Abstract
Objective
To determine if troponin I is more often elevated in children with suspected nonaccidental trauma (NAT) compared with uninjured children of similar age, and describe associations between troponin I elevation and NAT injuries.
Study design
Prospective 2-group study of children less than 2 years of age presenting to the emergency department with nonaccidental abdominal, thoracic, or intracranial injuries, and similarly aged uninjured children. Primary outcome was serum troponin I (≥0.04 ng/mL) using frozen blood samples from the 2 groups. Secondary outcomes included descriptive analyses of age, injury characteristics, and clinical appearance.
Results
There were 129 subjects; 60 injured patients and 69 uninjured patients. Groups had similar age and sex. Troponin I was elevated in 38% of injured children compared with 17% of uninjured children (P = .008). No uninjured patient over 3 months of age had elevated troponin I. Abdominal trauma, acute rib fractures, or the child’s ill-appearance in the emergency department were associated with having elevated troponin I.
Conclusions
Troponin I is more often elevated in children with suspected NAT than uninjured children. Elevation of troponin I in children greater than 3 months of age with suspected NAT is concerning for trauma. Occult cardiac injury is more likely to occur in children with inflicted abdominal trauma, acute rib fractures, or ill appearance.
Nonaccidental trauma (NAT) or child physical abuse is an important cause of morbidity and mortality among young children.1–4 Evaluation of victims of NAT is often complicated by the absence of a reliable history, a victim’s young age, and the potential for occult clinical findings. Tests that indicate specific types of injuries and the extent of injury can have significant diagnostic and forensic implications. For this reason, the standard of care for NAT evaluation in young children includes tests such as a skeletal survey, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and neuroimaging with magnetic resonance imaging or computed tomography (CT).4–8 Rarely are occult injuries detected with these tests associated with significant morbidity. However, their findings are important in determining whether the history correlates with the injury sustained and in describing the extent of injury. Thus, identification of occult injuries is a critical part of protecting the child from further harm.
NAT involving the skin or skeleton may be detectable on exam or with standard diagnostic techniques. Signs and symptoms of cardiac injury, however, are nonspecific and can easily be attributed to other causes. Tachycardia can be mistakenly explained by pain or anxiety, and both hypoxia and hypotension can be attributed to other injuries.9–15 Therefore, a biomarker specific for cardiac injury may have clinical utility in this setting.
Cardiac troponin I is a contractile protein that is unique to atrial and ventricular tissue.16 It is released into the circulation only after disruption of cardiac cellular membrane.16 Troponin I can be detected 4–6 hours after injury, peaks at 18–20 hours after injury, and can remain elevated in the serum for 7 days.16 Similar to a bruise illustrating damage to underlying blood vessels in the skin, elevated troponin I demonstrates cellular damage to the heart.
Previous studies have indicated that approximately 20% of infants less than 12 months of age can have a baseline elevation in troponin I (levels above the adult reference range). 17,18 Prior to commencement of this study, normal troponin I values had not been established for young children. This study aimed to determine if troponin I is more often elevated in children less than 24 months of age with suspected NAT compared with uninjured children of a similar age, and to describe associations between troponin I elevation and specific injuries.
Methods
We used a prospective 2-group design that enrolled children with suspected NAT and uninjured children. This institutional review board approved study took place at a level 1 pediatric trauma center with an annual emergency department (ED) census of 90 000 visits and approximately 200 cases of NAT each year. To avoid inclusion bias arising from unwillingness to participate in research among those with suspected NAT, we were granted a waiver of consent for use of remnant blood samples. Troponin I is not currently considered standard of care in the evaluation of NAT; therefore, troponin I results obtained as part of this investigation were not made available to clinical providers. If troponin I was obtained as part of a patient’s clinical care, the value was recorded and no separate research sample was processed.
The injured group was composed of children less than 2 years of age with suspected NAT (as documented by the medical record or evidenced by completion of standard physical abuse work-up). These children were evaluated in the ED, had blood samples obtained, and had clinical findings of thoracic trauma (bruising or abrasions to the chest, acute rib fractures, acute fractures of the sternum or scapula, acute clavicle fracture, pulmonary contusion, pulmonary hemorrhage, pneumothorax), viscous or solid organ abdominal injury, or head trauma (acute subdural hemorrhage and epidural hematoma). A report to Children’s Services for suspected physical abuse was not part of the inclusion criteria as these patients were captured at the initiation of the evaluation before a determination of abuse could be made.
