Abstract
Objective
The challenges posed by people living with multiple chronic conditions are unique for people with dementia and other significant cognitive impairment. There have been recent calls to action to review the existing literature on co-occurring chronic conditions and dementia in order to better understand the effect of cognitive impairment on disease management, mobility, and mortality.
Methods
This systematic literature review searched PubMed databases through 2011 (updated in 2016) using key constructs of older adults, moderate-to-severe cognitive impairment (both diagnosed and undiagnosed dementia), and chronic conditions. Reviewers assessed papers for eligibility and extracted key data from each included manuscript. An independent expert panel rated the strength and quality of evidence and prioritized gaps for future study.
Results
Four thousand thirty-three articles were identified, of which 147 met criteria for review. We found that moderate-to-severe cognitive impairment increased risks of mortality, was associated with prolonged institutional stays, and decreased function in persons with multiple chronic conditions. There was no relationship between significant cognitive impairment and use of cardiovascular or hypertensive medications for persons with these comorbidities. Prioritized areas for future research include hospitalizations, disease-specific outcomes, diabetes, chronic pain, cardiovascular disease, depression, falls, stroke, and multiple chronic conditions.
Conclusions
This review summarizes that living with significant cognitive impairment or dementia negatively impacts mortality, institutionalization, and functional outcomes for people living with multiple chronic conditions. Our findings suggest that chronic-disease management interventions will need to address co-occurring cognitive impairment.
Keywords: dementia, cognitive impairment, multiple chronic conditions, systematic literature review, public health, aging
Introduction
There are currently 44 million people living with dementia worldwide (5 million in the U.S. (Hebert et al., 2013), with numbers expected to more than triple by 2050 (Prince et al., 2013). Dementia is the largest single contributor to disability and needs for care among older adults out of any chronic disease (Wimo and Prince, 2010), and total monetary costs of dementia represent a similar financial burden as heart disease and cancer (Hurd et al., 2013). Dementia has recently been identified as an important chronic condition (CC) to target for public health interventions (Machlin and Soni, 2009; Goodman et al., 2013).
Current increases in the numbers of older adults and prevalence of CCs as leading causes of death have led to the recognition of the challenges faced by people living with multiple CCs (Goodman et al., 2013). A growing body of evidence indicates a higher burden of CCs in older adults with dementia (Hill et al., 2002; Bynum et al., 2004); recent U.S. Medicare beneficiary data suggest that one in four persons with dementia have co-occurring stroke (24%) and one in three have co-occurring coronary artery disease (33%) (National Academy on an Aging Society, 2000). People in more advanced stages of dementia often have difficulty recognizing and reporting symptoms and/or side effects, adhering to medication, and complying with treatment and follow-up recommendations because of deficits in memory, language, judgment, and reasoning ability (McGuire et al., 2006; Boustani et al., 2007; Arlt et al., 2008; Punthakee et al., 2012). Older adults with dementia also have higher health care expenditures than those without impairment (Hill et al., 2002; Frytak et al., 2008; Kuo et al., 2008; Zhao et al., 2008; Marengoni et al., 2009; Suehs et al., 2013). At the same time, persons with multiple CCs consume a disproportionate number of health care (Thorpe et al., 2010; Centers for Medicare and Medicaid Services (CMMS), 2012), and more care coordination and greater self-management skills are required for those with multiple CCs (Redelmeier et al., 1998; Committee on Quality of HealthCare in America, 2001).
A better understanding is needed to better understand the relationships between dementia and co-occurring CCs from a public health standpoint. The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018 ((Alzheimer’s Association (AA) and Centers for Disease Control and Prevention (CDC), 2013) has called for “A review of the literature on co-occurring CCs and dementia, including Alzheimer’s disease, to understand the effect of dementia on various outcomes such as depression, disease management, morbidity, and mortality.” This systematic literature review examines the effects of moderate-to-severe cognitive impairment (including both diagnosed and undiagnosed dementia) on co-occurring CCs to document what is currently known and what gaps remain in the research literature.
Methods
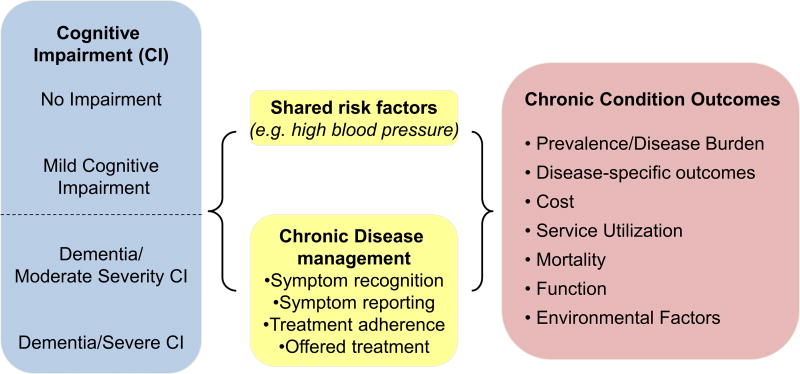
This review was guided by a multidisciplinary seven-member expert panel of health services and cognitive health researchers from around the U.S. The conceptual framework (Figure 1) was developed based on the National Institutes of Health (NIH) Cognitive and Emotional Health Project, which identified how moderate-to-severe cognitive impairment might influence co-occurring CCs through shared risk factors (such as hypertension) and/or disease management (such as symptom recognition, reporting, or treatment adherence) (NIH, 2001; Hendrie et al., 2006). Outcomes of interest include disease-specific outcomes (e.g., recurrent strokes for persons with moderate to severe cognitive impairment and a history of stroke), healthcare costs (e.g., outpatient or inpatient care costs), service utilization (e.g., hospitalizations and institutionalizations), mortality, and function.
