Abstract
Interactions between the brain and distinct adipose depots have a key role in maintaining energy balance, thereby promoting survival in response to metabolic challenges such as cold exposure and starvation. Recently, there has been renewed interest in the specific central neuronal circuits that regulate adipose depots. Here, we review anatomical, genetic and pharmacological studies on the neural regulation of adipose function, including lipolysis, non-shivering thermogenesis, browning and leptin secretion. In particular, we emphasize the role of leptin-sensitive neurons and the sympathetic nervous system in modulating the activity of brown, white and beige adipose tissues. We provide an overview of advances in the understanding of the heterogeneity of the brain regulation of adipose tissues and offer a perspective on the challenges and paradoxes that the community is facing regarding the actions of leptin on this system.
The maintenance of energy homeostasis is achieved by sophisticated brain circuits that rigorously maintain energy levels by affecting food intake and energy expenditure. The identification of the leptin gene (Lep) in 1994 ushered in the molecular era of obesity research and provided new opportunities to better understand the mechanisms regulating energy homeostasis1–3. Despite tremendous efforts to identify the multiple factors that contribute to excessive fat accumulation, the obesity epidemic continues to worsen, with more than one-third of US adults being obese4. Adipose tissue has a central role in leptin production and in the management of systemic energy stores. Understanding how the actions of leptin on the CNS influence adipose tissues, and how adipose tissue metabolism in turn is affected by the sympathetic nervous system (SNS), represents a novel opportunity to tackle the obesity problem.
Brown adipose tissue (BAT) and white adipose tissue (WAT) are specialized for non-shivering thermogenesis and lipid storage, respectively5,6 (FIG. 1). Under specific physiological conditions, clusters of brown-like adipocytes in white fat (‘brite’ adipocytes) can also develop through so-called browning or ‘beiging’ of WAT. The development of beige adipose tissue (BeAT) has an important role in systemic metabolic regulation6,7 (BOX 1). The activity of adipose cells is tightly regulated by the SNS. In particular, increased SNS outflow promotes fat mobilization8. Activation of the SNS stimulates non-shivering thermogenesis9 and participates in the development of BeAT7. Importantly, sympathetic tone is differentially regulated in the adipose depots. For example, in response to fasting, most tissues, including BAT, will experience a decrease in sympathetic tone, whereas sympathetic activity in WAT increases to allow fatty acid mobilization10 (FIG. 1a). By contrast, cold exposure increases sympathetic outflow to both BAT and WAT to provide substrates for non-shivering thermogenesis11 (FIG. 1a). This divergence implies that complex and distinct CNS circuits have evolved to coordinate adipose tissue metabolism.
Figure 1. Sympathetic regulation of white, brown and beige adipose tissues.
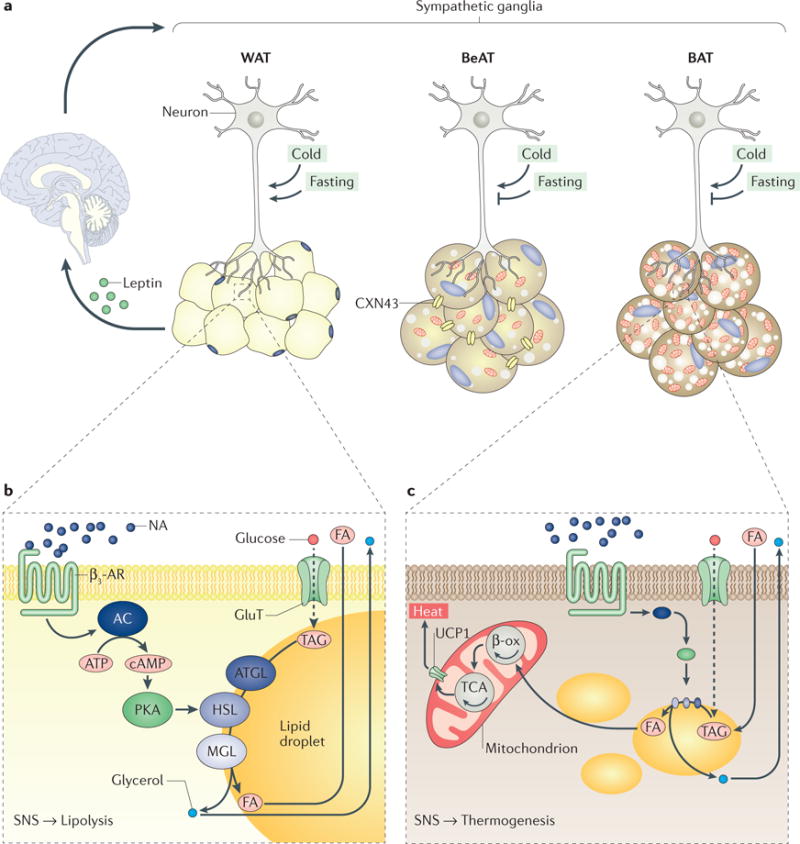
a Innervation of white adipose tissue (WAT), beige adipose tissue (BeAT) and brown adipose tissue (BAT). Neurons of the sympathetic nervous system (SNS) project (via their axons) to different fat depots and establish contacts with adipocytes. A cell-to-cell connexin 43 (CXN43) gap-junction system facilitates the propagation of the sympathetic signal among ‘brite’ adipocytes. Whether this system is also important for lipolysis is unknown. Leptin, which is secreted from WAT, also affects the SNS outflow via actions in the brain. Cold and fasting differently affect the sympathetic tone to different adipose tissues. b SNS-dependent WAT regulation. The SNS regulates WAT energy storage and mobilization. Evidence indicates the existence of a leptin-dependent neuro–adipose tissue connection that plays an important part in fine-tuning fuel usage. The neurons release noradrenaline (NA), which signals to β3-adrenoceptors (β3-ARs) located on adipocytes. This signalling promotes a well-characterized cascade of molecular events that trigger lipolysis (lipid breakdown). Briefly, the accumulation of cAMP promotes protein kinase A (PKA)-dependent activation of a well-established molecular cascade involving adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoglyceride lipase (MGL), which generate fatty acids (FAs) and glycerol. Glucose can also be incorporated into triacylglycerol (TAG) through de novo lipogenesis and subsequently catabolized to generate FAs and glycerol. cAMP also reduces leptin production (see FIG. 2). In white adipocytes, FA and glycerol are released into the circulation, whereas in brite adipocytes, FA can be used as a substrate for oxidative metabolism in mitochondria. c SNS-dependent BAT regulation. The SNS regulates BAT non-shivering thermogenesis. As in WAT, NA signals to β3-ARs and stimulates lipolysis. Lipolysis in BAT activates mitochondrial uncoupling protein 1 (UCP1) and thermogenesis following oxidation of FAs (via β-oxidation (β-ox) and the tricarboxylic acid cycle (TCA)). As for BeAT, it is assumed that brite adipocytes share features of WAT and BAT; that is, adrenergic stimulation leads to both increased lipolysis and thermogenesis in BeAT. Circulating glucose can also be used by brown adipocytes to generate heat after de novo lipogenesis. AC, adenylyl cyclase; GluT, glucose transporter.
Box 1. What is a ‘brite’ adipocyte?
In rodents, classic brown adipocytes are located in specific areas, including the interscapular region. Under specific physiological conditions, brown-like adipocytes can also develop within white adipose tissue (WAT). These brown-like adipocytes are generally referred to as recruitable, beige or ‘brite’ adipocytes. When a WAT depot shelters these brite cells, it becomes labelled as a beige adipose tissue (BeAT), owing to its colour. The process by which these cells develop is called browning or ‘beiging’. There is not yet a consensus on the required number of brite cells that need to be present in a specific depot to make it beige. The sole presence of mitochondrial brown fat uncoupling protein 1 (UCP1)-positive cells is generally sufficient to define the development of BeAT.
Classic brown and brite adipocytes share similar properties upon stimulation with adrenergic agonists. However, in contrast to classic brown adipocytes, brite adipocytes are not derived from myogenic factor 5 (MTF5)-positive progenitor cells and homeobox protein engrailed 1 (EN1)-expressing cells of the central dermomyotome. As such, brite adipocytes might be at once both, and neither, brown and white. It has been proposed that these cells are derived from different precursors, differentiate from a white adipocytic precursor or transdifferentiate from an existing mature white adipocyte. It has also been shown that brite adipocytes exhibit a distinct molecular signature that is not shared by either classic brown or white fat cells. Overall, brite adipocytes seem to be recruitable brown cells within WAT and therefore might participate in systemic energy homeostasis.
The brain regulates BAT, WAT and BeAT metabolism through efferent pathways; in turn, these tissues relay information to the brain about the status of energy stores through sensory innervation and hormone secretion8,12,13. Increased interest in this bidirectional interaction between the brain and adipose depots was catalysed by the cloning of Lep3. Leptin is an adipokine with multiple functions; for example, it regulates appetite, body weight, the maturation of the reproductive axis and neuroendocrine adaptations to fasting14,15. Leptin is also generally acknowledged to act within the brain to regulate glucose homeostasis16–18. The confirmation that functional brown adipocytes exist in adult humans19,20 has also spurred new studies on the neural regulation of BAT. While the role of leptin in regulating energy balance is well known, its role in the neural regulation of BAT, WAT and BeAT is still debated. Many extensive and up-to-date reviews are available on the neural control of adipose tissue or the leptin-sensitive neurons that maintain energy balance2,9,17,21–24. However, the two systems are generally separately discussed, when they could instead be considered as one. For instance, leptin contributes to the regulation of the sympathetic tone to BAT, WAT and BeAT; in turn, the transcription and secretion of leptin in adipocytes are controlled by the SNS25–29. Therefore, the goal of this Review is to discuss the role of leptin as a key regulator of the sympathetic outflow to adipose tissues. We also take this opportunity to stress the heterogeneity of the innervation of adipose tissue and to discuss the mechanisms by which the SNS regulates the synthesis and release of leptin. Ultimately, this Review is aimed at identifying some of the challenges ahead in the field of energy metabolism and underscoring important notions to consider in understanding obesity and other metabolic complications.
