Abstract
Introduction
In 2014, the Canadian Task Force on Preventive Health Care (CTFPHC) recommended against routine prostate cancer screening with the prostate-specific antigen (PSA) blood test.1 We surveyed Canadian primary care physicians (PCPs) to understand their opinions and attitudes towards prostate cancer screening in 2016.
Methods
Twenty PCPs piloted the survey to assess its accessibility. We distributed a flyer to 19 633 PCPs as an insert in a large mailed package inviting them to attend a national meeting, and later promoted the survey at the meeting. Multinomial logistic regression models examined factors associated with agreement of key guideline statements and the overall benefit of PSA screening.
Results
A total of 1254 PCPs responded (rate of 6.4%); 54.7% of physicians aware of the CTFPHC recommendations report screening less often as a result. Overall, 55.6% of PCPs feel that the risks of PSA screening outweigh the benefits. On multivariable analysis, physicians who did not read the guidelines, did not have an academic appointment, or were in practice for over 20 years were significantly more likely to disagree with the statement that men 55–69 years old should not be screened for prostate cancer with PSA.
Conclusions
Our national survey found that the prostate cancer screening practices of Canadian PCPs varies widely across physician demographic groups, with almost equal numbers for or against. This has significant ethical, medical, and legal implications. The poor response rate to highly incentivized survey request may suggest a reluctance or general apathy towards this subject because of the Task Force recommendations. Future efforts should provide physicians with objective guidance around PSA screening, incorporating input from all stakeholders, including PCPs, urologists, and patients.
Introduction
Prostate cancer is the most prevalent cancer among Canadian men, representing 24% of all new cancer diagnoses in Canada.1 For nearly three decades, screening for prostate cancer with prostate-specific antigen (PSA) testing has been an essential component of preventive care.2 More recently, the risks and benefits of PSA as a screening biomarker for prostate cancer have come under scrutiny, prompting a reevaluation of its role in clinical practice.3,4
First in 2008, and again in 2012, the United States Preventive Services Task Force (USPSTF)3 published recommendations against screening for prostate cancer based on two large, randomized, controlled trials. The USPSTF is a government-issued panel composed of clinical epidemiologists, internists, and primary care physicians (PCPs), who objectively analyze available data and make recommendations based on the perceived quality of the evidence. Following the 2012 updated recommendations, the Canadian government asked the Canadian Task Force on Preventive Health Care (CTFPHC)4 to undertake a comparable analysis. In 2014, a similar recommendation against screening for prostate cancer with the PSA test was published by the CTFPHC. The American and Canadian task forces cite both of the large, randomized trials in their recommendations, as neither was able to show an overall survival benefit in their screening arms despite evidence of false-positive biopsies, over-diagnosis of non-life-threatening cancers, and subsequent complications from investigation and treatment.5,6
These recommendations have been met with criticism from urologists, oncologists, and patient advocacy groups. In October 2014, the Canadian Urological Association (CUA) issued a press release addressing the CTFPHC recommendations, citing concerns that the task force failed to include key observational studies that point to PSA’s utility in both screening and risk-stratification of men aged less than 55 years old.7,8 The CUA also cited the failure to acknowledge the role of PSA screening in conservative management (“active surveillance”) of diagnosed low-risk cases,9 an established clinical practice in Canadian urology. In light of this ongoing controversy, the views and practices of Canadian PCPs in 2016 remain heterogeneous. In 2012, Allard et al published the results of a provincial survey of Ontario family physicians, immediately following publication of the USPSTF guidelines.10 They found a wide variation among Ontario PCPs, around both general PSA screening practices and their individual beliefs about the utility of screening for prostate cancer. Earlier studies conducted in British Columbia and Newfoundland and Labrador found similar results.11,12
We created a survey instrument to survey a national sample of Canadian PCPs to understand their knowledge of and agreement with the CTFPHC guidelines, their current screening practices, and their use of shared decision-making around PSA testing.
Methods
Our survey instrument was designed for distribution to PCPs across Canada who routinely see men of prostate cancer screening-appropriate age in their practice (Appendix 1; available at cuaj.ca). The survey questionnaire and content were developed systematically through multiple iterations, with input from experts in key stakeholder groups. Prior to distribution, a pilot survey was conducted using local area PCPs from both community and academic practices in Ontario and British Columbia, and feedback was collected regarding the content and accessibility of the instrument.