Figure 1.
Conceptual Framework. [Colour figure can be viewed at wileyonlinelibrary.com]
Review methods were derived from the Guide to Community Preventive Services “The Guide” (Briss et al., 2000; Norris et al., 2002) and previously conducted systematic reviews (Frederick et al., 2007; Snowden et al., 2011), and align with PRISMA guidelines (Moher et al., 2009). We searched peer-reviewed literature from inception (1967) through 2011 using PubMed. Subject headings and text words reflected key constructs of older adults, moderate-to-severe cognitive impairment, and CCs (Table 1). References to meta-analyses, review papers, and included articles were also examined to identify possible articles.
Table 1.
PubMed search terms
| Construct | Search terms | |
|---|---|---|
| Older adults | Aged[mh] | |
| Aged, 80 and over[mh] | ||
| Frail Elderly[mh] | ||
| elderly[tiab] | ||
| older adults[tiab] | ||
| seniors[tiab]) | ||
| Cognitive impairment | Dementia[mh] | |
| “cognitive impairment”[tiab] | ||
| MCI[tiab] | ||
| CIND[tiab] | ||
| Chronic conditions and geriatric syndromes | Accidental falls[Majr] | Depression[Majr] |
| Hyperlipidemias[Majr] | Schizophrenia[Majr] | |
| Pulmonary disease, chronic obstructive[Majr] | Multiple sclerosis[Majr] | |
| Emphysema[Majr] | Bone diseases[Majr] | |
| Diabetes mellitus[Majr] | Chronic pain[tiab] | |
| Cardiovascular diseases[Majr] | Osteoarthritis[Majr] | |
| cardiovascular disease[tiab] | Frailty[tiab] | |
| Intracranial arterial Diseases[Majr] | Epilepsy[Majr] | |
| Carotid artery diseases[Majr] | Brain ischemia[Majr] | |
| Hypertension[tiab] | Stroke[Majr] | |
| Heart disease[tiab] | Sleep disorders[Majr] | |
| Neoplasms[Majr] | Oral health[Majr] | |
| Parkinsonian disorders[Majr] | Urinary incontinence[Majr] | |
| Asthma[Majr] | Osteoporosis[Majr] | |
| Substance-related disorders[Majr] | Comorbidity[Majr] | |
| Stress disorders, Post-traumatic[Majr] | co-morbidity[tiab] | |
| Bipolar disorder[tiab] | comorbidity[tiab] | |
| Manic depressive disorder[tiab] | Chronic disease[Majr] | |
| Study design | Cross-sectional studies[Mesh] OR cross-sectional stud*[tiab] OR cohort studies[Mesh] OR cohort stud*[tiab] OR case–control studies[Mesh] OR case–control stud*[tiab] OR longitudinal stud*[tiab] OR longitudinal stud*[tiab] OR prospective stud*[tiab] OR retrospective stud*[tiab] | |
[Mesh], Medical subject headings; [mh] is used to search a MeSH heading; [majr] is used to search a MeSH heading that is a major topic of an article; [tiab], Title or abstract.
Note: Limits of English-only studies were also set.
Inclusion/exclusion criteria
Study inclusion criteria were: (i) sample size of 100 or more; (ii) participants aged 50 and older, (iii) data on populations both with and without moderate to severe cognitive impairment; (iv) a valid and reliable measure of dementia or moderate-to-severe cognitive impairment; (v) at least one other CC or geriatric syndrome or measure of comorbidity; and (vi) a description of the prevalence or effect of cognitive impairment on co-occurring CCs.
To define cognitive impairment, the article must include a valid and reliable clinical diagnosis of dementia (e.g., the Diagnostic Statistical Manual criteria, fourth edition (DSM-IV) (American Psychiatric Association, 2000); the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for Alzheimer’s disease (McKhann et al., 1984); or the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia (Erkinjuntti, 1994) or valid and reliable measures of moderate-to-severe cognitive impairment that captured memory and at least one other cognitive domain such as executive function or language. These could include both multidimensional measures (e.g., Mini-Mental Status Examination (MMSE)) (Folstein et al., 1975) or multiple individual measures of cognitive function with at least one focused on memory. The review focused on moderate-to-severe cognitive impairment, including persons with and without official dementia diagnoses. We included the latter group given the high rate of persons without a formal diagnosis; currently, less than half of those in high income countries and less than 10% in low-income and middle-income countries have received a dementia diagnosis (Prince et al., 2013).