Leptin and adipose functions
The CNS regulates peripheral tissues through the SNS and the parasympathetic nervous system (PNS)30. The monoamine noradrenaline is the main neurotransmitter released by the sympathetic nerves, whereas acetylcholine is the main neurotransmitter of the PNS31. Sympathetic ganglion cells are innervated by preganglionic fibres that primarily emerge from the intermediolateral column (IML) of the spinal cord. By contrast, parasympathetic ganglion cells receive their preganglionic input from neurons in the brainstem, such as the dorsal nucleus of the vagus nerve31. Another distinction between the SNS and PNS is that sympathetic ganglion neuron bodies are located in the bilateral chain of sympathetic ganglia, whereas the terminal ganglia of the PNS are very near, or even within, the innervated organ31.
Adipose tissue receives an SNS innervation; however, the role of the PNS in adipose innervation is unsettled8,32,33. The number of fibres expressing choline O-acetyltransferase (ChAT; a marker of parasympathetic neurons) present in inguinal WAT is negligible compared with the number of fibres expressing tyrosine hydroxylase (TH; a marker of sympathetic neurons)8,34. The SNS regulates the non-shivering thermo genesis function of BAT9, energy storage and mobilization by WAT and the development of BeAT7 (FIG. 1). The importance of the sympathetic control of fat mobilization was supported by the early observation that in adrenal demedullated rats, chemical sympathectomy using N-ortho-chloro-benzyl-N′,N″-dimethylguanidine prevents cold-induced lipolysis, an effect that is reversed by treatment with with noradrenaline35. Subsequent work showed that noradrenaline turnover is increased in adipose tissue in cold-exposed or fasted rodents36–38. Moreover, optogenetic nerve stimulation of SNS fibres in mice increased fatty acid release39. Below, we discuss how leptin influences the sympathetic regulation of BAT, WAT and BeAT functions in response to metabolic challenges.
Neural control of leptin levels
Leptin is predominantly secreted from visceral white adipocytes in rodents40 and from subcutaneous adipose tissue in humans41. There is a general consensus that leptin levels are tightly correlated with adiposity in both rodents and humans42. Although leptin is required for regulating food intake, it is not viewed as a short-term ‘satiety’ signal, as food consumption takes several hours to influence circulating levels of leptin43. The expression and protein levels of leptin are subject to many regulatory factors. For instance, both Lep expression and circulating leptin levels show a circadian rhythm; in rats, they peak during the late-dark, active period ( zeitgeber time 21) and reach their nadir during the late-light, inactive period (zeitgeber time 9)44. However, the changes in leptin expression across the circadian cycle are very modest (about 1.5-fold)44 compared with changes in response to metabolic challenges (such as fasting or cold exposure), which rapidly affect leptin synthesis independent of changes in fat mass26,40,45 (FIG. 2). For instance, fasting mice for 24 h is sufficient to reduce Lep mRNA levels fourfold40, whereas 4 h of cold exposure reduces Lep mRNA levels to undetectable levels26. Likewise, fasting reduces circulating levels of leptin (by about 60–70%) in obese and normal-weight human participants45 and in rats46. As discussed below, the SNS has been implicated as a key acute regulator for both leptin synthesis and secretion; other factors that also regulate circulating leptin levels (such as insulin and glucocorticoids) are not discussed here45,47,48 (FIG. 2).
Figure 2. Sympathetic regulation of leptin production.
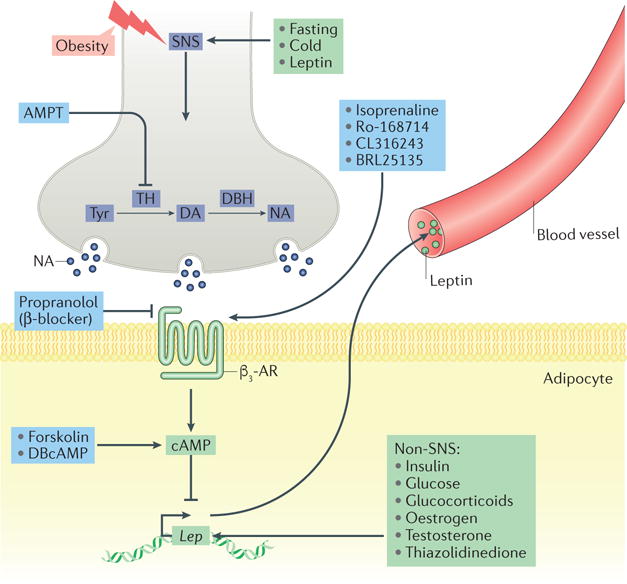
The sympathetic nervous system (SNS) is a key acute regulator of leptin production. Metabolic challenges affect leptin production through a β3-adrenergic receptor (β3-AR)–cAMP-dependent mechanism. Leptin regulates its own expression through a negative feedback loop from adipose tissue to the brain. Other non-SNS-related factors — exogenous and endogenous — also increase or decrease Lep expression. AMPT, α-methyl-p-tyrosine; DA, dopamine; DBcAMP, dibutyryl-cAMP; DBH, dopamine β-hydroxylase; NA, noradrenaline; TH, tyrosine hydroxylase; Tyr, tyrosine.
Acute treatment with catecholamines (such as noradrenaline) reduces leptin levels through a β3-adrenergic receptor (β3-AR)–cAMP-dependent mechanism (FIG. 2). This finding is supported by evidence that β3-AR agonists (including CL316243 and BRL35153A)27–29,49–52 and cAMP analogues (for example, dibutyryl-cAMP (DBcAMP)) or activators (such as forskolin)28,53 suppress Lep expression in rodent gonadal adipocytes and leptin levels (FIG. 2). By contrast, sympathetic blockade with the TH inhibitor α-methyl-p-tyrosine (AMPT) increases Lep expression51,54 (FIG. 2). Furthermore, chemical destruction of dopaminergic and noradrenergic neurons with 6-hydroxydopamine (6-OHDA) raises leptin levels (about 15-fold) in rats just 18–20 h after treatment55. However, data from pharmacologic sympathectomy studies must be interpreted with caution; for example, such treatments may cause neurotoxic lesions of the midbrain dopamine neurons56. Nevertheless, (male) individuals with spinal cord injuries also have inappropriately high leptin levels, even at low body mass index57, suggesting that sympathetic denervation also impairs leptin regulation in humans. In addition, leptin seems to regulate its own expression by acting on the SNS: exogenous leptin reduces Lep expression in gonadal adipose tissue but not in mice lacking dopamine β-hydroxylase (DBH), the enzyme responsible for converting dopamine to noradrenaline58 (FIG. 2). Newly developed genetic tools that enable targeted manipulations of molecules involved in sympathetic signalling represent a promising way to delineate the tissue-specific regulation of leptin production59. Overall, these observations indicate that SNS activity decreases leptin expression independently of adiposity, and most likely through activation of β3-AR25–29, although other receptors may also regulate leptin. Notably, the VGF-derived neuropeptide TLQP21, which is involved in energy balance regulation60, might potentiate β3-AR-dependent lipolysis through an adipocyte complement 3a receptor 1 (C3aR1)-dependent mechanism61; however, whether TLQP21 also affects leptin production requires further investigation.
Less is known about SNS-mediated regulation of Lep transcription. Several transcription factors directly regulate leptin expression: the transcription factor AP-2β (TFAP2B) inhibits leptin expression by directly binding to the Lep promoter62, whereas neurofibromin (NF1) and forkhead box protein L2 (FOXL2) both have positive effects on Lep expression63,64. In addition, the Lep promoter contains functional binding sites for CCAAT/enhancer-binding proteins (CEBPs), the transcription factor SP1, the glucocorticoid receptor (GR), cAMP-responsive element-binding protein (CREB) and sterol regulatory element-binding protein 1C (SREBP1C)65–71. These transcription factors are unlikely to mediate the SNS-dependent regulation of Lep expression, however, as they do not seem to mediate the effects of fasting or feeding on leptin levels72. Moreover, the potential transcription factors repressing leptin in response to cold are still unknown. The SNS-dependent regulation of leptin production might also be coupled to a lipid-sensing mechanism1, although the intracellular factors mediating this have yet to be identified.
Cold exposure and fasting each cause profound, yet reversible, decreases in leptin levels26,45,73–75, suggesting that the regulation of leptin does not reflect adiposity but, rather, energy demand. As cold exposure and fasting are important modulators of sympathetic tone, regulation of leptin by these metabolic stressors is probably dependent on the SNS45,75–77. Supporting this idea, treatment with β-blockers, such as propranolol, attenuates fasting-induced reductions in leptin51, whereas AMPT increases leptin levels even in fasted animals51,54 (FIG. 2). Paradoxically, leptin and its receptor seem to be required for the effect of the SNS on leptin expression: 24 h fasting leads to a ~70% decrease in leptin levels in lean Fa/Fa rats expressing wild-type leptin receptor but does not affect leptin levels in leptin-receptor-deficient (fa/fa) Zucker rats75,78. Together, these studies support the model that the nervous system can modulate leptin levels independently of adiposity.
Lipolysis
WAT contributes to energy homeostasis by storing energy (mainly as triglycerides) and by mobilizing free fatty acids when energy is needed79 through a process known as lipolysis (FIG. 1b). The breakdown of lipids involves a tightly regulated hydrolytic process in which intracellular cAMP activates a well-established molecular cascade involving adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), monoglyceride lipase (MGL) and perilipins80 (FIG. 1b). Dysregulation of adipose tissue metabolism leads to various complications, including ectopic lipid accumulation, lipotoxicity, insulin resistance and obesity79. WAT also has an important endocrine function, as it secretes adipokines79 and microRNAs81 that can influence systemic metabolism. Evidence suggests that increased visceral WAT (such as gonadal or retroperitoneal WAT) is associated with metabolic dysfunction, whereas increased subcutaneous WAT (for example, inguinal WAT) is protective79. The latter notion is supported by the fact that overexpression of adiponectin, which leads to a massive increase in subcutaneous adipose tissue, protects against diet-induced insulin resistance in mice82. The notion of healthy versus unhealthy WAT expansion has also recently been carefully reviewed79. Investigations are still ongoing to better understand the relative contributions and functions of the different WAT depots to energy homeostasis.