The population approached to complete the survey represented a sampling of PCPs from across Canada, excluding Quebec. A flyer was distributed as an insert to a mailed invitation to attend a national primary care physician conference. We used a raffle prize draw to incentivize participants to complete the survey. A total of 19 633 physicians (out of 30 902 PCPs in Canada, excluding Quebec) received the mailing including our invitation, informing them of the purpose of the study and directing them to a web address where the survey was located, hosted by Fluid Surveys (www.fluid-surveys.com). The survey was kept open for three months to ensure enough time for respondents to access the questionnaire (June–August 2016). We then re-opened and promoted the survey at the national conference, asking those who had yet to complete the questionnaire to do so.
Descriptive statistics included frequency distribution data and histogram representation of survey responses. Stratification of the cohort allowed for data comparisons across different demographic categories. Mann-Whitney U and Kruskal-Wallis tests were used to identify significant variations in agreement with guideline statements and overall benefit of PSA screening across strata. We used multinomial logistic regression models to understand the relationship between physician demographic and practice type, agreement with guideline statements and interpretation of the overall risk-benefit relationship of PSA screening. Statistical significance was set at p<0.05 based on a two-tailed comparison. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, U.S.). Ethical approval was granted for the study (REB Ref. 670-1601-Uro-009).
Results
One thousand fifty-eight physicians accessed the survey after the mail-out flyer, with an additional 196 during the second enrollment period at the conference (total of 1254 respondents; response rate 6.4%). Forty-seven of these were excluded, as no responses were recorded. Seventeen respondents were excluded after reporting they were not currently working as PCPs in a Canadian practice or seeing men of screening age. A total of 1190 responses were included in the final analysis (93% completion rate).
Demographics
Demographic data from both the first and second enrollment periods were similar, and so were combined for the overall analysis (Table 1).
Table 1.
Respondent demographics (n=1190)
| No (%) | |
|---|---|
| Gender | |
| Female | 549 (46.1) |
| Male | 641 (53.9) |
| Age | |
| <35 years old | 332 (27.9) |
| 35–44 years old | 374 (31.4) |
| 45–54 years old | 218 (18.4) |
| 55–64 years old | 187 (15.7) |
| >65 years old | 79 (6.6) |
| Province/territory | |
| Alberta | 216 (18.2) |
| British Columbia | 309 (26.0) |
| Manitoba | 40 (3.4) |
| New Brunswick | 35 (2.9) |
| Newfoundland | 18 (1.5) |
| Northwest Territories | 1 (0.1) |
| Nova Scotia | 31 (2.6) |
| Nunavut | 2 (0.2) |
| Ontario | 398 (37.3) |
| Prince Edward Island | 5 (0.4) |
| Quebec | 17 (1.4) |
| Saskatchewan | 64 (5.4) |
| Yukon | 5 (0.4) |
| Years in practice | |
| <5 | 402 (33.8) |
| 5–10 | 231 (19.4) |
| 10–20 | 202 (17) |
| >20 | 355 (29.8) |
| Catchment area size | |
| Small population centre (≤29 999 people) | 313 (26.3) |
| Medium population centre (30 000–99 999 people) | 249 (20.9) |
| Large population centre (≥100 000 people) | 628 (52.8) |
| Practice type | |
| Group practice | 1020 (85.7) |
| Solo practice | 170 (14.3) |
| Academic affiliation | |
| Yes | 544 (45.7) |
| No | 646 (54.3) |
Sources of information on screening guidance
Our questionnaire asked respondents to identify where they turn to for guidance regarding best practice in cancer screening; 45.4% reported using government agencies to inform their screening practice (e.g., CTFPHC), whereas 26.1% use specialist organizations (e.g., CUA), and 25.3% look to national or provincial colleges.
Understanding of and agreement with CTFPHC guidelines
Most respondents (81.5%) were aware of the 2014 CTFPHC guidelines at the time of the survey, 80.9% of whom reported having read the document. Of those reading the guideline, 78.1% perceived the guidelines to be either “clear” or “very clear.” Of those who were aware of the recommendations, 54.7% reported screening less as a result, 4.7% screen more often, and 40.5% reported no change in their screening practices (of whom 23.1% report not routinely using the PSA test).