Using the definition of CCs as “conditions that last a year or more and require ongoing medical attention and/or limit activities of daily living (ADL’s)” (Warshaw, 2006; US DHHS, 2010) the expert panel created a list of common CCs (Table 2) from The NIH Cognitive and Emotional Health Project (Hendrie et al., 2006), the U.S. DHHS multiple chronic conditions (MCC) Strategic Framework (US DHHS, 2010), and disease categories in PubMed. Geriatric syndromes (e.g., frailty and polypharmacy) were included as well given their large impact on quality of life and disability (Inouye et al., 2007). Geriatric syndromes are conditions that are common in older adults but do not fit into discrete disease categories (Ahmed et al., 2007; Inouye et al., 2007; Chaudhry et al., 2010). To be included in this review, the article had to include people living with at least one CC condition or geriatric syndrome (Table 2), or evidence of co-morbid conditions via a valid and reliable measure of comorbidity (e.g., Charlson’s Comorbidity Index) (Charlson et al., 1987).
Table 2.
Chronic conditions and geriatric syndromes
| Chronic conditions | Geriatric syndromes |
|---|---|
| Asthma | Falls, fractures, and other injuries |
| Arthritis | Frailty |
| Bone diseases (e.g., osteoporosis) | Functional impairments |
| Brain diseases (e.g., Parkinson’s) | Polypharmacy/High-risk medications |
| Cancer | Urinary incontinence |
| Cardiovascular diseases (e.g., CAD, hypertension, and hyperlipidemias) | |
| Cerebrovascular diseases (e.g., stroke) | |
| Chronic kidney disease | |
| Chronic pain | |
| Dental problems | |
| Depression and other chronic mental illness (PTSD, schizophrenia, and bipolar) | |
| Diabetes | |
| Emphysema/COPD | |
| Neurological conditions (epilepsy and multiple sclerosis) | |
| Sleep disorders | |
| Substance abuse | |
| Risk factors if a chronic conditions is present (e.g., obesity, smoking, and gait) |
Articles included both community-based and institutionalized populations from around the globe. We excluded articles not published in English, that only used an ICD diagnosis for cognitive impairment or dementia, that defined cognitive impairment as delirium, traumatic brain injury, developmental disorders, or MCI, and that reported only reviews, commentaries, or meta-analyses.
Data collection
We used a two-step screening process to assess whether articles met inclusion criteria. We first reviewed titles and abstracts. Articles that could not be included or excluded based on abstracts were retrieved to examine the full text in a second screening phase. Screening of titles and abstracts was conducted jointly by members of the research team (LS, LB, and CC) until 80% agreement was reached; subsequent title and abstract screening was conducted separately. Articles were screened by individual reviewers. A different research team member abstracted data from accepted articles to double check whether an article met eligibility criteria. The reviewers met weekly to discuss title/abstract and article screening. The principal investigator (MS) made final determinations when questions or disagreements between reviewers could not be resolved.
Rating the evidence
We used a standardized form to systematically collect data from each article, including study design, sample size, setting, co-occurring chronic disease, outcome measures, results, and indicators of study quality (e.g., adjustment for possible confounders). Data were compiled in summary tables for evidence rating (see Appendices). We grouped articles into chronic condition-outcome pairings to categorically rate the evidence, and panelists rated the overall evidence within each group; for example, rating the evidence for how cognitive impairment impacted mortality for persons with multiple CCs, stroke, or depression.
Expert panel members rated both the quality and strength of evidence across articles in each chronic condition-outcome pairing using a qualitative approach from previous systematic literature reviews (Frederick et al., 2007; Snowden et al., 2011). Indicators of quality included sample size and generalizability, appropriate measurement of cognitive impairment and of CCs, use of multivariable analysis, and controlling specifically for age, gender, and education. For quality rating, panel members independently rated each condition-outcome pairing as “good”, “fair”, or “limited”.
Indicators of strength of evidence included the study quality and design, the number of studies in the chronic condition-outcome pairing, consistency across studies, and statistical findings. For strength of evidence ratings, the panel members independently rated each condition-outcome pairing as “strong”, “sufficient”, or “insufficient” (recording whether the insufficient rating was due to an insufficient number of at least fair quality studies or a sufficient number of available studies but inconclusive data).
Final determination of quality and strength of evidence was based on 80% agreement among panel members (i.e., agreement among six out of the seven panelists). The panel met to discuss areas of disagreement, and panel members were allowed to change their votes after the discussion; however, to reduce persuasion bias, they were not required to reach consensus.
Identifying and prioritizing gaps where evidence was rated “insufficient”
The panel also identified and prioritized the areas for which there was insufficient evidence. We used Q-sort ranking (Brown, 1996), a method for rank-ordering items, to prioritize the gaps in which panelists assigned a rank (“5”=highest priority to “1”=least priority) to each of the 42 outcomes and CCs/geriatric syndromes with insufficient evidence in order of priority for further research (“5”=highest priority to “1”=least priority). Using the Q-sort method, informants were given a limited, normally distributed allotment of 1s, 2s, 3s, 4s, and 5s to create a normal distribution of rankings.