They were excluded if the child had received cardiopulmonary resuscitation prior to or during the ED evaluation, or had a recent accidental thoracic, abdominal, or intracranial trauma. Additionally, injured children were excluded if their medical documentation revealed a history of cardiac disease, positive blood cultures, or systemic illness requiring intensive care within the past month, hematology/oncology conditions, or if the child was receiving medications that could cause cardiac dysfunction.
Children were enrolled into the injured group between June 2010 and December 2012. Study staff utilized the ED’s electronic medical record to screen for children aged less than 2 years who had an abdominal CT or AST, ALT, or hepatic profile ordered. If inclusion criteria were met, the clinical laboratory was contacted to request freezing of a remnant blood sample (within 8 hours of ED evaluation). Frozen samples were collected and stored at −20° C until they were thawed and assayed for troponin I.
All remnant study control blood samples analyzed in the clinical laboratory are frozen to −20° C within 8 hours of arrival and stored for at least 1 week prior to being discarded. The uninjured group was selected from these already available frozen samples. Included samples were from children less than 2 years of age evaluated in the ED, test referral centers, or inpatient services between June 2010 and December 2012. Initial selection of potentially eligible samples from uninjured children was done by the medical director of the clinical laboratory based on age and appropriateness of sample (sufficient quantity, correct tube). The study principal investigator then reviewed the medical record for these potential patients to assess for exclusion criteria. Patients were excluded if they had underlying cardiac disease, concurrent severe systemic illness, or recent intracranial, thoracic, or abdominal injury; if they were seen in pediatric or neonatal intensive care units, cardiology, or hematology/oncology clinics; or if they had troponin I, creatine kinase, creatine kinase (MB fraction), brain natriuretic peptide, lactate, blood gas, or positive blood cultures measured within the prior 1 month. After eligibility was determined, troponin I analysis was performed on the blood samples per laboratory protocol.
Data describing the injured children were extracted from the medical record by 1 investigator. Extracted data included demographics (age and sex), clinical appearance (descriptive appearance documented by the treating physician), laboratory/radiographic results, associated injuries, potential cardiac sequelae (defined as arrhythmia, unexplained hypotension, or consultation with cardiology), and whether or not a report of suspected abuse was made after evaluation completion. The clinical laboratory provided age and sex of uninjured children. Missed eligible patients were identified via review of hospital child abuse team patient lists.
Samples from both groups were frozen per institutional laboratory protocol. Troponin I was measured using Abbott Architect (Abbott Diagnostics, Abbott Park, Illinois) and Siemens Vista 1500 (Siemens, Malvern, Pennsylvania). Troponin I plasma samples were separated from cells and frozen within 8 hours at −20°C and were suitable for troponin I analysis according to the manufacturer’s insert. Based on institutional laboratory control standards for pediatric age patients at the time of the study, troponin I levels ≥.04 ng/mL were considered elevated. The above assays yield troponin values with less than 20% coefficient of variance. All undetectable levels were reported as <0.015 ng/mL.
The Mann–Whitney U test was used to compare medians, and the χ2 test and Fisher exact test were used to compare proportions. The magnitude of differences with 95% CI was calculated. All statistical analyses were conducted using SPSS 22.0 (IBM Corporation, Armonk, New York). Graphics were created using R (gplot).
Results
A total of 70 injured children were initially identified as potentially eligible for the study. Five children in this group had blood samples with quantity insufficient for troponin I analysis and 5 children were excluded because of a history of cardiopulmonary resuscitation associated with the visit. Therefore, 60 children were included in the injured group. (Twelve eligible patients were missed; they were not clinically different from included patients.) Three hundred fifty children were initially identified as potential patients for the uninjured group; 281 met exclusion criteria and 69 were included in the study.
Median age for injured children was 4 months (IQR 5) and median age for uninjured children was 4 months (IQR 8); 48% of uninjured children were age 3 months or younger, and 49% of injured children were age 3 months or younger. Thirty-eight percent of injured children and 42% of uninjured children were female. Seventeen injured patients (28%) had troponin I measured as a component of their clinical care; the remaining had troponin I completed for research purposes only. There were no clinical differences of importance between the group who had troponin I obtained as part of clinical care and the group who had troponin I obtained for research purposes.