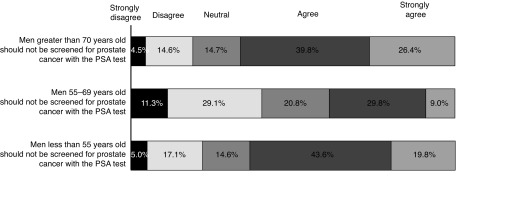
We then asked respondents to state their level of agreement with the CTFPHC report’s recommendations on screening for three separate age groups of men (Fig. 1). There was little agreement regarding men aged 55–69 years, with 38.8% of respondents agreeing that men in this cohort should not be screened. Notably, 10.6% of respondents reported disagreement with all three guideline statements, whereas 35% agreed with all CTFPHC recommendations.
Fig. 1.
Respondent agreement with routine screening in men of different age groups. PSA: prostate-specific antigen.
Screening practice patterns
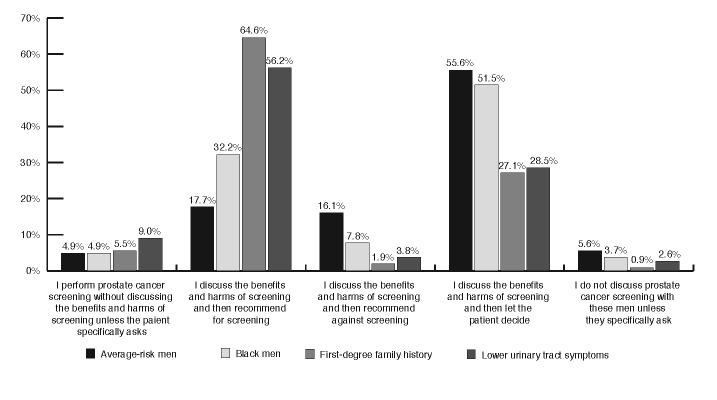
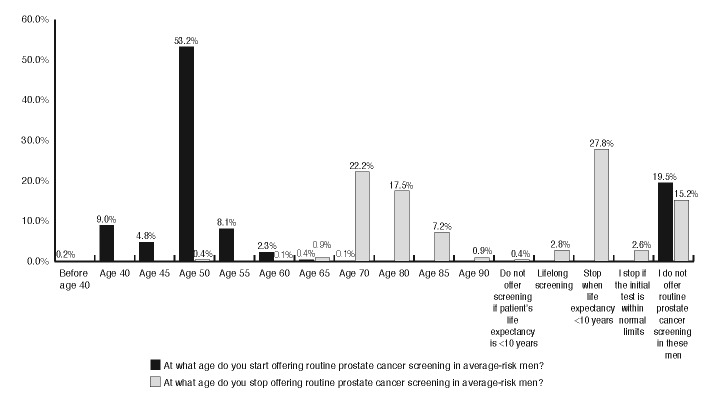
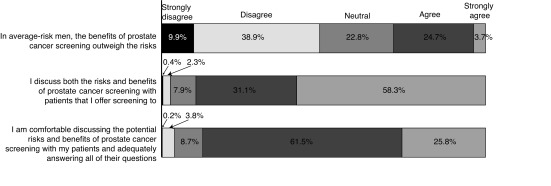
Respondents were asked to outline their screening practices for men with different prostate cancer risk profiles (Fig. 2). We also inquired more generally about screening methods. The majority of respondents (52.6%) reported using both PSA and digital rectal examination (DRE), with 14.5% using DRE alone and 10.2% using only PSA testing without physical examination. Fig. 3 illustrates the patient ages at which physician’s initiate and terminate routine prostate cancer screening. When the initial test is normal, the frequency of PSA testing by those who recommend screening was either annually (22.9%), every two years (31.6%), or not again (27.6%). Finally, we asked respondents to provide their overall level of agreement with the statement that in average-risk men, PSA screening’s benefits outweigh its risks (Fig. 4).
Fig. 2.
Screening practices in men with different risk profiles.
Fig. 3.
At what ago do you start/stop offering routine cancer screening in average-risk men?