The importance of the SNS in regulating WAT lipolysis is supported by the abundance of adrenergic receptors at the surface of the adipocytes that are upstream of the lipolytic cascade (FIG. 1). Moreover, bilateral adrenal demedullation does not prevent lipolysis83,84, suggesting that catecholamines originating from SNS neurons are required for the breakdown of lipids. Work conducted primarily in Siberian hamsters suggests that SNS innervation of WAT is both sufficient and necessary for the regulation of WAT lipolysis8. Consistent with this was the identification of an anatomic bidirectional connection between postganglionic neurons and WAT that controls lipolysis8,85,86 (BOX 2). Functional evidence includes early observations that hemiplegic patients mobilize lipids only from their intact leg and that dogs with spinal cord injury do not mobilize lipids87. WAT surgical denervation studies in different species, including rodents and cats, were also key in determining the sufficiency of intact nervous input to adipose tissue for lipolysis87.
Box 2. Sympathetic and sensory innervation of adipose tissues.
Adipose tissues are richly innervated by the sympathetic nervous system (SNS). The understanding of the innervation patterns of the different fat depots is still evolving, and more studies are needed to better characterize and map how the brain and adipose tissues communicate with each other. Using a combination of fluorescent tract tracers and retrograde labelling (using pseudorabies virus (PRV)), sympathetic thoracolumbar ganglia were observed to project to both gonadal white adipose tissue (WAT) and inguinal WAT in Siberian hamsters and rats109,208 (FIG. 4). However, anatomical studies also demonstrated that different subsets of sympathetic neurons regulate lipolysis in visceral versus subcutaneous fat pads (FIG. 4). Moreover, the pattern of SNS innervation of fat pads differs in mice, with minimal fibres projecting to gonadal WAT compared with inguinal WAT99. The regional specificity of SNS innervation is further emphasized by functional changes (reflected by differences in noradrenaline turnover) observed following treatment with various stimuli. For example, in Siberian hamsters, cold exposure, but not fasting, increases noradrenaline turnover to retroperitoneal WAT, whereas central administration of the melanocortin receptor agonist melanotan II (MTII) increases noradrenaline turnover only to subcutaneous WAT209. Fluorogold studies have suggested that neurons in ganglia thoracic spinal segment 13 (T13)– lumbar spinal segment 3 (L3) project to both gonadal and inguinal WAT210. However, it is difficult to determine whether these ganglia are part of the SNS, owing to the lack of tyrosine hydroxylase (TH) counterstaining in these studies. Sympathetic thoracic chain ganglia innervating WAT were recently described in mice using TH-specific antibody staining211. Thus, coupling tract tracers and retrograde labelling to TH immunostaining will be useful to further determine the nature of the SNS ganglia projection to adipose tissues. Interestingly, the SNS ganglia that innervate inguinal WAT are similar to those innervating brown adipose tissue (BAT) (at T1–T3 and T12–L3 vertebral levels)109, whereas the SNS ganglia that innervate mesenteric and inguinal WAT were shown to exhibit some regional differences throughout the T5–L13 sympathetic chain levels208 (FIG. 4). However, there is a caveat associated with PRV tracing studies: glial or phagocytic cells of the immune compartment might accumulate PRV particles, populate the SNS ganglia and be incorrectly interpreted as neurons211. Moreover, studies in rats failed to identify innervation of inguinal BAT from ganglion cells in the T13 and the L1–L3 ganglia110, again reinforcing the need for further investigations into species-specific innervation differences. Although PRV retrograde tracing experiments should be interpreted with caution, the tracing studies nevertheless provide an anatomical picture supporting the differential central sympathetic regulation of different WAT depots. This regional specificity highlights the importance of considering each fat depot as a unique organ in terms of SNS regulation and function. As recently emphasized212, mice and humans share topological similarity of adipose tissues; however, the pattern of SNS innervation of these fat depots is largely unknown. The differences in innervation may also contribute to differences in leptin expression and regulation between visceral fat and subcutaneous fat, with leptin being predominantly produced by visceral fat in rodents40.
Leptin can influence the sympathetic tone to WAT. Central administration of leptin reduces gonadal fat weight independently of changes in food intake88, decreases lipogenesis in gonadal WAT89 and increases sympathetic tone towards inguinal WAT90. More recently, a leptin-dependent neuro–adipose tissue connection was shown to regulate lipolysis39. Using optical projection tomography together with multiphoton microscopy, axon bundles in the inguinal WAT were shown to come into close proximity to adipocytes (FIG. 1a). Importantly, optogenetic activation of these neurons increased levels of noradrenaline and activated HSL, a key enzyme for lipolysis, in inguinal WAT. Furthermore, the effect of leptin on HSL phosphorylation was prevented by surgical denervation of the tissue, suggesting that the local sympathetic innervation of inguinal WAT is required for the lipolysis-stimulating effects of exogenous leptin39.
Although there are other conflicting findings suggesting that injection of 6-OHDA in adipose tissue does not prevent the effects of leptin on lipolysis91,92, these studies suggest that leptin influences triglyceride breakdown in WAT through actions on the SNS. Studies in cultured rat adipocytes from different fat pads also indicate that leptin can directly stimulate lipolysis93–95 (although these effects are very modest when compared with that of noradrenergic signalling). Although these studies relied on high exogenous or pharmacological doses of leptin, they do suggest that leptin-induced lipolysis plays an important part in fine-tuning fuel usage in response to metabolic challenges. However, it remains paradoxical that the physiological decrease in leptin levels observed with fasting is concomitant with elevated lipolytic rates in WAT. Indeed, if the function of leptin is to stimulate lipolysis, why would leptin levels decrease in situations where lipolysis needs to be activated? One possibility is that the effects of leptin on lipolysis depend on insulin levels96 and on the sympathetic tone.
Browning
Under specific sustained conditions (for example, with chronic adrenergic stimulation using CL316243 or cold exposure), specific WAT depots, particularly those in the subcutaneous compartment, can develop clusters of brite adipocytes (BOX 1; FIG. 1a) with the characteristics of brown fat cells (including expression of mitochondrial brown fat uncoupling protein 1 (UCP1), multilocularity and high mitochondria content)7. Development of these brite cells improves insulin sensitivity and systemic metabolism7, yet the thermogenic potential of BeAT is still controversial11,97.
It is still unclear whether leptin directly regulates the browning of WAT and how this process might influence the regional synthesis and secretion of leptin. However, there is evidence that central leptin positively influences gonadal WAT browning98. Using volume fluorescence-imaging techniques to visualize the neural arborizations in mouse inguinal WAT, it was recently shown that sympathetic fibres are in close apposition to over 90% of adipocytes34. Supporting these observations, investigators recently developed an Adipo-Clear model in which fat pads were delipidated to enable 3D whole-tissue imaging and found dense staining of sympathetic fibres in close contact with the majority of adipocytes in inguinal WAT99. Interestingly, blocking the outgrowth of neural fibres to inguinal WAT using genetic or pharmacologic tools prevented the sympathetic arborization of the tissue and the ability of cold to promote the development of brite adipocytes34. Moreover, genetic deletion of the three β-ARs also prevented cold-induced browning of inguinal WAT34. These results confirm that the sympathetic innervation of inguinal WAT is required for cold-induced development of BeAT. Although there is a clear link between the activation of adrenergic receptors and BeAT development in mice7, the mechanisms by which the SNS positively regulates browning of WAT are still unknown. One way the sympathetic signal propagates among brite adipocytes is through a cell-to-cell connexin 43 gap-junction system100 (FIG. 1a). It is tempting to speculate that the propagation of a sympathetic neuronal signal in response to SNS activation allows these gap-junction channels to also negatively regulate leptin secretion in BeAT. However, there is not yet any direct evidence that leptin production and BeAT development are inversely related.
Fasting leads to the inhibition of the thermogenic programme (that is, changes in gene expression) in BeAT101 and preferentially suppresses the browning of retroperitoneal WAT. Similar to leptin regulation, these fasting-induced adipose effects are likely to be mediated by the SNS51,54. However, it is also possible that fasting-induced alterations in browning are secondary to hypoglycaemia or hypoinsulinaemia and represent part of an evolutionary mechanism to reduce fuel consumption under low-energy conditions. Notably, changes in sympathetic outflow induced by fasting differ between fat depots101: noradrenaline levels are increased in BAT and inguinal WAT, are reduced in retroperitoneal WAT, and are unchanged in gonadal WAT. Interestingly, fasting prevents the ability of cold exposure to promote browning, again suggesting that there may be an evolutionary mechanism to promote energy conservation during fasting101. Whether and how the fasting-induced inhibition of browning is linked to the fasting-induced inhibition of leptin is yet to be demonstrated. In addition, the central pathways responsible for driving SNS outflow to WAT, for suppressing browning and leptin secretion and for inducing lipolysis are yet to be determined.
Non-shivering thermogenesis
The ability of brown adipocytes to generate heat, a process referred to as non-shivering thermogenesis, is mediated by UCP1, a mitochondrial protein that has the unique ability to uncouple the process of substrate oxidation from electron transport5,9 (FIG. 1c). Although several anatomical depots of brown fat have been identified in rodents102, most of the current knowledge relates to interscapular BAT. The most powerful activator of BAT thermogenesis is undoubtedly cold exposure103. Upon cold exposure, BAT takes up and metabolizes different substrates (such as glucose and free fatty acids)104. These substrates are then incorporated into lipid vesicles directly, or through de novo lipogenesis, and are ultimately used by mitochondria to generate heat in response to adrenergic stimulation105.