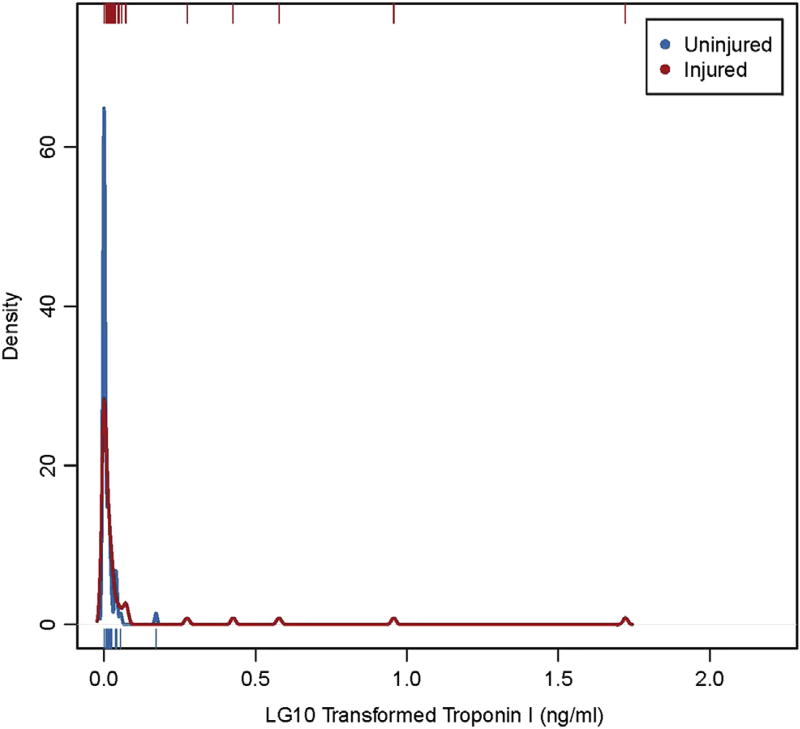
Troponin I levels were non-normally distributed in both groups, with 5 outliers in each group. The median troponin I for the uninjured group was <0.015 ng/mL (IQR 0.03, range <0.015–0.49 ng/mL). The median troponin I for the injured group was 0.02 ng/mL (IQR 0.06, range <0.015–51.67 ng/mL). Troponin I was elevated (≥0.04 ng/mL) significantly more often in injured children compared with uninjured children (38% vs 17%; difference 21%, 95% CI 6–36, P = .008). Additionally, median troponin I was higher in injured patients compared with uninjured patients (Figure; available at www.jpeds.com; 95% CI 0.00–0.04; P = .018).
Figure.
Empirical density plot with rug illustrating the significant difference in troponin I distribution for injured and uninjured patients. The small lines at the top and bottom of the graph show the location of each unique value. The curve shows how many patients are clustered at each location.
Characteristics associated with elevated troponin levels in injured children were younger age, abdominal trauma as evidenced by elevated AST/ALT and/or abdominal injury on CT, and ill-appearance in the ED (Table). In both groups, troponin I was more often elevated among the very young infants than among older infants and toddlers. The median age of children with elevated troponin I was 2 months (IQR 2, range <1–18 months) and the median age of children with nonelevated troponin I was 6 months (IQR 9, range <1–23 months). No uninjured patient over 3 months of age had an elevated troponin I.
Table.
