-medications-by-province-territory/
- 69.Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of U.S. men and women. Hypertension. 2014;63:934–41. doi: 10.1161/HYPERTENSIONAHA.113.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature Reviews Urology. 2010;7(5):245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reaume MN, Graham GE, Tomiak E, et al. Canadian guideline on genetic screening for hereditary renal cell cancers. Can Urol Assoc J. 2013;7:319–23. doi: 10.5489/cuaj.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho TH, Jonasch E. Genetic kidney cancer syndromes. J Natl Compr Canc Netw. 2014;12:1347–55. doi: 10.6004/jnccn.2014.0129. [DOI] [PubMed] [Google Scholar]
- 73.Purdue MP, Ye Y, Wang Z, et al. A genome-wide association study of renal cell carcinoma among African Americans. Cancer Epidemiol Biomarkers Prev. 2014;23:209–14. doi: 10.1158/1055-9965.EPI-13-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gudmundsson J, Sulem P, Gudbjartsson DF, et al. A common variant at 8q24.21 is associated with renal cell cancer. Nat Commun. 2013;4 doi: 10.1038/ncomms3776. [DOI] [PubMed] [Google Scholar]