Fig. 4.
Respondents’ agreement with a shared-decision approach to screening.
Shared decision-making
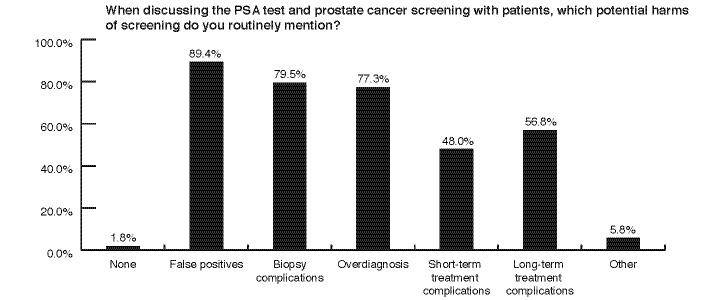
The vast majority of respondents believe in a shared decision-making approach to PSA testing (Fig. 4); 89.4% discuss the risks and benefits of screening with men, and 87.3% of physicians reported feeling comfortable having such a discussion with a patient in their practice. Respondents counsel their patients around many of the risks associated with PSA testing (Fig. 5).
Fig. 5.
Counselling patients on risks of prostate-specific antigen screening.
Multivariable analysis
Multinomial logistic regression models were constructed to better understand whether key demographics and practice-types of PCPs affected their agreement with guideline statements and overall perceived benefit of PSA screening (Table 2a). The relative odds of agreeing rather than being neutral was 2.07 (95% confidence interval [CI] 1.39–3.09) times for PCPs with an academic appointment compared to PCPs without an academic appointment. Conversely, those with over 20 years in practice were more likely to disagree with this recommendation (odds ratio [OR] 2.43; 95% CI 1.29–4.60). In men 55–69 years old, PCPs who had read the guidelines document were less likely to disagree than be neutral with the CTFPHC’s recommendation (OR 0.59; 95% CI 0.37–0.95), as were those with an academic appointment (OR 0.67; 95% CI 0.46–0.98); however, physicians with 10–20 years’ experience were less likely to agree than be neutral with this recommendation (OR 0.58; 95% CI 0.36–0.96). Those with more than 20 years’ experience were also more likely to disagree than be neutral that men aged 55–69 should not be screened (OR 2.73; 95% CI 1.63–4.57). We examined which demographics predicted a PCP’s agreement with the statement “in average risk men (i.e., no risk factors for prostate cancer), the benefits of prostate cancer screening outweigh the risks” (Table 2b). Similar to the guideline statements, those PCPs who had read the guideline document were more likely to disagree than be neutral with this statement (OR 1.88; 95% CI 1.25–2.85). Physicians with greater than 20 years’ experience (OR 3.55; 95% CI 2.03–6.19) were more likely to agree than be neutral with the above statement, and those with 10–20 years’ experience were both more likely to agree (OR 2.00; 95% CI 1.12–3.58) and less likely to disagree (OR 0.59; 95% CI 0.36–0.95) that the benefits of PSA screening outweigh the risks, compared to physicians with neutral responses.
Table 2a.