Demographic and injury characteristics by elevated troponin level
| Not elevated (n = 37) |
Elevated (n = 23) |
Difference | 95% CI
|
P value | ||||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
|
|
|
|||||||
| Age (mo) – median, (IQR) | 4 | (4) | 3 | (3) | −1 | −3.5 | 1.48 | <.0001 |
| Patient characteristics–N () | ||||||||
| Male | 22 | (59.5) | 15 | (65.2) | (5.8) | (−19.3) | (30.8) | .656 |
| Head injury only | 13 | (35.1) | 8 | (34.8) | (−0.4) | (−25.2) | (24.5) | .978 |
| Liver function test elevation >80 | 6 | (16.2) | 10 | (43.5) | (27.3) | (3.8) | (50.7) | .020 |
| Intracranial injury | 21 | (56.8) | 15 | (65.2) | (8.5) | (−16.7) | (33.6) | .515 |
| Retinal hemorrhages | 6 | (16.2) | 8 | (34.8) | (18.6) | (−4.2) | (41.4) | .098 |
| Rib fractures (healing or acute) | 14 | (37.8) | 12 | (52.2) | (14.3) | (−11.4) | (40.0) | .276 |
| Rib fractures (acute) | 3 | (8.1) | 7 | (30.4) | (22.3) | (1.6) | (43.1) | .035 |
| Chest bruising | 7 | (18.9) | 5 | (21.7) | (2.8) | (−18.2) | (23.9) | .791 |
| Signs of chest injury | 16 | (43.2) | 12 | (52.2) | (8.9) | (−17.0) | (34.8) | .500 |
| Abdominal injury on CT | 0 | (0.0) | 6 | (26.1) | (26.1) | (8.1) | (44.0) | .002 |
| Extremity fracture | 7 | (18.9) | 3 | (13.0) | (−5.9) | (−24.5) | (12.8) | .727 |
| Classic metaphyseal lesion | 3 | (8.1) | 3 | (13.0) | (4.9) | (−11.4) | (21.3) | .666 |
| Ill Appearance | 6 | (16.2) | 12 | (52.2) | (36.0) | (12.3) | (59.6) | .003 |
After the clinical evaluation was completed, there was sufficient concern for abuse to warrant a report to Children’s Services in 47 patients (78%). Twenty-one (45%) of those patients had elevation of troponin I. Only 1 patient who was not reported to Children’s Services had elevation in troponin I with a level of 0.14 ng/mL; that patient was a 3-week-old infant who fell from its mother’s arms onto concrete steps.
Discussion
Our results are consistent with previous findings of elevated troponin I in very young patients.17,18 Physiologic elevation of troponin I in infants is purportedly due to programmed apoptosis.18 A study by Bailey et al established pediatric reference intervals for endocrine and biochemical markers, including troponin I.19 Their reference interval illustrates the potential for elevated levels in the first 2 weeks of life that decline until reaching normal (undetectable) adult levels at 3 months of age. Our data are consistent with this. None of the uninjured children greater than 3 months of age had elevated troponin I. This suggests that in children greater than 3 months of age with concerns for NAT, elevated troponin I may be related to inflicted injury rather than physiologic elevation.
When patients younger than 3 months of age are excluded because of potential physiologic elevation of troponin I, none of the uninjured group had elevation of troponin I, and 26% of the injured group had elevations. This proportion of positive results from troponin I elevation is consistent with the proportion of injuries revealed with other accepted screening tools for NAT. Skeletal surveys identify fractures in 10%-28% of patients20–24 with suspected NAT. Neuroimaging identifies occult intracranial injury in approximately 30% of patients with concerns for physical abuse.7,8 The similar yield from troponin I in identifying occult cardiac cellular injury in our specific population illustrates potential as an additional screening test in the evaluation of NAT. Elevation of troponin I is not diagnostic of inflicted trauma but could be used to describe the extent of injury. It is, however, important to note that although elevation in troponin I illustrates cellular injury to the heart, it does not imply a mechanism of injury. Physiological conditions that could cause cellular damage to the heart need to be considered prior to determining if the etiology is trauma.
Our study highlighted specific injury characteristics associated with elevation in troponin I. Abdominal injury, as evidenced by elevated AST/ALT or abnormal abdominal CT, was associated with elevated troponin I. This may be due to the “hydraulic ram effect,” which is the upward displacement of abdominal viscera causing cardiac injury.10,25 It is not surprising that acute rib fractures were also significantly associated with elevation in troponin I. The clinician’s notation of a child’s ill-appearance was also associated with elevated troponin I levels. In children being evaluated for NAT, signs of acute rib injury, abdominal trauma, and ill-appearance indicate further evaluation for occult cardiac injury using troponin I.