Brown adipose depots are richly innervated by sympathetic fibres106. Retrograde transneuronal viral tracing using pseudorabies virus (PRV) was instrumental in mapping the sympathetic innervation of BAT107,108. Sympathetic preganglionic neurons innervating interscapular BAT are located in the IML of the T1–T6 spinal segments109,110, and the sympathetic postganglionic neurons that project into interscapular BAT are located in the T1–T5 paravertebral ganglia, with a large number in the stellate ganglion111 (BOX 2). Again, these data must be interpreted with caution, as PRV can infect different neuronal terminals. Furthermore, PRV trans-synaptic transduction means that the tracing is not limited to first-order neurons. The activity of brown fat and its thermogenic capacity (that is, its ability to expand its thermogenic machinery by increasing levels of proteins involved in fat oxidation and thermogenesis) are dependent on noradrenaline105. Denervation of interscapular BAT prevents or impairs cold-induced thermogenesis9, indicating the importance of sympathetic innervation.
Early studies suggested that leptin had a thermogenic effect. For example, many hypothalamic neurons involved in regulating non-shivering thermogenesis are also leptin sensitive112, and leptin-deficient mice have a lowered core temperature when housed in conditions below thermoneutrality113. Exogenous hypothalamic leptin also enhances glucose uptake by BAT and raises BAT temperature114–117. Moreover, in rodents, leptin administration exerts a mild pyrexic effect113,118–120 and increases the noradrenaline turnover to BAT121; activity in sympathetic nerves innervating BAT122, kidneys, adrenals and hindlimb123; glucose uptake to heart and muscle117; glucose turnover in the liver124; and arterial pressure125–127. Leptin positively regulates Ucp1 mRNA expression in rodent BAT121,128–132, and this effect is blocked by sympathetic denervation128 and in mice lacking DBH58.
More recent findings, however, suggest that leptin is not a major regulator of non-shivering thermogenesis. In particular, the lowered core temperature of leptin-deficient mice is caused by an increase in heat loss (that is, increased thermal conductance) from these animals rather than by defects in non-shivering thermogenesis113,133. Leptin-deficient mice defend a lower body temperature, suggesting that leptin may have pyrexic, rather than thermic, effects113. Thus, leptin might affect thermoregulation through vasomotor or thermal conductance mechanisms rather than by directly affecting BAT thermogenesis. Moreover, increasing evidence suggests that the effects of exogenous leptin treatments do not accurately reflect endogenous physiology. For example, animals fed a high-fat diet (HFD) are resistant to exogenous leptin but remain completely sensitive to endogenous leptin134. Furthermore, a pegylated leptin receptor antagonist (PLA) that acted as a competitive antagonist of the leptin receptor increased feeding and body weight in both lean and diet-induced obese mice134. Moreover, this analogue also blocked signal transducer and activator of transcription 3 (STAT3) signalling, the best-known readout of leptin action, suggesting that, at a minimum, the development of leptin resistance could raise the set point for leptin in specific neurons, causing neurons to require a higher concentration of leptin to activate STAT3 signalling. Together, these studies highlight the need for developing new tools aimed at modulating endogenous leptin.
Leptin and neural control of adipose
Some of the neurons within the central pathways that are connected to adipose depots express leptin receptors. PRV studies have identified brain regions that are polysynaptically connected to interscapular BAT and different WAT depots86,110,135–138; these brain regions include brainstem nuclei (specifically, the nucleus of the solitary tract (NTS) and raphe nuclei, such as the raphe pallidus nucleus (RPa)), midbrain nuclei (such as the periaqueductal grey (PAG)) and forebrain nuclei (such as the arcuate nucleus (ARC), dorsomedial hypothalamus (DMH), lateral nucleus (LH), paraventricular nucleus (PVH), suprachiasmatic nucleus (SCN) and the preoptic area (POA) of the hypothalamus) (FIG. 3). Importantly, these structures contain neurons that express considerable levels of leptin receptors139–141.
Figure 3. Leptin-sensitive neurons regulating adipose depots.
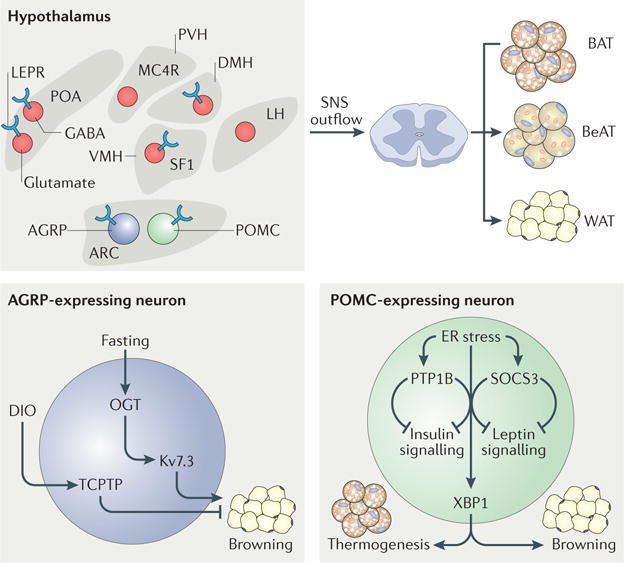
Neurons in several hypothalamic nuclei express leptin receptors, and many of these nuclei are in turn involved in adipose regulation. The arcuate nucleus (ARC)–paraventricular nucleus (PVH) melanocortin system, the thermoregulatory circuit (the preoptic area (POA)– dorsomedial hypothalamus (DMH)–raphe pallidus nucleus) and the steroidogenic factor 1 (SF1)-expressing neurons (of the ventromedial hypothalamus (VMH)) all have roles in regulating the sympathetic nervous system (SNS) outflow to white adipose tissue (WAT), brown adipose tissue (BAT) and beige adipose tissue (BeAT). O-GlcNAc transferase (OGT) and T cell protein-tyrosine phosphatase (TCPTP) expressed in the neuropeptide Y or agouti-related protein (AGRP)-expressing neurons of the ARC are key regulators of BeAT development and are influenced by fasting and diet-induced obesity (DIO). The endoplasmic reticulum (ER) stress response factor X-box binding protein 1 (XBP1) expressed in the pro-opiomelanocortin (POMC)-expressing neurons of the ARC is a key regulator of non-shivering thermogenesis in BAT and BeAT development. LEPR, leptin receptor; LH, lateral hypothalamus; Kv7.3, voltage-gated Kv7.3 channel; MC4R, melanocortin receptor 4; PTP1B, protein-tyrosine phosphatase 1B; SOCS3, suppressor of cytokine signalling 3.
Although we now have a better appreciation of the brain circuits involved in WAT regulation, traditionally most of the understanding was based on neuroanatomical studies in Siberian hamsters and, to a lesser extent, in rats. Whether mice and humans also show similar circuitry in terms of innervation is largely unknown. Here, we discuss the different leptin-sensitive brain circuits potentially involved in modulating BAT thermogenesis, WAT lipolysis and BeAT development and metabolism.
Melanocortin system
Recent work has revealed the importance of the leptin–melanocortin system in WAT, BeAT and BAT metabolism. The central melanocortin system includes pro-opiomelanocortin (POMC) neurons producing melanocortins such as α-melanocyte-stimulating hormone (α-MSH). POMC neurons are primarily located in the ARC but can also be found in the NTS142. A distinct population of adjacent neurons expresses agouti-related protein (AGRP) in the ARC143. These subsets of melanocortin neurons are important targets of leptin and express leptin receptors144,145. They act on melanocortin receptor 4 (MC4R) and MC3R, which are widely expressed in the brain146–148. In particular, MC4Rs are highly expressed in the PVH, where they control energy balance through a well-described ARC–PVH melanocortin circuit149–151 (FIG. 3).
The first demonstration of an interaction between the melanocortin system and BAT thermogenesis was made in the late 1990s152,153. Central administration of the synthetic melanocortin receptor antagonist SHU9119 completely blocked leptin-induced increases in BAT Ucp1 mRNA expression in rats116. Moreover, central administration of the MC4R agonist MTII increased BAT Ucp1 mRNA expression — an effect that was blocked by surgical sympathetic denervation154. Transgenic mouse models further clarified the role of the leptin–melanocortin system in BAT thermogenesis. For example, compared with wild-type mice, mice lacking MC4R showed a smaller upregulation of BAT Ucp1 in response to HFD or cold exposure (stressors that affect leptin production)155. However, whether leptin directly acts on the melanocortin circuit to regulate BAT activity needs further validation. Interestingly, MC3R and/or MC4R are required for exogenous leptin-induced increases in renal, but not BAT, sympathetic nerve activity in anaesthetized rats153.
Many MC4R-expressing neurons in the PVH are polysynaptically connected to BAT138; however, conditional re-expression of MC4R specifically in the PVH of otherwise MC4R-null mice did not restore impaired energy expenditure in these animals, as assessed by indirect calorimetry156. Subsequent studies found that MC4R expression in sympathetic, but not parasympathetic, autonomic preganglionic neurons is important for regulating thermogenesis in mice157 (FIG. 3). Deletion of Mc4r in the sympathetic preganglionic neurons using ChAT– Cre mice impaired HFD-induced and cold-induced thermogenesis in BAT and reduced the development of BeAT157. These findings support the idea that cholinergic preganglionic neurons might be involved in the effects of leptin and melanocortin on BAT metabolism.
Although the specific MC4R-expressing neuronal populations that regulate BAT thermogenesis are unknown, recent work has suggested a role for ARC neurons. As mentioned, the ARC is a heterogeneous region of the hypothalamus in which melanocortin neurons with opposing functions reside in close proximity to one another158,159 (FIG. 3). Overexpression of X-box binding protein 1 (XBP1), an important molecule in the endoplasmic reticulum (ER) stress response, in POMC neurons is sufficient to increase thermogenesis in BAT and browning of inguinal WAT160 (FIG. 3). Thus, XBP1 in POMC neurons may regulate the development of BeAT and hence contribute to improvements in whole-body metabolism. Interestingly, blocking the connexin 43 gap-junction system in WAT prevents the ability of neuronal POMC XBP1 to affect BeAT development, suggesting that the effects of the melanocortin system on BeAT metabolism require an intact adipocyte gap-junction system100. Furthermore, insulin and leptin act together on POMC neurons to promote BeAT formation161. Deletion of the tyrosine-protein phosphatases, PTP1B and T cell protein-tyrosine phosphatase (TCPTP; also known as PTPN2), which regulate insulin signalling, in POMC neurons prevents HFD-induced obesity by increasing BeAT development and energy expenditure161 (FIG. 3). Together, these studies indicate that POMC neurons are important for regulating BAT, WAT and BeAT metabolism.