Results of multinomial logistic regression, part 1
| Men <55 years old should not be screened for prostate cancer with PSA | Men 55–69 years old should not be screened for prostate cancer with PSA | Men >70 years old should not be screened for prostate cancer with PSA | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Disagree | Agree | Disagree | Agree | Disagree | Agree | |||||||||||||
|
| ||||||||||||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||
|
| ||||||||||||||||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Read guidelines (yes vs. no) | 0.94 | 0.54 | 1.64 | 1.21 | 0.74 | 1.96 | 0.59 | 0.37 | 0.95 | 1.03 | 0.63 | 1.68 | 0.66 | 0.35 | 1.23 | 0.87 | 0.51 | 1.48 |
| Academic appointment (yes vs. no) | 1.20 | 0.75 | 1.93 | 2.07 | 1.39 | 3.09 | 0.67 | 0.46 | 0.98 | 1.12 | 0.78 | 1.60 | 0.97 | 0.58 | 1.61 | 1.95 | 1.30 | 2.92 |
| Catchment area | ||||||||||||||||||
| Small | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||
| Medium | 1.31 | 0.65 | 2.64 | 1.06 | 0.60 | 1.87 | 1.22 | 0.71 | 2.09 | 1.14 | 0.68 | 1.90 | 0.76 | 0.36 | 1.61 | 1.05 | 0.58 | 1.91 |
| Large | 1.57 | 0.89 | 2.77 | 0.98 | 0.62 | 1.55 | 1.18 | 0.76 | 1.83 | 1.06 | 0.70 | 1.61 | 0.80 | 0.44 | 1.44 | 0.87 | 0.54 | 1.40 |
| Gender (female vs. male) | 0.63 | 0.39 | 1.03 | 0.81 | 0.54 | 1.22 | 0.74 | 0.50 | 1.08 | 0.67 | 0.46 | 0.96 | 0.89 | 0.53 | 1.50 | 1.09 | 0.72 | 1.64 |
| Years in practice | ||||||||||||||||||
| <5 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||
| 5–10 | 1.53 | 0.76 | 3.08 | 0.73 | 0.42 | 1.25 | 1.53 | 0.91 | 2.57 | 0.91 | 0.56 | 1.47 | 1.70 | 0.81 | 3.56 | 0.99 | 0.57 | 1.74 |
| 10–20 | 1.80 | 0.90 | 3.61 | 0.60 | 0.34 | 1.05 | 1.17 | 0.70 | 1.96 | 0.58 | 0.36 | 0.95 | 1.96 | 0.94 | 4.09 | 0.87 | 0.49 | 1.54 |
| >20 | 2.43 | 1.29 | 4.60 | 0.56 | 0.33 | 0.94 | 2.73 | 1.63 | 4.57 | 0.99 | 0.60 | 1.63 | 2.41 | 1.24 | 4.68 | 0.83 | 0.50 | 1.40 |
| Province | ||||||||||||||||||
| Maritime + territories | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||
| BC | 1.17 | 0.47 | 2.95 | 0.86 | 0.39 | 1.91 | 1.01 | 0.49 | 2.05 | 1.02 | 0.50 | 2.08 | 0.77 | 0.26 | 2.29 | 0.41 | 0.16 | 1.05 |
| Prairies | 0.68 | 0.27 | 1.72 | 0.85 | 0.39 | 1.85 | 1.05 | 0.52 | 2.13 | 1.00 | 0.49 | 2.03 | 0.59 | 0.20 | 1.78 | 0.53 | 0.21 | 1.35 |
| Central | 0.63 | 0.26 | 1.56 | 1.03 | 0.48 | 2.20 | 0.67 | 0.34 | 1.34 | 1.26 | 0.64 | 2.48 | 0.46 | 0.15 | 1.37 | 0.71 | 0.28 | 1.76 |
Odds of agreeing or disagreeing with guidelines statements, compared to giving a neutral response. Bold values are statistically significant (p<0.05). CI: confidence interval; OR: odds ratio; PSA: prostate-specific antigen; Ref: reference.
Table 2b.
Results of multinomial logistic regression, part 2
Odds of agreeing or disagreeing that the benefits of PSA screening in average-risk men outweigh the risks, compared to a neutral response
| In average-risk men (i.e., no risk factors for prostate cancer) the benefits of prostate cancer screening outweigh the risks | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Disagree | Agree | |||||
|
| ||||||
| OR | 95% CI | OR | 95% CI | |||
|
| ||||||
| Lower | Upper | Lower | Upper | |||
| Read guidelines (yes vs. no) | 1.88 | 1.25 | 2.85 | 1.36 | 0.85 | 2.18 |
| Academic appointment (yes vs. no) | 1.51 | 1.08 | 2.13 | 0.83 | 0.56 | 1.24 |
| Catchment area | ||||||
| Small | Ref | Ref | ||||
| Medium | 0.93 | 0.57 | 1.52 | 0.87 | 0.48 | 1.56 |
| Large | 0.87 | 0.58 | 1.30 | 0.94 | 0.58 | 1.52 |
| Gender (female vs. male) | 1.37 | 0.97 | 1.94 | 0.84 | 0.55 | 1.29 |
| Years in practice | ||||||
| <5 | Ref | Ref | ||||
| 5–10 | 0.69 | 0.44 | 1.09 | 1.54 | 0.86 | 2.77 |
| 10–20 | 0.59 | 0.36 | 0.95 | 2.00 | 1.12 | 3.58 |
| >20 | 1.04 | 0.65 | 1.65 | 3.55 | 2.03 | 6.19 |
| Province | ||||||
| Maritime + territories | Ref | Ref | ||||
| BC | 0.99 | 0.50 | 1.96 | 0.93 | 0.44 | 1.96 |
| Prairies | 1.06 | 0.54 | 2.08 | 0.69 | 0.32 | 1.45 |
| Central | 1.40 | 0.73 | 2.70 | 0.55 | 0.26 | 1.14 |
Bold values are statistically significant (p<0.05). CI: confidence interval; OR: odds ratio; PSA: prostate-specific antigen; Ref: reference.