Review of medical records for children being evaluated for suspected NAT did not reveal any documentation of concern for clinically significant cardiac injury. One reason for this may be due to the fact that signs and symptoms of cardiac injury are often nonspecific and can be attributed to other injuries. Another consideration is that although a biochemical sign of myocardial damage is present, the injury may not be severe enough to indicate a need for clinical intervention. Regardless of the lack of apparent end-organ sequelae, testing for occult injury is the standard of care in evaluation of NAT. Identification of occult findings is invaluable in determining if a given history adequately explains the injuries, describing the extent of injury, and can be critical in efforts to protect a child from further harm. Similar to the use of skeletal surveys and neuroimaging to detect occult injuries, troponin I can identify occult cardiac injury and has the potential to be a very important part of NAT evaluations.
There are limitations to our study that are worth noting. First, criteria for abnormal vital signs and abnormal clinical appearance were based on physician documentation rather than comparison of vital sign measurements to age-specific norms. This method was chosen because identifying a child as ill-appearing is often made by clinical gestalt rather than by specific vital signs. Additionally, vital signs may change during the course of the evaluation and can be affected by fear, anxiety, and pain.
Second, troponin I was obtained only for research purposes in 72% of patients being evaluated for suspected NAT, and the results were not available to the treating team. It is unknown if the treating physician would have changed his/her management plan or documentation of potential cardiac injury if he/she had knowledge of the child’s elevated troponin I.
Patients were included in the injured group if they underwent an evaluation for suspected NAT regardless of whether or not a report to Children’s Services was made. After the evaluation was completed, there was insufficient suspicion for NAT in 22% of cases to make a report. We believe that if we had focused exclusively on a population with reported NAT, we would likely demonstrate an even greater difference between the injured and uninjured groups.
After enrollment was completed, review of child abuse team patient lists revealed 12 children who were missed eligible patients as they presented during periods of time when the study researchers were not available. Whether inclusion of these patients would have changed our results is unknown, but there is no reason to suspect a systematic bias.
Lastly, although troponin I definitively indicates cardiac cellular damage, this study was not designed to determine if the cardiac injury was from blunt trauma to the heart or cellular injury resulting from hypoxia, ischemia, or hypovolemia caused by the inflicted injury. Regardless of the mechanism of elevation of troponin I, one can interpret that the inflicted injury to the child resulted in cardiac cellular damage.
Patients with signs of abdominal trauma, acute rib fractures, or ill-appearance are most likely to benefit from evaluation for occult cardiac injury with troponin I. A larger study is needed to further investigate the utility of troponin I as a screening tool for cardiac injury in suspected NAT and to determine the clinical significance of elevated levels of troponin I in this population.
Acknowledgments
Supported by the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grant Program and the National Institutes of Health/National Center for Research Resources (Institutional Clinical and Translational Science Award 5UL1RR026314-03).
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CT
Computed tomography
- ED
Emergency department
- NAT
Nonaccidental trauma
Footnotes
The authors declare no conflicts of interest.
References
- 1.Jenny C, Isaac R. The relation between child death and child maltreatment. Arch Dis Child. 2006;91:265–9. doi: 10.1136/adc.2004.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roaten JB, Partrick DA, Nydam TL, Bensard DD, Hendrickson RJ, Sirotnak AP, et al. Nonaccidental trauma is a major cause of morbidity and mortality among patients at a regional level 1 pediatric trauma center. J Pediatr Surg. 2006;41:2013–5. doi: 10.1016/j.jpedsurg.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Krug EG, Mercy JA, Dahlberg LL, Zwi AB. World report on violence and health. Geneva: World Health Organization; 2002. [Google Scholar]