Deletion of O-GlcNAc transferase (OGT) specifically from AGRP-expressing neurons reduces the excitability of these neurons (by inhibiting the activity of voltage-dependent potassium channels) and promotes the development of BeAT101 (FIG. 3). By contrast, acute chemogenetic activation of AGRP-expressing neurons reduces energy expenditure by suppressing the browning of retroperitoneal and inguinal WAT without affecting the thermogenic programme in BAT101. These observations further demonstrate that the process of WAT browning is highly dynamic and specific to different fat depots. Mice lacking synaptic GABA release from hypothalamic neurons expressing rat insulin promoter (RIP), which include AGRP-expressing neurons, also show reduced energy expenditure162 (FIG. 3). Importantly, the ability of exogenous leptin to increase energy dissipation was attenuated in these mice, suggesting that GABAergic transmission from hypothalamic RIP-expressing neurons is required for exogenous leptin to regulate energy expenditure. Deletion of TCPTP in AGRP-expressing neurons prevented HFD-induced obesity and increased energy expenditure and BeAT development in mice163 (FIG. 3). Interestingly, deletion of TCPTP from the AGRP-expressing neurons of obese mice restored a lean metabolic phenotype163. Although still preliminary, taken together, these studies suggest that leptin has an important role in the melanocortin-dependent regulation of adipose function. Nevertheless, the direct role of leptin signalling in these circuits remains to be addressed.
Thermoregulatory circuits
The preoptic area (POA) of the hypothalamus contains neurons responsible for thermoregulation, including those that regulate BAT thermogenesis23, through direct connections with the DMH164 and the RPa165 (FIG. 3). The fact that leptin-deficient mice are hypothermic (or anapyrexic113) and are unable to defend their body temperature during an acute cold exposure166,167, together with the observation of high levels of leptin receptors within the POA140,168, suggests that leptin might influence body temperature by directly acting on POA neurons. Indeed, leptin does affect sympathetic outputs to BAT, as determined by increased BAT temperature and energy expenditure, through GABAergic and glutamatergic neurons of the POA168,169. Leptin receptors also play a part in the thermic effect of systemic inflammatory stressors such as lipopolysaccharide administration170. More recently, however, pharmacogenetic activation of leptin-receptor-expressing POA neurons in mice was reported to induce hypothermia and to reduce energy expenditure through a glutamatergic, but not GABAergic, mechanism112. This involvement of glutamate is further supported by the observation that deleting vesicular glutamate transporter 2 (VGLUT2), which is needed for glutamate transmission, from leptin-receptor-expressing neurons in mice drastically reduces energy expenditure and body temperature171. As discussed above, the ultimate thermoregulatory effects of leptin are more likely to involve vasomotor mechanisms than BAT thermogenesis. Additional work is needed to better understand the direct implication of endogenous leptin on the POA–BAT axis.
Leptin may also regulate thermoregulation through direct actions on the DMH23,172–174 (FIG. 3). Disinhibition of the DMH, through unilateral microinjection of bicuculline methiodide (a GABA type A receptor antagonist), evokes non-shivering thermogenesis172,173, whereas inhibition of DMH neurons prevents BAT thermogenesis175. Leptin receptor-expressing DMH neurons project to areas involved in the SNS regulation of body temperature, including the PVH, PAG and RPa168,176 (FIG. 3). Exogenous leptin activates many DMH neurons177,178, and intra-DMH injections of leptin increase body temperature169,179 in both lean and obese mice180. Similarly, chemogenetic activation of leptin receptor-expressing neurons in the DMH stimulates BAT thermogenesis and energy expenditure169. Interestingly, these effects are blunted or reduced by β3-AR antagonists169,180. Recently, a subpopulation of DMH neurons that co-express leptin receptors and prolactin-releasing peptide (PrRP) was also shown to mediate the effects of leptin on BAT sympathetic nerve activity and temperature in mice120 (FIG. 3). However, this is unlikely to occur in rats, as PrRP is only weakly expressed in the rat DMH181. Leptin may also influence the activity of the RPa. Pharmacological activation of the serotonin receptor 1A (5-HT1A receptor) in the RPa using the specific agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) inhibits leptin-evoked stimulation of BAT sympathetic nerve activity122 (FIG. 3). However, these effects do not suggest that leptin acts directly on RPa serotonergic cells, as these neurons are not leptin sensitive182.
Steroidogenic-factor-1-expressing neurons of the ventromedial hypothalamus
The role of the ventromedial hypothalamus (VMH) in regulating non-shivering thermogenesis was unclear for many years. Early studies showed that manipulations of the VMH altered temperature and noradrenaline turnover in BAT183,184 and fat utilization in WAT185–188; however, there was no evidence of direct connections between the VMH and BAT108. However, a recent study using PRV tract tracing from BAT shows a substantial percentage of PRV-labelled neurons in the VMH109, helping to account for earlier works showing a functional connection between the VMH and BAT185–188.
Leptin microinjection into the VMH increases glucose uptake in different tissues, including BAT — an effect that is blunted by sympathetic denervation117,189,190. Furthermore, administration of leptin in the VMH, but not the ARC, PVH or DMH, increases circulating levels of catecholamines, and this effect is abrogated in VMH-lesioned rats116. Leptin receptors are expressed throughout the VMH and are especially enriched in the dorsomedial division178, where steroidogenic factor 1 (SF1)-expressing neurons reside in adult mice191 (FIG. 3). Genetic deletion of leptin receptors in the dorsomedial VMH using SF1–Cre mice impaired HFD-induced thermogenesis and BAT Ucp1 expression in mice192–194. In particular, SF1 in the VMH is required for thermogenic responses after HFD exposure194. Whether leptin also acts through these neurons to regulate WAT lipolysis and BeAT development remains to be determined.
Peripheral afferent neurons
Although fairly preliminary and controversial, there is evidence that peripheral afferent neurons innervate adipose tissues (FIG. 4). The sensory-neuron markers substance P and calcitonin gene-related peptide (CGRP)195 are expressed in neural fibres innervating BAT196,197 and WAT198, and anterograde labelling experiments using both traditional dye and viral tracers show innervation of WAT by afferent neurons in the dorsal root ganglia (DRG)13,199. However, the function of this afferent innervation is still unclear. Injection of leptin into one gonadal WAT pad in rats was shown to increase the sympathetic nerve activity of the contralateral epididymal WAT pad within seconds200,201. Interestingly, intra-inguinal WAT injection of leptin also increases the electrophysiological activity of afferent neurons in WAT202. This suggests the presence of a leptin-sensitive afferent pathway that mediates SNS outflow to WAT to regulate adipocyte function, including lipolysis203. It is also possible that sensory innervation carries a negative feedback signal to regulate lipid mobilization86. Together, these studies suggest the presence of a leptin-regulated adipose–afferent reflex.
Figure 4. Sympathetic and sensory innervation of adipose tissues.
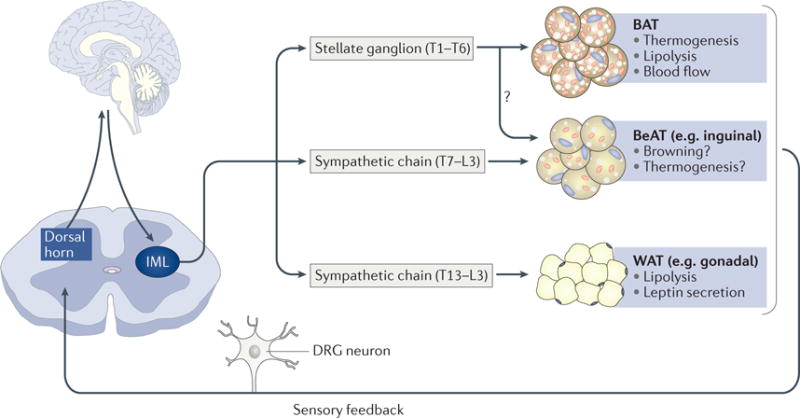
Sympathetic innervations of brown adipose tissue (BAT) originate from the stellate ganglion (T1–T6). Although still debated (owing to divergence in the species studied), sympathetic thoracolumbar ganglia (T7–L3) project to gonadal and inguinal white adipose tissue (WAT) (which is the most characterized inducible beige adipose tissue (BeAT) depot). It is also suggested that there are similarities between inguinal WAT and BAT innervation (with BAT-innervating and WAT-innervating neurons possibly residing in T1–T3 ganglia). Sensory feedback from adipose tissues via the dorsal root ganglion (DRG) to the brain regulates WAT lipolysis and leptin secretion and BAT non-shivering thermogenesis. Additional studies are needed to validate these afferent innervations in mice. IML, intermediolateral column of spinal cord; L, lumbar spinal segment; T, thoracic spinal segment.
Conclusions and future directions
In this Review, we have highlighted the inherent complexities of the CNS regulatory circuits coordinating adipose tissue function. We have also briefly discussed some controversies and paradoxes that the neuroscience community is facing in regard to leptin and the neural regulation of WAT, BAT and BeAT.
Although our knowledge of the sympathetic regulation of adipose metabolism is ever expanding, many challenges remain (BOX 3), including how to translate findings in rodents to the clinic. Although there are many similarities between rodent and human WAT depots, important differences also exist. For example, there is no omental WAT in rodents and no perigonadal WAT in humans, and there are important differences in the distribution of subcutaneous WAT between rodents and humans8. The molecular profile of adipocytes also differs across species. For instance, whereas rodent adipocytes express high levels of β3-AR, human adipocytes express very few β-ARs and instead express high levels of α2-ARs204. Moreover, the highest levels of leptin are found in the visceral WAT of rodents and subcutaneous WAT in humans40,41,205. Our understanding of leptin and the neural control of adipose metabolism must take these interspecies differences into account206.