Discussion
Despite the disappointing response rate, our survey was able to collate the opinions and practices of over 1200 Canadian PCPs and demonstrates the impact of the CTFPHC guidelines on prostate cancer screening in this country. As a result of simply being aware of the CTFPHC guideline, the majority (54.7%) of respondents state they have decreased the amount of screening they perform in their practice. As PCPs form the front line of cancer screening, this will have an undeniable impact on the number of men referred for biopsy and subsequently the incidence of prostate cancer diagnoses in Canada in future years. The vast majority of our respondents had no issue with the clarity of the guidelines document, with 78.1% stating they were “clear” or “very clear.” Additionally, we found that PCPs generally agreed with statements put forward by the CTFPHC, particularly that men under the age of 55 and over the age of 70 should not receive prostate cancer screening; however, it is worth noting that this general agreement does not hold true in the key demographic of men 55–69 years old.
Despite the general acceptance of these recommendations, there still exists a significant amount of variation among Canadian PCPs’ screening practices. This clearly has both medical and legal implications. Among average-risk patients, there is an even split in approach, with equal numbers of respondents recommending for and against PSA screening after a risk/benefit discussion with the patient. In addition, PCPs are screening men with lower urinary tract symptoms, although these are not associated with an increased risk of prostate cancer diagnosis and this is clearly discussed in the CTFPHC recommendations. While it is difficult to answer why this is the case, it may represent a misunderstanding among physicians around current known prostate cancer risk factors.
The multinomial logistic regression models that were constructed allowed us to examine which respondent demographics and practice types predicted agreement with guideline statements and the overall benefit of PSA screening (Tables 2a, 2b). When looking at these analyses together, we can see that those PCPs with more years in practice seem to disagree with the CTFPHC’s recommendations against PSA screening, and this same group of physicians are also more likely to agree with the notion that the benefit of PSA screening outweighs the risks overall. This finding is interesting, as it is these physicians who were in practice before and during the initiation of PSA screening. It may be that the number of men presenting with locally advanced and meta-static prostate cancer encountered by this subgroup in the pre-screening era has dissuaded them from ceasing PSA screening despite CTFPHC recommendations.
Our survey results are compatible with recent observed trends in prostate cancer screening in both the U.S. and Canada. Bhindi et al described a decrease in the number of men being referred for prostate biopsy to a high-volume centre in the wake of the 2012 USPTF recommendations.13 They found that the detection rate of low-grade, but also intermediate- and high-grade prostate cancers, dropped from 2008 to 2013 in their time-series analysis. Similarly, studies from the U.S. show that prostate cancer screening decreased following the 2008 USPTF recommendations,14,15 as did the incidence of low-grade prostate cancer diagnoses.16,17 These are expected epidemiological findings after the publication of a guideline against screening, but worryingly, recent population data from the Surveillance, Epidemiology, and End Results (SEER) database indicates the rate of lethal cancers may be rising at the same time.18 Our study adds to this body of literature by addressing the perceptions and practices of PCPs, with whom the ultimate responsibility for carrying out PSA screening sits.