Results
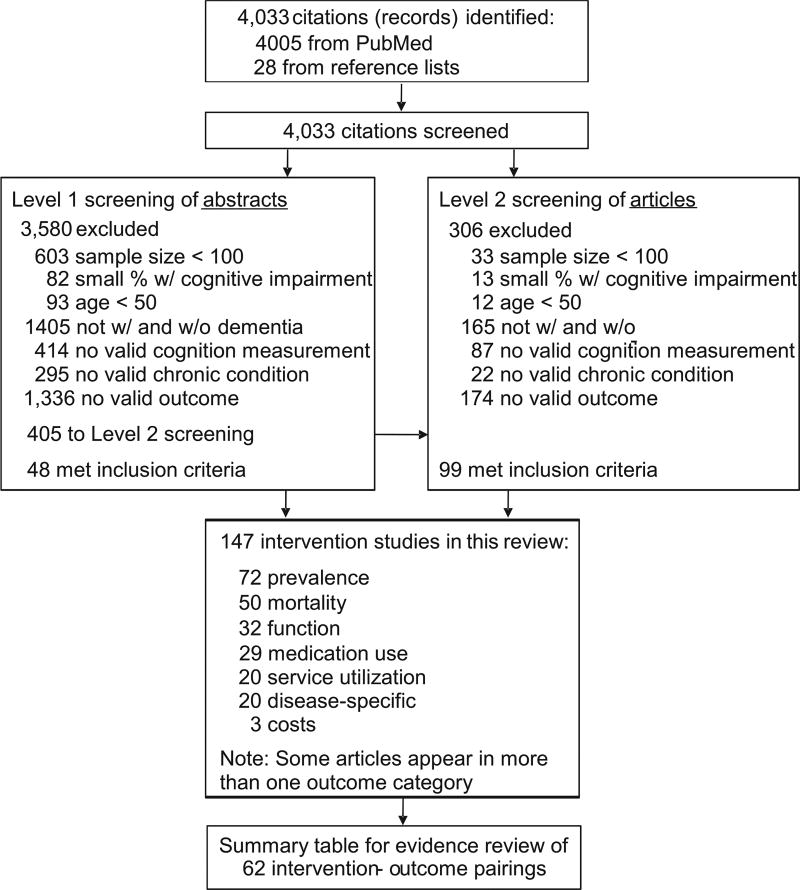
Four thousand thirty-three articles were identified in the literature review: 4,005 in the PubMed search and 28 from reference lists of review articles or meta-analyses (Figure 2). Forty-eight articles met inclusion criteria after Level 1 screening of abstracts, and 99 articles met inclusion criteria after Level 2 screening of abstracts, yielding 147 included articles. Articles were excluded primarily due to small sample size or the omission of both persons with and without dementia/cognitive impairment.
Figure 2.
Literature search flow chart.
The 147 included articles were grouped into 62 chronic condition-outcome pairings, or categories, for rating the evidence. Nine percent (N = 7) of the categories were rated as having sufficient evidence (Table 3); all of which were rated at least fair quality. Sufficient evidence was found for the impact of moderate-to-severe cognitive impairment on mortality, length of stay in institutional settings, function, and cardiovascular medication use, for persons with certain co-occurring CCs. The other pairings were deemed to have insufficient evidence, due to lack of studies (two or fewer) or inconclusive evidence (mixed results within or across studies) (Table 4).
Table 3.
Summary of evidence rating for chronic condition-outcome pairings with sufficient evidence
CVD, cardiovascular disease; MCC, multiple chronic conditions; NH, nursing home.
Some of the 147 studies are listed in more than one chronic condition-outcome pairing. Some articles include both community and clinical populations.
Quality ratings included Good, Fair, and Limited. Effectiveness ratings included Strong, Sufficient, and Insufficient. Seven expert panelists rated quality and effectiveness individually and then met to reach consensus.
CVD medications includes those besides anti-hypertensives.
Table 4.