Box 3. Key outstanding questions.
Throughout this Review, we highlight how leptin plays an important role in brain– adipose crosstalk. However, there are many questions that still require attention:
What are the differences in adipose innervation between males and females? Could differences in sympathetic outflow explain the differences in fat distribution between sexes?
What are the intracellular mechanisms through which activation of adrenergic receptors regulates leptin expression? How do increased levels of cAMP lead to inhibition of leptin expression and secretion?
Can we develop genetic tools to neuromodulate specific adipose tissues? This would first require the development of more comprehensive maps of the sympathetic innervation of brown adipose tissue (BAT), beige adipose tissue (BeAT) and white adipose tissue (WAT).
How similar are the afferent innervations of adipose tissue between Siberian hamsters, rats, mice and humans? It is imperative to fully characterize the innervation patterns in mice to enable the use of the available and expanding library of transgenic tools.
Is there a similar neural circuit regulating adipose tissue in humans? The fact that there are differences in the expression of adrenergic receptors between rodents and humans suggests that alternative mechanisms for BAT, BeAT and WAT regulation might exist in humans.
What are the central neural circuits regulating BAT, BeAT and WAT? Is leptin required for the differential effects of metabolic stresses such as fasting and cold on adipose tissues?
Another important challenge is to identify the mechanisms by which the SNS differentially regulates adipose metabolism across different metabolic challenges. Many studies have focused on understanding how high-energy availability (for example, with HFDs) affects adipose metabolism. Although these studies were fundamental in defining important aspects of the pathophysiology of obesity, we believe that we now need to put more emphasis on examining the impact of other metabolic challenges, including states of negative energy balance, such as fasting, exercise, cachexia and cold exposure. Clearly, the physiological functions of leptin also need to be reconsidered in non-obese settings1,15,207.
Lastly, more studies are warranted on the neural control of various fat depots. The sympathetic activity of adipose tissue is unique compared with that of other tissues, as evidenced by the fact that fasting decreases the sympathetic tone to most tissues, including BAT, but increases the sympathetic activity towards WAT10. Adding to the complexity of adipose physiology, WAT can undergo adipose-depot-specific browning upon SNS activation7. Therefore, it is imperative to identify how the CNS differentially regulates each of the fat depots. In the future, it would be useful to develop genetic tools enabling the selective manipulation of the SNS outflow to specific adipose depots. The identification of genes uniquely expressed in stellate or thoracolumbar ganglia could represent a first step in developing such tools. As so aptly described by the late Timothy Bartness, “the trafficking of sympathetic outflow to peripheral tissues remains one of the great mysteries of regulatory biology” (REF.10). Thus, developing these genetic tools represents a promising avenue to better understand the regional functions of the sympathetic outflow to adipose tissues.
Acknowledgments
The authors apologize to all colleagues whose work could not be cited owing to space limitations. This work was supported by US National Institutes of Health grants DK053301, R01 DK088423 and R01 DK100659 to J.K.E. A.C. is a Canadian Diabetes Association postdoctoral fellow.
Glossary
- SNS outflow
The release of neurotransmitters derived from sympathetic neurons in peripheral tissues
- Fat mobilization
A process, in response to signals for energy, in which fatty acids are released from triglyceride stores in fat cells and delivered to organs
- Dermomyotome
The epithelial cell layer constituting the dorsal part of the somite lying under the ectoderm. It gives rise to the dorsal dermis and to the skeletal muscle of the myotome
- Bilateral chain
A symmetric chain of sympathetic ganglia located just ventral and lateral to the spinal cord
- Terminal ganglia
Parasympathetic ganglia situated within or close to an innervated organ and the site where preganglionic nerve fibres terminate
- Noradrenaline turnover
A surrogate index of changes in sympathetic activation, based on the assumption that tissue levels of noradrenaline decline at a rate proportional to initial noradrenaline concentrations. Increased noradrenaline turnover implies increased sympathetic activation
- Zeitgeber time
A quantification of time based on the period of a zeitgeber. Under standard light–dark cycles, the time at lights-on is usually defined as zeitgeber time 0 for diurnal organisms, and the time at lights-off is defined as zeitgeber time 12 for nocturnal animals
- β-Blockers
β-Adrenergic blocking agents that prevent the effects of the hormone adrenaline
- Ectopic lipid accumulation
An accumulation of lipids in lean tissues such as kidneys, liver, muscle and heart that results in lipotoxicity and metabolic diseases
- Lipotoxicity
The accumulation of lipid intermediates in non-adipose tissue, leading to cellular dysfunction and death
- Multilocularity
The appearance of cytoplasmic lipids arranged in numerous small droplets
- Thoracolumbar ganglia
Ganglia arising from the thoracolumbar (T1–L2) regions of the spinal cord
- Fluoro-gold
A fluorescent retrograde axonal tracer
- Thermoneutrality
The state of thermal balance at which an organism does not need to generate heat in order to maintain a normal core temperature
- Pyrexic
Relating to factors that raise body temperature above normal
- Leptin resistance
Reduced sensitivity with respect to the metabolic responses to exogenously administered leptin
- Calorimetry
The process of measuring the amount of energy as heat released, or absorbed, by an organism
- O-GlcNAc transferase
(OGT). An enzyme that catalyses the addition of an N-acetylglucosamine group in O-glycosidic linkage to serine or threonine residues of intracellular proteins
- Hypothermic
A condition that leads to a decrease in body core temperature — be that regulated, accidental or forced — when the body dissipates more heat than it generates
- Anapyrexic
Relating to decreases in body temperature below normal
- Lipopolysaccharide
Large molecules found in the outer membrane of Gram-negative bacteria. They elicit strong immune responses
- Omental
Adipose tissue located in the omentum, a layer of peritoneum that surrounds abdominal organs
- Cachexia
A syndrome characterized by uncontrolled weight loss, muscle atrophy, fatigue and weakness
Footnotes
Author contributions
A.C. wrote the manuscript and prepared the figures. J.K.E., A.C., L.G. and S.L. researched, discussed and reviewed and/or edited the manuscript before submission.
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neuroscience thanks A. Domingos, J. Friedman and S. Morrison for their contribution to the peer review of this work
References
- 1.Friedman J. The long road to leptin. J Clin Invest. 2016;126:4727–4734. doi: 10.1172/JCI91578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman J. 20 years of leptin: leptin at 20: an overview. J Endocrinol. 2014;223:T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. This study reports the cloning and sequencing of the mouse Lep gene and has ushered in the molecular era of obesity research. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. US Department of Health and Human Services; 2015. [PubMed] [Google Scholar]
- 5.Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab. 2013;24:408–420. doi: 10.1016/j.tem.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbe SM, et al. Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci. 2015;9:150. doi: 10.3389/fnsys.2015.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1445–R1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- 11.Labbe SM, et al. Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am J Physiol Endocrinol Metab. 2016;311:E260–E268. doi: 10.1152/ajpendo.00545.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol. 1987;253:R942–R944. doi: 10.1152/ajpregu.1987.253.6.R942. [DOI] [PubMed] [Google Scholar]
- 14.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 15.Flier JS. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 17.Munzberg H, Qualls-Creekmore E, Berthoud HR, Morrison CD, Yu S. Neural control of energy expenditure. Handb Exp Pharmacol. 2016;233:173–194. doi: 10.1007/164_2015_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. This study demonstrates the presence of functional BAT in humans and has contributed to the recent renewed interest in the role of BAT in regulating energy balance. [DOI] [PubMed] [Google Scholar]
- 21.Caron A, Richard D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann NY Acad Sci. 2017;1391:35–53. doi: 10.1111/nyas.13263. [DOI] [PubMed] [Google Scholar]
- 22.Contreras C, et al. The brain and brown fat. Ann Med. 2015;47:150–168. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Compr Physiol. 2014;4:1677–1713. doi: 10.1002/cphy.c140013. This review summarizes the functional organization and neurochemical influences governing BAT sympathetic nerve activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison MB, Myers MG., Jr 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223:T25–35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Matheny M, Scarpace PJ. β3-adrenergic-mediated suppression of leptin gene expression in rats. Am J Physiol. 1997;272:E1031–E1036. doi: 10.1152/ajpendo.1997.272.6.E1031. [DOI] [PubMed] [Google Scholar]
- 26.Trayhurn P, Duncan JS, Rayner DV. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J. 1995;311:729–733. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trayhurn P, Duncan JS, Rayner DV, Hardie LJ. Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the β3-adrenoceptor agonists BRL 35135A and ZD2079. Biochem Biophys Res Commun. 1996;228:605–610. doi: 10.1006/bbrc.1996.1704. [DOI] [PubMed] [Google Scholar]
- 28.Gettys TW, Harkness PJ, Watson PM. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 29.Mantzoros CS, et al. Activation of β3 adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes. 1996;45:909–914. doi: 10.2337/diab.45.7.909. [DOI] [PubMed] [Google Scholar]
- 30.Furness JB. The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci. 2006;130:1–5. doi: 10.1016/j.autneu.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 31.McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. 2007;71:78. doi: 10.5688/aj710478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreier F, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. J Clin Invest. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giordano A, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Ding X, Cao Y, Wang H, Zeng W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 2017;26:686–692.e3. doi: 10.1016/j.cmet.2017.08.016. This study reveals a dense network of sympathetic fibres in close apposition with adipocytes. It provides evidence that catecholamines deriving from these sympathetic fibres, but not from the adrenal medulla or resident macrophages, are essential for the development of BeAT. [DOI] [PubMed] [Google Scholar]
- 35.Gilgen A, Maickel RP, Nikodijevic O, Brodie BB. Essential role of catecholamines in the mobilization of free fatty acids and glucose after exposure to cold. Life Sci. 1962;1:709–715. doi: 10.1016/0024-3205(62)90138-8. [DOI] [PubMed] [Google Scholar]
- 36.Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977;196:1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 37.Migliorini RH, Garofalo MA, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol. 1997;272:R656–R661. doi: 10.1152/ajpregu.1997.272.2.R656. [DOI] [PubMed] [Google Scholar]