There are limitations to our survey, primarily the low response rate, and subsequently, possible non-response bias. Measures were taken to prevent this, such as piloting the survey medium, ensuring a long collection period (three months), and using a generous incentive. Multiple studies have investigated the decline in physician survey response rates, citing survey burden and fatigue, perceived ineligibility, and lack of interest.19–21 Due to the nature of the survey distribution, we were unable to send reminder notices, and this may have also contributed to the low response rate. We do not believe that the structure or content of the questionnaire itself contributed to this limitation, as the completion rate from those who accessed the survey was very high (93%). We are concerned that many PCPs are just not interested in the topic or do not deal with men during their at-risk years for prostate cancer. This apparent apathy should serve as a call to urologists, radiation and medical oncologists, and allied healthcare professionals to increase our efforts to engage PCPs in the prostate cancer screening conversation. Proposed avenues to accomplish this include the organization of community forums on the issue with urologist and PCP discussion and debate, academic and clinical collaborative efforts, and increased physician engagement with social media. A final limitation is that Quebec PCPs were not surveyed in this study because the flyers were not mailed to physicians in this province.
The gap between the CUA position and the perceptions of PCPs in our survey around prostate cancer screening appears to be wide. The results presented here show that there may be a disconnect in understanding between two groups of physicians crucial to the health of this population. Although the evidence for PSA screening is mixed, the rational and passionate arguments on both sides of the issue imply that the optimal, patient-centred approach to this problem lies somewhere between screening for all men or none. Our understanding of PSA has become more nuanced over the past two decades, and the evidence would suggest that careful patient selection and thoughtful timing of PSA testing (so-called “smart screening”) can lead to fewer unnecessary biopsies and increased detection of high-risk cancers.22 While it is correct to look to high-level evidence for guidance on cancer screening, like the randomized, control trials in this field, the unfortunate contamination of these studies mean that we must be cautious when interpreting their findings.23 To make sweeping recommendations based on the results of these few studies at face value will see us return to a time when a diagnosis of prostate cancer often had a much bleaker presentation and outcome.24
The landscape of prostate cancer screening continues to be in flux. A 2017 revision of the USPSTF recommendations saw this group’s stance against PSA screening soften, changing their rating from a “D” to a “C” grade.25 They cite evidence from long-term followup in the European Randomized study of Screening for Prostate Cancer (ERSPC) trial demonstrating improved cancer-specific survival (CSS),26 and reduced metastatic disease burden27 in the trial’s screening arm. They also acknowledge the increasing acceptance of active surveillance in men with low-risk prostate cancer.28 This change in recommendation immediately brought public and media attention to the issue,29 and time will tell what impact this decision will have on PSA screening in Canada.
Following the USPTF’s reclassification of its recommendation regarding PSA screening,30 the CUA released a statement outlining its 2017 position on the subject.31 It highlights the USPTF’s acknowledgement of the need to integrate a shared decision-making approach with the individual patient, aligning with the CUA’s own position on prostate cancer screening. Unlike the USPTF, however, the CUA advocates for an individualized risk-based approach to determine the age to commence and stop screening, accounting for patient age, PSA level, and current life expectancy.
Conclusion
Our study demonstrates the impact the CTFPHC recommendations on prostate cancer screening have had on screening practices in Canada. More than half of Canadian PCPs reported being less willing to offer men screening with the PSA test. This has significant practice pattern, medical, and legal implications, particularly if it results in a stage shift in diagnosis, with an increase of Canadian men presenting with metastatic disease. The low overall response rate of our survey must be considered when interpreting the responses. Despite this, the data presented here represents a diverse cohort of over 1200 Canadian PCPs. Our findings inform future work to monitor changes in prostate cancer care and emphasize the urgency for our urological opinion leaders to provide all PCPs in Canada with clear, unified guidance.