Summary of evidence rating for chronic condition-outcome pairings with insufficient evidence due to not enough studies. Categories with insufficient evidence due to mixed or inconclusive findings are listed in italics)
| Outcome | Chronic condition | # of studies | Sample size | Author, Year |
|---|---|---|---|---|
| Mortality | ||||
| Mortality | Depression | 4 | 3,283 | Arfken et al., 1995; Lavretsky et al., 2010; Millán-Calenti et al., 2011; St. John and Montgomery, 2009 |
| Mortality | Cancer | 2 | 3,278 | Robb et al., 2010; Roe et al., 2010 |
| Mortality | Atrial Fib | 1 | 2,837 | Miyasaka et al., 2007 |
| Mortality | CHF | 1 | 142 | Haydar et al., 2004 |
| Mortality | COPD | 1 | 134 | Antonelli-Incalzi et al., 2006 |
| Mortality | Hip fracture | 1 | 558 | Cree et al., 2000 |
| Mortality | Hypertension | 1 | 2,496 | Gombojav et al., 2011 |
| Service utilizations/institutionalizations | ||||
| Hospitalizations | MCC | 3 | 13,334 | Feil et al., 2003; McCormick et al., 2001; Welmerink et al., 2010 |
| Hospitalizations | Cancer | 1 | 3,020 | Roe et al., 2010 |
| Hospitalizations | CHD | 2 | 7,188 | Bursi et al., 2006; Welmerink et al., 2010 |
| Hospitalizations | Diabetes | 1 | 789 | Sinclair et al., 2000 |
| Length of stay, hospital | MCC | 2 | 1,343 | Lang et al., 2006; Zekry et al., 2009 |
| Other medical care | MCC | 2 | 779 | McCormick et al., 1994; McCormick et al., 2001 |
| Other medical care | Cancer | 1 | 258 | Robb et al., 2010 |
| Other medical care | Diabetes | 2 | 1,188 | Quinn et al., 2009; Sinclair et al., 2000 |
| Other medical care | Myocardial infarction | 1 | 1,832 | Bursi et al., 2006 |
| Institutionalization/NH | MCC | 4 | 2,361 | Guhne et al., 2006; Meerman et al., 2008; Smith et al., 2000; Zekry et al., 2009 |
| Institutionalization/NH | Diabetes | 1 | 789 | Sinclair et al., 2000 |
| Institutionalization/NH | Hip fracture | 1 | 558 | Cree et al., 2000 |
| Institutionalization/NH | Parkinson’s disease | 1 | 178 | Parashos et al., 2002 |
| Length of stay, institution/NH | Diabetes | 1 | 399 | Quinn et al., 2009 |
| Amount of care at institutions | MCC | 1 | 198 | Lyketsos et al., 2007 |
| Home care | MCC | 1 | 435 | Zekry et al., 2009 |
| Home care | Diabetes | 1 | 789 | Sinclair et al., 2000 |
| Caregiver burden | MCC | 1 | 198 | Lyketsos et al., 2007 |
| Medicare qualified stay | Diabetes | 1 | 399 | Quinn et al., 2009 |
| Hospice | CHF | 1 | 142 | Haydar et al., 2004 |
| Advanced medical planning | CHF | 1 | 142 | Haydar et al., 2004 |
| Use of social services | Diabetes | 1 | 789 | Sinclair et al., 2000 |
| Costs | ||||
| Cost | MCC | 2 | 1.101 | McCormick et al., 2001; Scuvée-Moreau et al., 2002 |
| Cost | Diabetes | 1 | 399 | Quinn et al., 2009 |
| Medications | ||||
| Medications-polypharmacy | MCC | 7 | 8,527 | Doruk et al., 2010; Lopponen et al., 2005; Lyketsos et al., 2005; Lyketsos et al., 2007; Millán-Calenti et al., 2011; Wang et al., 2010b; Zekry et al., 2008 |
| Medications-polypharmacy | Falls | 3 | 411 | Eriksson et al., 2008; Morris et al., 1987; Van Iersel et al., 2006 |
| High-risk medications | Falls | 4 | 590 | Allan et al., 2009; Eriksson et al., 2008; Morris et al., 1987; Van Iersel et al., 2006 |
| Antidepressants | Depression | 2 | 3,228 | Blazer et al., 2005; Janzing et al., 2000 |
| Diabetes medications | Diabetes | 2 | 1,886 | Okura et al., 2009; Sinclair et al., 2000 |
| Osteoporosis medications | Hip and other fractures | 1 | 15,718 | Vik et al., 2007 |
| Anti Parkinson’s medications | Parkinson’s disease | 1 | 130 | Aarsland et al., 2001a |
| Part D Medicare coverage | MCC | 1 | 13,160 | Zivin et al., 2009 |
| Disease-specific | ||||
| Disease-specific | MCC | 6 | 7,940 | Eriksson et al., 2008; García-Lara et al., 2010; Magaziner et al., 2005; Rothman et al., 2008; Sambrook et al., 2007; Wang et al., 2010c |
| Disease-specific | Cancer | 1 | 258 | Robb et al., 2010 |
| Disease-specific | Falls | 4 | 1,723 | Chen et al., 2005; Chen et al., 2010; Fleming and Brayne, 2008; van Iersel et al., 2006 |
| Disease-specific | Depression | 1 | 121 | Janzing et al., 2000 |
| Disease-specific | CVD | 1 | 1,832 | Bursi et al., 2006 |
| Disease-specific | Diabetes | 1 | 1,097 | Okura et al., 2009 |
| Disease-specific | Hypertension | 1 | Vinyoles et al., 2008 | |
| Disease-specific | Parkinson’s disease | 3 | 555 | Aarsland et al., 2001a, 2001b; Melton et al., 2006 |
| Disease-specific | Stroke | 3 | Desmond et al., 1998; Harris et al., 1994; Melkas et al., 2009 | |
| Function | ||||
| Function | CVD | 3 | 852 | Freels et al., 2002; Haydar et al., 2004; Lopponen et al., 2005 |
| Function | Depression | 3 | 3,517 | Feng et al., 2010; Fuhrer et al., 1992a, 1992b; Millán-Calenti et al., 2011 |
| Function | Diabetes | 3 | 2,285 | Okura et al., 2009; Quinn et al., 2009; Sinclair et al., 2000 |
| Function | Falls | 3 | 490 | Allan et al., 2009; Eriksson et al., 2008; van Iersel et al., 2006 |
| Function | Stroke | 2 | 303 | Harris et al., 1994; Stott et al., 2001 |
| Function | Cancer | 1 | 258 | Robb et al., 2010 |
| Function | Hypertension | 1 | 782 | Huang et al., 2009 |
CHF, congestive heart failure; CVD, cardiovascular disease; NC, no consensus; MCC, multiple chronic conditions; NH, nursing home.