- 4.Kellogg ND American Academy of Pediatrics CoCAaN. Evaluation of suspected child physical abuse. Pediatrics. 2007;119:1232–41. doi: 10.1542/peds.2007-0883. [DOI] [PubMed] [Google Scholar]
- 5.Lindberg D, Makoroff K, Harper N, Laskey A, Bechtel K, Deye K, et al. Utility of hepatic transaminases to recognize abuse in children. Pediatrics. 2009;124:509–16. doi: 10.1542/peds.2008-2348. [DOI] [PubMed] [Google Scholar]
- 6.Jenny C, American Academy of Pediatrics CoCAaN Evaluating infants and young children with multiple fractures. Pediatrics. 2006;118:1299–303. doi: 10.1542/peds.2006-1795. [DOI] [PubMed] [Google Scholar]
- 7.Laskey AL, Holsti M, Runyan DK, Socolar RR. Occult head trauma in young suspected victims of physical abuse. J Pediatr. 2004;144:719–22. doi: 10.1016/j.jpeds.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Rubin DM, Christian CW, Bilaniuk LT, Zazyczny KA, Durbin DR. Occult head injury in high-risk abused children. Pediatrics. 2003;111:1382–6. doi: 10.1542/peds.111.6.1382. [DOI] [PubMed] [Google Scholar]
- 9.Sybrandy KC, Cramer MJM, Burgersdijk C. Diagnosing cardiac contusion: old wisdom and new insights. Heart. 2003;89:485–9. doi: 10.1136/heart.89.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Chami MF, Nicholson W, Helmy T. Blunt Cardiac Trauma. J Emerg Med. 2008;35:127–33. doi: 10.1016/j.jemermed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Feghali NT, Prisant LM. Blunt myocardial injury. Chest. 1995;108:1673–7. doi: 10.1378/chest.108.6.1673. [DOI] [PubMed] [Google Scholar]
- 12.Kamdar G, Santucci K, Emerson BL. Management of Pediatric Cardiac Trauma in the ED. Clin Pediatr Emerg Med. 2011;12:323–32. [Google Scholar]
- 13.Peclet MH, Newman KD, Eichelberger MR, Gotschall CS, Garcia VF, Bowman LM. Thoracic trauma in children: an indicator of increased mortality. J Pediatr Surg. 1990;25:961–5. doi: 10.1016/0022-3468(90)90238-5. Discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 14.Woosley CR, Mayes TC. The pediatric patient and thoracic trauma. Semin Thorac Cardiovasc Surg. 2008;20:58–63. doi: 10.1053/j.semtcvs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Bromberg BI, Mazziotti MV, Canter CE, Spray TL, Strauss AW, Foglia RP. Recognition and management of nonpenetrating cardiac trauma in children. J Pediatr. 1996;128:536–41. doi: 10.1016/s0022-3476(96)70366-9. [DOI] [PubMed] [Google Scholar]
- 16.Kanaan UB, Chiang VW. Cardiac troponins in pediatrics. Pediatr Emerg Care. 2004;20:323–9. doi: 10.1097/01.pec.0000125664.35690.51. [DOI] [PubMed] [Google Scholar]
- 17.Soldin SJ, Murthy JN, Agarwalla PK, Ojeifo O, Chea J. Pediatric reference ranges for creatine kinase, CKMB, troponin I, iron, and cortisol. Clin Biochem. 1999;32:77–80. doi: 10.1016/s0009-9120(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 18.Quivers ES, Murthy JN, Soldin SJ. The effect of gestational age, birth weight, and disease on troponin I and creatine kinase MB in the first year of life. Clin Biochem. 1999;32:419–21. doi: 10.1016/s0009-9120(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 19.Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, et al. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013;59:1393–405. doi: 10.1373/clinchem.2013.204222. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty EG, Perez-Rossello JM, Levine MA, Hennrikus WL. Evaluating children with fractures for child physical abuse. Pediatrics. 2014;133:e477–89. doi: 10.1542/peds.2013-3793. [DOI] [PubMed] [Google Scholar]
- 21.Rangel EL, Cook BS, Bennett BL, Shebesta K, Ying J, Falcone RA. Eliminating disparity in evaluation for abuse in infants with head injury: use of a screening guideline. J Pediatr Surg. 2009;44:1229–34. doi: 10.1016/j.jpedsurg.2009.02.044. Discussion 34-5. [DOI] [PubMed] [Google Scholar]
- 22.Degraw M, Hicks RA, Lindberg D. Incidence of fractures among children with burns with concern regarding abuse. Pediatrics. 2010;125:e295–9. doi: 10.1542/peds.2009-1478. [DOI] [PubMed] [Google Scholar]
- 23.Duffy SO, Squires J, Fromkin JB, Berger RP. Use of skeletal surveys to evaluate for physical abuse: analysis of 703 consecutive skeletal surveys. Pediatrics. 2011;127:e47–52. doi: 10.1542/peds.2010-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen KK, Campbell KA. How useful are skeletal surveys in the second year of life? Child Abuse Negl. 2009;33:278–81. doi: 10.1016/j.chiabu.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Elie M. Blunt cardiac injury. Mount Sinai J Med. 2006;73:542–52. [PubMed] [Google Scholar]