Supplementary Information
Footnotes
Supplementary material available at cuaj.ca
Competing interests: Dr. Greenberg is an advisor for J&J and has received payment from AstraZeneca. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Statistics CCSACOC. Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 2.Bunting PS, Goel V, Williams JI, et al. Prostate-specific antigen testing in Ontario: Reasons for testing patients without diagnosed prostate cancer. CMAJ. 1999;160:70–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 4.Krahn M. Prostate cancer screening: Going beyond the clinical evidence. CMAJ. 2014;186:1201. doi: 10.1503/cmaj.141252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schröder FH, Hugosson J, Carlsson S, et al. Screening for prostate cancer decreases the risk of developing metastatic disease: Findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2012;62:745–52. doi: 10.1016/j.eururo.2012.05.068. [DOI] [PubMed] [Google Scholar]
- 8.Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117:1210–9. doi: 10.1002/cncr.25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy DG, Ahlering T, Catalona WJ. The Melbourne Consensus Statement on the early detection of prostate cancer. BJU Int. 2014;113:186–8. doi: 10.1111/bju.12556. [DOI] [PubMed] [Google Scholar]
- 10.Allard CB, Dason S, Lusis J, et al. Prostate cancer screening: Attitudes and practices of family physicians in Ontario. Can Urol Assoc J. 2012;6:188–93. doi: 10.5489/cuaj.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoag NA, Davidson RA, Pommerville PJ. Prostate cancer screening practices and attitudes among primary care physicians in Victoria, British Columbia. BCMJ. 2008;50:456–60. [Google Scholar]
- 12.Curran V, Solberg S, Mathews M, et al. Prostate cancer screening attitudes and continuing education needs of primary care physicians. J Cancer Educ. 2009;20:162–6. doi: 10.1207/s15430154jce2003_10. [DOI] [PubMed] [Google Scholar]
- 13.Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate-specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519–24. doi: 10.1016/j.juro.2014.11.096. [DOI] [PubMed] [Google Scholar]
- 14.Shoag J, Halpern JA, Lee DJ, et al. Decline in prostate cancer screening by primary care physicians: An analysis of trends in the use of digital rectal examination and prostate-specific antigen testing. J Urol. 2016;196:1047–52. doi: 10.1016/j.juro.2016.03.171. [DOI] [PubMed] [Google Scholar]
- 15.Sammon JD, Abdollah F, Choueiri TK, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314:2077–9. doi: 10.1001/jama.2015.7273. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314:2054–61. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Ma J, Siegel R, et al. Prostate cancer incidence rates 2 years after the US Preventive Services Task Force recommendations against screening. JAMA Oncol. 2016;2:1657–60. doi: 10.1001/jamaoncol.2016.2667. [DOI] [PubMed] [Google Scholar]
- 18.Thomas CR, Jr, Shyr Y. Determining penetration of prostate-specific antigen screening recommendations. JAMA Oncol. 2017;3:707. doi: 10.1001/jamaoncol.2016.5978. [DOI] [PubMed] [Google Scholar]
- 19.Cull WL, O’Connor KG, Sharp S, et al. Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40:213–26. doi: 10.1111/j.1475-6773.2005.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Templeton L, Deehan A, Taylor C, et al. Surveying general practitioners: Does a low response rate matter? Br J Gen Pract. 1997;4:91–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Wiebe ER, Kaczorowski J, MacKay J. Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58:e225–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Leapman MS, Carroll PR. What is the best way not to treat prostate cancer? Urol Oncol. 2017;35:42–50. doi: 10.1016/j.urolonc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–6. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 24.Weiner AB, Matulewicz RS, Eggener SE, et al. Increasing incidence of metastatic prostate cancer in the United States (2004–2013) Prostate Cancer Prostatic Dis. 2016;19:395–7. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 25.Bibbins-Domingo K, Grossman DC, Curry SJ. The US Preventive Services Task Force 2017 draft recommendation statement on screening for prostate cancer. JAMA. 2017;317:1949–50. doi: 10.1001/jama.2017.4413. [DOI] [PubMed] [Google Scholar]
- 26.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of followup. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzzoni C, Auvinen A, Roobol MJ, et al. Metastatic prostate cancer incidence and prostate-specific antigen testing: New insights from the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2015;68:885–90. doi: 10.1016/j.eururo.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 29.Chustecka Z. “Individualize,” says prostate cancer screening from USPSTF. Medscape. Apr, 2017. [Accessed November 2, 2017]. pp. 1–5. Available at http://www.medscape.com/viewarticle/878438.
- 30.UPSTF. United States Preventative Services Task Force Draft Recommendation Statement on Prostate Cancer Screening. [Accessed November 14, 2017]. Available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/prostate-cancer-screening1.
- 31.Rendon RA, Mason RJ, Marzouk K, et al. Canadian Urological Association recommendations on prostate cancer screening and early diagnosis. Can Urol Assoc J. 2017;11:298–309. doi: 10.5489/cuaj.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.