- 38.Garofalo MA, Kettelhut IC, Roselino JE, Migliorini RH. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J Auton Nerv Syst. 1996;60:206–208. doi: 10.1016/0165-1838(96)00037-9. [DOI] [PubMed] [Google Scholar]
- 39.Zeng W, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94. doi: 10.1016/j.cell.2015.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice. FEBS Lett. 1995;368:488–490. doi: 10.1016/0014-5793(95)00719-p. [DOI] [PubMed] [Google Scholar]
- 41.Hube F, et al. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm Metab Res. 1996;28:690–693. doi: 10.1055/s-2007-979879. [DOI] [PubMed] [Google Scholar]
- 42.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 43.Koopmans SJ, Frolich M, Gribnau EH, Westendorp RG, DeFronzo RA. Effect of hyperinsulinemia on plasma leptin concentrations and food intake in rats. Am J Physiol. 1998;274:E998–E1001. doi: 10.1152/ajpendo.1998.274.6.E998. [DOI] [PubMed] [Google Scholar]
- 44.Sukumaran S, Xue B, Jusko WJ, Dubois DC, Almon RR. Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology. Physiol Genomics. 2010;42A:141–152. doi: 10.1152/physiolgenomics.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 46.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 47.Murakami T, Iida M, Shima K. Dexamethasone regulates obese expression in isolated rat adipocytes. Biochem Biophys Res Commun. 1995;214:1260–1267. doi: 10.1006/bbrc.1995.2422. [DOI] [PubMed] [Google Scholar]
- 48.Saladin R, et al. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- 49.Moinat M, et al. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 1995;373:131–134. doi: 10.1016/0014-5793(95)01030-i. [DOI] [PubMed] [Google Scholar]
- 50.Deng C, et al. Effects of β-adrenoceptor subtype stimulation on obese gene messenger ribonucleic acid and on leptin secretion in mouse brown adipocytes differentiated in culture. Endocrinology. 1997;138:548–552. doi: 10.1210/endo.138.2.4922. [DOI] [PubMed] [Google Scholar]
- 51.Trayhurn P, Duncan JS, Hoggard N, Rayner DV. Regulation of leptin production: a dominant role for the sympathetic nervous system? Proc Nutr Soc. 1998;57:413–419. doi: 10.1079/pns19980060. [DOI] [PubMed] [Google Scholar]
- 52.Giacobino JP. Role of the β3-adrenoceptor in the control of leptin expression. Horm Metab Res. 1996;28:633–637. doi: 10.1055/s-2007-979868. [DOI] [PubMed] [Google Scholar]
- 53.Slieker LJ, et al. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem. 1996;271:5301–5304. doi: 10.1074/jbc.271.10.5301. [DOI] [PubMed] [Google Scholar]
- 54.Rayner DV, Simon E, Duncan JS, Trayhurn P. Hyperleptinaemia in mice induced by administration of the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine. FEBS Lett. 1998;429:395–398. doi: 10.1016/s0014-5793(98)00642-5. [DOI] [PubMed] [Google Scholar]
- 55.Sivitz WI, et al. Sympathetic inhibition, leptin, and uncoupling protein subtype expression in normal fasting rats. Am J Physiol. 1999;277:E668–E677. doi: 10.1152/ajpendo.1999.277.4.E668. [DOI] [PubMed] [Google Scholar]
- 56.Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. 2000;81:1582–1586. doi: 10.1053/apmr.2000.9173. [DOI] [PubMed] [Google Scholar]
- 58.Commins SP, et al. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology. 1999;140:4772–4778. doi: 10.1210/endo.140.10.7043. [DOI] [PubMed] [Google Scholar]
- 59.Pereira MM, et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat Commun. 2017;8:14967. doi: 10.1038/ncomms14967. This study reports the development of a novel tool allowing chemical genetic ablation of genes of interest strictly in peripheral tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartolomucci A, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci USA. 2006;103:14584–14589. doi: 10.1073/pnas.0606102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cero C, et al. The neuropeptide TLQP-21 opposes obesity via C3aR1-mediated enhancement of adrenergic-induced lipolysis. Mol Metab. 2017;6:148–158. doi: 10.1016/j.molmet.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuke T, et al. Transcription factor AP-2β inhibits expression and secretion of leptin, an insulin-sensitizing hormone, in 3T3-L1 adipocytes. Int J Obes. 2010;34:670–678. doi: 10.1038/ijo.2009.295. [DOI] [PubMed] [Google Scholar]
- 63.Wrann CD, et al. FOSL2 promotes leptin gene expression in human and mouse adipocytes. J Clin Invest. 2012;122:1010–1021. doi: 10.1172/JCI58431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu YH, Dallner OS, Birsoy K, Fayzikhodjaeva G, Friedman JM. Nuclear factor-Y is an adipogenic factor that regulates leptin gene expression. Mol Metab. 2015;4:392–405. doi: 10.1016/j.molmet.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de la Brousse FC, Shan B, Chen JL. Identification of the promoter of the mouse obese gene. Proc Natl Acad Sci USA. 1996;93:4096–4101. doi: 10.1073/pnas.93.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong DW, Bi S, Pratley RE, Weintraub BD. Genomic structure and promoter analysis of the human obese gene. J Biol Chem. 1996;271:3971–3974. doi: 10.1074/jbc.271.8.3971. [DOI] [PubMed] [Google Scholar]
- 67.He Y, Chen H, Quon MJ, Reitman M. The mouse obese gene. Genomic organization, promoter activity, and activation by CCAAT/enhancer-binding protein α. J Biol Chem. 1995;270:28887–28891. doi: 10.1074/jbc.270.48.28887. [DOI] [PubMed] [Google Scholar]
- 68.Hwang CS, Mandrup S, MacDougald OA, Geiman DE, Lane MD. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein α. Proc Natl Acad Sci USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller SG, et al. The adipocyte specific transcription factor C/EBPα modulates human ob gene expression. Proc Natl Acad Sci USA. 1996;93:5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JB, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen XL, Hartzell DL, McGraw RA, Hausman GJ, Dean RG. Analysis of a 762-bp proximal leptin promoter to drive and control regulation of transgene expression of growth hormone receptor in mice. Biochem Biophys Res Commun. 1999;262:187–192. doi: 10.1006/bbrc.1999.1176. [DOI] [PubMed] [Google Scholar]
- 72.Wrann CD, Rosen ED. New insights into adipocyte-specific leptin gene expression. Adipocyte. 2012;1:168–172. doi: 10.4161/adip.20574. This review highlights the transcriptional mechanisms that regulate adipocyte-specific expression of leptin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker DJ, Ongemba LN, Brichard V, Henquin JC, Brichard SM. Diet- and diabetes-induced changes of ob gene expression in rat adipose tissue. FEBS Lett. 1995;371:324–328. doi: 10.1016/0014-5793(95)00943-4. [DOI] [PubMed] [Google Scholar]
- 74.MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1995;92:9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hardie LJ, Rayner DV, Holmes S, Trayhurn P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem Biophys Res Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. [DOI] [PubMed] [Google Scholar]
- 76.Szkudelski T. Intracellular mediators in regulation of leptin secretion from adipocytes. Physiol Res. 2007;56:503–512. doi: 10.33549/physiolres.931038. [DOI] [PubMed] [Google Scholar]
- 77.Rayner DV. The sympathetic nervous system in white adipose tissue regulation. Proc Nutr Soc. 2001;60:357–364. doi: 10.1079/pns2001101. [DOI] [PubMed] [Google Scholar]
- 78.Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats: reduced sensitivity compared with lean animals. Diabetes. 1996;45:1446–1450. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- 79.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 80.Caron A, Richard D, Laplante M. The roles of mTOR complexes in lipid metabolism. Annu Rev Nutr. 2015;35:321–348. doi: 10.1146/annurev-nutr-071714-034355. [DOI] [PubMed] [Google Scholar]
- 81.Thomou T, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishizawa Y, Bray GA. Ventromedial hypothalamic lesions and the mobilization of fatty acids. J Clin Invest. 1978;61:714–721. doi: 10.1172/JCI108984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi A, Shimazu T. Hypothalamic regulation of lipid metabolism in the rat: effect of hypothalamic stimulation on lipolysis. J Auton Nerv Syst. 1981;4:195–205. doi: 10.1016/0165-1838(81)90044-8. [DOI] [PubMed] [Google Scholar]
- 85.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–R1493. doi: 10.1152/ajpregu.1998.275.5.R1488. [DOI] [PubMed] [Google Scholar]
- 86.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 87.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 88.Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 89.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007;416:193–197. doi: 10.1016/j.neulet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Rooks CR, et al. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am J Physiol Regul Integr Comp Physiol. 2005;289:R92–R102. doi: 10.1152/ajpregu.00858.2004. [DOI] [PubMed] [Google Scholar]
- 92.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–R1621. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 93.Jaubert AM, Penot G, Niang F, Durant S, Forest C. Rapid nitration of adipocyte phosphoenolpyruvate carboxykinase by leptin reduces glyceroneogenesis and induces fatty acid release. PLoS ONE. 2012;7:e40650. doi: 10.1371/journal.pone.0040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez VM, Macarulla MT, Echevarria E, Portillo MP. Lipolysis induced by leptin in rat adipose tissue from different anatomical locations. Eur J Nutr. 2003;42:149–153. doi: 10.1007/s00394-003-0405-7. [DOI] [PubMed] [Google Scholar]
- 95.Siegrist-Kaiser CA, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100:2858–2864. doi: 10.1172/JCI119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6:1052–1065. doi: 10.1016/j.molmet.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warner A, et al. Activation of β3-adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am J Physiol Endocrinol Metab. 2016;311:E901–E910. doi: 10.1152/ajpendo.00204.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Plum L, et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Chi J, et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 2018;27:226–236.e3. doi: 10.1016/j.cmet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y, et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 2016;24:420–433. doi: 10.1016/j.cmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruan HB, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308:E1085–E1105. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 103.Foster DO. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can J Biochem Cell Biol. 1984;62:618–622. doi: 10.1139/o84-082. [DOI] [PubMed] [Google Scholar]
- 104.Labbe SM, et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015;29:2046–2058. doi: 10.1096/fj.14-266247. [DOI] [PubMed] [Google Scholar]
- 105.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. This is a comprehensive and detailed review of BAT biology. [DOI] [PubMed] [Google Scholar]
- 106.Murano I, Barbatelli G, Giordano A, Cinti S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat. 2009;214:171–178. doi: 10.1111/j.1469-7580.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bartness TJ, Song CK. Innervation of brown adipose tissue and its role in thermogenesis. Can J Diabetes. 2005;29:420–428. [Google Scholar]
- 108.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen NL, et al. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol. 2017;312:R132–R145. doi: 10.1152/ajpregu.00344.2016. This paper provides neuroanatomical and functional evidence of the existence of SNS crosstalk for thermoregulation and BeAT development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cano G, et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. This study demonstrates the central circuitry responsible for cold-induced increase in sympathetic outflow to interscapular BAT. [DOI] [PubMed] [Google Scholar]