Article citations for each chronic condition-outcome pairing are available by contacting the corresponding author. Some of the 147 studies are listed in more than one chronic condition-outcome pairing.
At least six expert panelists rated insufficient evidence due to not enough studies (typically 2 or less). Categories with mixed evidence are presented in italics.
Some studies are described in more then one article (e.g., References 132 and 133 describe the same study)
Mortality
Twenty-nine studies (Kukull et al., 1994; Arfken et al., 1995; Bruce et al., 1995; Gale et al., 1996; Agüero-Torres et al., 1999; Foley et al., 1999; Kammoun et al., 2000; Helmer et al., 2001; Stump et al., 2001; Freels et al., 2002; Ganguli et al., 2002; Feil et al., 2003; Nguyen et al., 2003; Tschanz et al., 2004; Cacciatore et al., 2005; Fitzpatrick et al., 2005; Magaziner et al., 2005; Bursi et al., 2006; Guhne et al., 2006; Llinàs-Regla et al., 2007; Lyketsos et al., 2007; Meerman et al., 2008; Rothman et al., 2008; Zekry et al., 2009; Lavretsky et al., 2010; Wang et al., 2010a; Gombojav et al., 2011; Millán-Calenti et al., 2011; Nikolova et al., 2011) compared mortality rates for persons with multiple CCs and with and without moderate-to-severe cognitive impairment or dementia. Seventeen used community-based samples and 12 used clinic-based samples. Most studies had a sample size greater than 500 (79%) with 52,644 subjects across all studies. A majority of studies used a multivariable analysis (90%) and almost half of these (46%) adjusted for age, gender, and education (the remaining studies adjusted for just one or two of these demographic covariates).
Mortality was measured in terms of mortality rates, risk for mortality (e.g., hazard ratios (HRs)), or survival rates. Percent mortality ranged from 40.2% to 82% for persons with moderate-to-severe cognitive impairment and 16.0% to 37.7% for persons without significant cognitive impairment (Stump et al., 2001; Nguyen et al., 2003; Tschanz et al., 2004; Guhne et al., 2006; Llinàs-Regla et al., 2007; Lavretsky et al., 2010; Wang et al., 2010a). Six studies (Bruce et al., 1995; Stump et al., 2001; Fitzpatrick et al., 2005; Llinàs-Regla et al., 2007; Meerman et al., 2008; Lavretsky et al., 2010) reported unadjusted relative risks (RR) for mortality (95% confidence interval (CI)), ranging from 2.3 (1.7–3.2) (Llinàs-Regla et al., 2007) to 7.4 (4.9–11.4) (Lavretsky et al., 2010).
Fourteen studies (Helmer et al., 2001; Stump et al., 2001; Freels et al., 2002; Feil et al., 2003; Nguyen et al., 2003; Tschanz et al., 2004; Fitzpatrick et al., 2005; Bursi et al., 2006; Guhne et al., 2006; Llinàs-Regla et al., 2007; Meerman et al., 2008; Rothman et al., 2008; Lavretsky et al., 2010; Gombojav et al., 2011) reported the adjusted HR for significant cognitive impairment on risk of mortality (95% CI) ranging from 1.5 (1.1–2.1) (Rothman et al., 2008) to 2.99 (2.53–3.53) (Tschanz et al., 2004); covariates may have included demographics, CCs, and six other frailty criteria (Rothman et al., 2008). The percent attributable risk (PAR%) of death related to dementia diagnosis ranged from 11.8% (Guhne et al., 2006; Llinàs-Regla et al., 2007) to 16.6% (Tschanz et al., 2004). Tschanz (Tschanz et al., 2004) and Feil (Feil et al., 2003) compared mortality RR and PAR for dementia versus other CCs: In addition, two studies (Feil et al., 2003; Tschanz et al., 2004) found that dementia or moderate-to-severe cognitive impairment had a greater RR of mortality than other CCs (e.g., an RR two to three times higher) and higher mortality PAR% than other CCs.
In addition, persons with moderate-to-severe cognitive impairment had shorter average survival times, ranging from 3.0 to 7.1years compared with 4.0 to 11.0years for persons without cognitive impairment (Agüero-Torres et al., 1999; Fitzpatrick et al., 2005; Guhne et al., 2006; Lavretsky et al., 2010; Wang et al., 2010a). Persons with moderate-to-severe cognitive impairment also had lower survival rates: the adjusted 5-year survival for persons with moderate-to-severe cognitive impairment was 16.1% versus 28.5% for normal cognition (Wang et al., 2010a); unadjusted survival rates were 22% versus 39% (Bursi et al., 2006) and lower (poorer) scores on the MMSE decreased likelihood of survival (Bruce et al., 1995).