- 111.Morrison SF, Ramamurthy S, Young JB. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J Neurosci. 2000;20:9264–9271. doi: 10.1523/JNEUROSCI.20-24-09264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu S, et al. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J Neurosci. 2016;36:5034–5046. doi: 10.1523/JNEUROSCI.0213-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fischer AW, et al. Leptin raises defended body temperature without activating thermogenesis. Cell Rep. 2016;14:1621–1631. doi: 10.1016/j.celrep.2016.01.041. This recent study suggests that leptin is pyrexic rather than thermogenic. [DOI] [PubMed] [Google Scholar]
- 114.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 115.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 116.Satoh N, et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48:1787–1793. doi: 10.2337/diabetes.48.9.1787. [DOI] [PubMed] [Google Scholar]
- 117.Haque MS, et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- 118.De Fanti BA, Milagro FI, Lamas O, Martinez-Anso E, Martinez JA. Immunomanipulation of appetite and body temperature through the functional mimicry of leptin. Obes Res. 2002;10:833–837. doi: 10.1038/oby.2002.112. [DOI] [PubMed] [Google Scholar]
- 119.Luheshi GN, Gardner JD, Rushforth DA, Loudon AS, Rothwell NJ. Leptin actions on food intake and body temperature are mediated by IL-1. Proc Natl Acad Sci USA. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dodd GT, et al. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 2014;20:639–649. doi: 10.1016/j.cmet.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collins S, et al. Role of leptin in fat regulation. Nature. 1996;380:677. doi: 10.1038/380677a0. [DOI] [PubMed] [Google Scholar]
- 122.Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R832–837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- 123.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 125.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17:599–606. doi: 10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 127.do Carmo JM, et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension. 2011;57:918–926. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scarpace PJ, Matheny M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am J Physiol. 1998;275:E259–E264. doi: 10.1152/ajpendo.1998.275.2.E259. [DOI] [PubMed] [Google Scholar]
- 129.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 130.Sarmiento U, et al. Morphologic and molecular changes induced by recombinant human leptin in the white and brown adipose tissues of C57BL/6 mice. Lab Invest. 1997;77:243–256. [PubMed] [Google Scholar]
- 131.Harris RB, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 132.Commins SP, et al. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 133.Kaiyala KJ, Ogimoto K, Nelson JT, Muta K, Morton GJ. Physiological role for leptin in the control of thermal conductance. Mol Metab. 2016;5:892–902. doi: 10.1016/j.molmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ottaway N, et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab. 2015;21:877–882. doi: 10.1016/j.cmet.2015.04.015. This study shows that the anorexigenic effects of leptin are maintained in hyperleptinaemic diet-induced obesity mice through the use of a novel leptin receptor antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R886–R900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull. 2001;54:375–385. doi: 10.1016/s0361-9230(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 137.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 138.Song CK, et al. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295:R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mercer JG, et al. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 140.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 141.Laque A, et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab. 2013;304:E999–E1011. doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 143.Ollmann MM, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 144.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 145.Elias CF, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 146.Kishi T, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 147.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 148.Begriche K, Sutton GM, Butler AA. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav. 2011;104:546–554. doi: 10.1016/j.physbeh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 150.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 151.Begriche K, Girardet C, McDonald P, Butler AA. Melanocortin-3 receptors and metabolic homeostasis. Prog Mol Biol Transl Sci. 2013;114:109–146. doi: 10.1016/B978-0-12-386933-3.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Satoh N, et al. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett. 1998;249:107–110. doi: 10.1016/s0304-3940(98)00401-7. [DOI] [PubMed] [Google Scholar]
- 153.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 154.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- 155.Voss-Andreae A, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 156.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 157.Berglund ED, et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife. 2015;4:e09800. doi: 10.7554/eLife.09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lam BYH, et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab. 2017;6:383–392. doi: 10.1016/j.molmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Williams KW, et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 2014;20:471–482. doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dodd GT, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kong D, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dodd GT, et al. A hypothalamic phosphatase switch coordinates energy expenditure with feeding. Cell Metab. 2017;26:375–393.e7. doi: 10.1016/j.cmet.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 164.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 165.Yoshida K, et al. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur J Neurosci. 2003;18:1848–1860. doi: 10.1046/j.1460-9568.2003.02919.x. [DOI] [PubMed] [Google Scholar]
- 166.Trayhurn P, Thurlby PL, James WP. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings] Proc Nutr Soc. 1976;35:133A. [PubMed] [Google Scholar]
- 167.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rezai-Zadeh K, et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014;3:681–693. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Steiner AA, et al. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 2004;18:1949–1951. doi: 10.1096/fj.04-2295fje. [DOI] [PubMed] [Google Scholar]
- 171.Xu Y, et al. Glutamate release mediates leptin action on energy expenditure. Mol Metab. 2013;2:109–115. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 173.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 174.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 175.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gautron L, Lazarus M, Scott MM, Saper CB, Elmquist JK. Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. J Comp Neurol. 2010;518:2090–2108. doi: 10.1002/cne.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Elias CF, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 179.Marsh AJ, et al. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 180.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iijima N, et al. Cytochemical study of prolactin-releasing peptide (PrRP) in the rat brain. Neuroreport. 1999;10:1713–1716. doi: 10.1097/00001756-199906030-00016. [DOI] [PubMed] [Google Scholar]
- 182.Lam DD, et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshida T, Bray GA. Catecholamine turnover in rats with ventromedial hypothalamic lesions. Am J Physiol. 1984;246:R558–R565. doi: 10.1152/ajpregu.1984.246.4.R558. [DOI] [PubMed] [Google Scholar]
- 184.Saito M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation accelerates norepinephrine turnover in brown adipose tissue of rats. Life Sci. 1987;41:193–197. doi: 10.1016/0024-3205(87)90493-0. [DOI] [PubMed] [Google Scholar]
- 185.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- 186.Holt SJ, Wheal HV, York DA. Hypothalamic control of brown adipose tissue in Zucker lean and obese rats. Effect of electrical stimulation of the ventromedial nucleus and other hypothalamic centres. Brain Res. 1987;405:227–233. doi: 10.1016/0006-8993(87)90292-7. [DOI] [PubMed] [Google Scholar]
- 187.Hugie T, Halvorson I, Thornhill J. Brown adipose tissue temperature responses following electrical stimulation of ventromedial hypothalamic and lateral preoptic areas or after norepinephrine infusion to Long Evans or Sprague-Dawley rats. Brain Res. 1992;575:57–62. doi: 10.1016/0006-8993(92)90422-6. [DOI] [PubMed] [Google Scholar]
- 188.Ruffin M, Nicolaidis S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Res. 1999;846:23–29. doi: 10.1016/s0006-8993(99)01922-8. [DOI] [PubMed] [Google Scholar]
- 189.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 190.Toda C, et al. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58:2757–2765. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- 192.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 194.Kim KW, et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci USA. 2011;108:10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hill CE, Gould DJ, Strigas J, Burcher E, Vidovic M. Sensory nerves play an efferent role in the function of the arterioles, but not the dilator muscle, of the rat iris. J Auton Nerv Syst. 1996;58:89–100. doi: 10.1016/0165-1838(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 196.Norman D, Mukherjee S, Symons D, Jung RT, Lever JD. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J Neurocytol. 1988;17:305–311. doi: 10.1007/BF01187853. [DOI] [PubMed] [Google Scholar]
- 197.De Matteis R, Ricquier D, Cinti S. TH-, NPY-, SP- and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27:877–886. doi: 10.1023/a:1006996922657. [DOI] [PubMed] [Google Scholar]
- 198.Giordano A, Morroni M, Santone G, Marchesi GF, Cinti S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J Neurocytol. 1996;25:125–136. doi: 10.1007/BF02284791. [DOI] [PubMed] [Google Scholar]
- 199.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst. 1998;73:19–25. doi: 10.1016/s0165-1838(98)00109-x. [DOI] [PubMed] [Google Scholar]
- 201.Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett. 1999;262:125–128. doi: 10.1016/s0304-3940(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 202.Murphy KT, et al. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–E1347. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shi Z, et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol. 2012;112:1008–1014. doi: 10.1152/japplphysiol.01164.2011. [DOI] [PubMed] [Google Scholar]
- 204.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–1091. [PubMed] [Google Scholar]
- 205.Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–347. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 206.Tchkonia T, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Flier JS, Maratos-Flier E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 2017;26:24–26. doi: 10.1016/j.cmet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 208.Nguyen NL, Randall J, Banfield BW, Bartness TJ. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2014;306:R375–R386. doi: 10.1152/ajpregu.00552.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 210.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol. 1995;268:R744–R751. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- 211.Pirzgalska RM, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309–1318. doi: 10.1038/nm.4422. This recent study demonstrates that sympathetic-neuron-associated macrophages can metabolize catecholamines, thus identifying a new molecular target for the treatment of obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang F, et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 2018;27:252–262.e3. doi: 10.1016/j.cmet.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