Several studies reported non-significant or negative relationships. For example, in 2005, Magaziner et al., (2005) reported an unadjusted RR for mortality of 0.61 (0.53–0.71) and adjusted RR for mortality of 0.63 (0.51–0.77) for persons with dementia and multiple CCs. This study included persons living in nursing homes (N = 2,153, mean age > 80), almost half of which were diagnosed with dementia using DSM criteria. In this study, the group without dementia included people who were difficult to diagnose.
The expert panel also found sufficient evidence that moderate-to-severe cognitive impairment increases mortality in persons with Parkinson’s disease (Ebmeier et al., 1990; Marder et al., 1991; Mitchell and Rockwood, 2000; Levy et al., 2002; Parashos et al., 2002; Buter et al., 2008; Lo et al., 2009; Hobson et al., 2010, N = 4,513) and stroke (Tatemichi et al., 1994; Desmond et al., 1998; Desmond et al., 2002; Liebetrau et al., 2003; Melkas et al., 2009; Oksala et al., 2009, N = 2,302). These were the only specific co-occurring CCs for which sufficient evidence was found to examine mortality outcomes. Other articles included people with dementia or moderate-to-severe cognitive impairment from various etiologies (stroke, Parkinson’s disease, and other).
Service utilization
Three studies (Smith et al., 2000; Magaziner et al., 2005; Rothman et al., 2008, (N = 3,423) examined length of stay in an institution for persons with multiple CCs (Inouye et al., 2007). In 2008, Rothman et al., (2008) reported an adjusted HR for long-term nursing home stay of 3.7 (95% CI) 2.5–5.4) for persons with cognitive impairment (MMSE < 24) versus those without impairment. In 2000, Smith et al., (2000) found an unadjusted median length of stay in nursing homes for those with dementia (diagnosed using DSM-III-R criteria) of 946 days compared with those without dementia at 579days. Lastly, Magaziner and colleagues reported a decreased likelihood of being discharged to home (adjusted RR of 0.23 (95% CI 0.17–0.31)) for those with dementia (DSM-III-R criteria) compared with those without.
Function
Nineteen studies (Agüero-Torres et al., 1998; Zhu et al., 1998; Agüero-Torres et al., 2002; Covinsky et al., 2003; Lyketsos et al., 2005; Magaziner et al., 2005; Lyketsos et al., 2007; Cankurtaran et al., 2008; Rothman et al., 2008; Zekry et al., 2008; Huang et al., 2009; Sousa et al., 2009; Stewart et al., 2009; Zekry et al., 2009; Feng et al., 2010; Wang et al., 2010b; Gombojav et al., 2011; Millán-Calenti et al., 2011; Nikolova et al., 2011) (N = 37,466) examined the impact of significant cognitive impairment and co-occurring multiple comorbidities on function. Function was typically defined as basic activities of daily living (ADL’s) or instrumental ADL’s (IADLs). Nine studies included samples with a dementia diagnosis and seven included institutionalized persons. Half of the studies used multivariable analysis, with half of these adjusting for age, sex, and education. Persons with dementia and multiple CCs had significantly more IADL and ADL impairments than those without significant cognitive impairment.
Medication use
Seven studies (Freels et al., 2002; Lopponen et al., 2005; Bursi et al., 2006; Rastas et al., 2007; Andersson et al., 2008; Barzilay et al., 2008; Cankurtaran et al., 2008) (N = 5,245) examined the degree to which dementia affected cardiovascular medication use (other than that for hypertension) in persons with co-occurring cardiovascular disease (CVD). Only one (Barzilay et al., 2008) found statistically significant differences between people with dementia (VaD, AD, and not otherwise specified) and use of CVD medications such as beta-blockers, ACE inhibitors, or aspirin.
In addition, the expert panel agreed on sufficient evidence that moderate-to-severe cognitive impairment had no impact on hypertension medication use in people with co-occurring hypertension (Freels et al., 2002; Hanon et al., 2006; Barzilay et al., 2008; Vinyoles et al., 2008; Huang et al., 2009; Stewart et al., 2009; Gombojav et al., 2011) with five of the seven studies (Freels et al., 2002; Hanon et al., 2006; Huang et al., 2009; Stewart et al., 2009; Gombojav et al., 2011) (N = 8,236) reporting no statistically significant differences for persons with and without moderate-to-severe cognitive impairment.
Prioritizing the gaps in the evidence
The expert panel identified 55 pairings with insufficient or no evidence (Table 4). These included 18 outcomes (e.g., cost and function) and 10 CCs or geriatric syndromes (e.g., cancer and chronic obstructive pulmonary disease (COPD)) with insufficient evidence, and no evidence was found for the impact of significant cognitive impairment on 14 additional conditions/syndromes: asthma, arthritis, bone diseases, chronic kidney disease, chronic pain, dental problems, other chronic mental illness, neurological conditions, sleep disorders, substance abuse, risk factors if another CC was present (e.g., obesity and smoking), frailty, functional impairments, and urinary incontinence.
After the expert panel prioritized these gaps, the following were identified as highest priority for future research: service utilization-hospitalizations and disease-specific outcomes, and diabetes, chronic pain, CVD, depression, falls or fractures, stroke, and multiple CCs (the presence of moderate-to-severe cognitive impairment and more than two other CCs).
Updating the review
We were recently asked to update the literature review to include more recent articles published between mid-2011 and June of 2016. Twenty-two additional articles met our criteria and two new chronic condition-outcome pairings had sufficient evidence. The first looked at the impact of cognitive impairment and co-occurring CVD on mortality. Two studies (O’Donnell et al., 2012 and Huijts et al., 2013) found an over 50% increase in mortality risk for people living with moderate-to-severe cognitive impairment (adjusted HRs 1.53 (95% CI 1.02–2.30) to 1.68 (1.49–1.90)). The second new outcome was disease-specific; namely that living with dementia and diabetes increased the risk for severe hypoglycemia compared with persons living with diabetes and without dementia (Abbatecola et al., 2015; Prinz et al., 2015). Fourteen new articles (Chang et al., 2012; Chen et al., 2014; Huijts et al., 2013; Llibre et al., 2014; Katsoulis et al., 2014; Lee et al., 2015; Mignardot et al., 2014; Murao et al., 2014; O’Donnell et al., 2012; Park et al., 2016; Sanyal et al., 2014; Warchol-Celinska et al., 2015; van Asch et al., 2013) fell into existing chronic condition-outcome pairings and did not change the quality or strength of the evidence, and four articles (Hawkins et al., 2012; Jacobs et al., 2012; Hajduk et al., 2013; Provencher et al., 2015) were found in separate areas where there was previously no evidence.
Discussion
This review found sufficient evidence that moderate-to-severe cognitive impairment (including both diagnosed and undiagnosed dementia) in the presence of co-occurring CCs had a significant effect on increasing mortality, increasing length of stay in institutional settings, and decreasing function. In addition, there was sufficient evidence that moderate-to-severe cognitive impairment was not associated with cardiovascular or hypertension medication use. For all other outcome-chronic condition pairings, the panel found insufficient evidence or no evidence.
Previous studies in older adults have identified multiple risk factors for co-occurring cognitive decline and other CCs such as CVD (NIH, 2001). Comparisons between older adults with significant cognitive impairment and those without suggest that the former tend to be sicker and more likely to suffer from multiple CCs and greater functional impairment (Hill et al., 2002; Bynum et al., 2004; Frytak et al., 2008; Kuo et al., 2008; Zhao et al., 2008; Marengoni et al., 2009). The current review partially confirmed these earlier findings (specifically for risks of mortality, institutional length of stay, and decreased function) and further affirmed that significant cognitive impairment itself may have an impact on the subsequent development of other CCs and syndromes in older adults. Most of the sufficient evidence found in this review was for moderate-to-severe cognitive impairment and multiple CCs; there was insufficient evidence to support a relationship between significant cognitive impairment and course of specific CCs (e.g., depression).
Moderate-to-severe cognitive impairment (including both diagnosed and undiagnosed dementia) is associated with clinical deterioration. Significant cognitive impairment also makes it more likely that individuals will not recognize or respond appropriately to early symptoms of CCs, geriatric syndromes, and decreasing function. Greater severity of illness at diagnosis and the inability to maintain medication regimens and management of side effects may lead, at least in part, to the increased costs of health care for older adults with significant cognitive impairment compared with those without (Gutterman et al., 1999). Policies and procedures that improve surveillance among those with significant cognitive impairment may encourage timely diagnosis and treatment that with sufficient support can reduce severity and promote improved health. In addition, the assessment of cognitive abilities in those with multiple CCs would potentially assist health care providers in improved disease management. Although it is perhaps reassuring that difficulty managing medications does not appear to affect access to them, affected individuals may need additional supports to maintain control over their conditions.
Reviewing existing evidence and identifying gaps is the first step toward assisting clinicians, investigators, and policy makers at international, national, and local levels to understand the effects of significant cognitive impairment on CCs, and then design and deliver relevant evidence-based programs for disease management. Several national entities such as the NIH’s Cognitive and Emotional Health Project (NIH, 2001; Hendrie et al., 2006) and the CDC’s Healthy Brain Initiative (CDC and the AA, 2007; AA and CDC, 2013) emphasize the identification and use of existing datasets to answer these important research gaps. Our research team and advisory committee created an inventory of existing major datasets that include measures of both cognitive impairment and CCs (Bell et al., 2015); these datasets can be used to conduct secondary data analyses to address research gaps identified by our review team (e.g., Snowden et al, 2015). Findings from this review can also inform Healthy People 2020 objectives’ (US DHHS ODPHP, 2010) to increase the proportion of older adults with one or more CCs who report confidence in managing their conditions, and to decrease the numbers of preventable hospitalizations (many for co-occurring CCs) for people living with Alzheimer’s disease and other dementias.
The strengths of this systematic review are its focus on the effects of significant cognitive impairment on CCs, the use of a formal process derived from the Guide to Community Preventive Services (Briss et al., 2000; Norris et al., 2002), an experienced study team (Frederick et al., 2007; Snowden et al., 2011), and input from a multidisciplinary expert panel to identify relevant studies, assess quality, and summarize the evidence. There is far more literature to date that looks at how CCs impact moderate-to-severe cognitive impairment. In practice, it is important to consider both, or bidirectionality (Mercer et al., 2012)-that physical conditions can affect mental conditions, and vice versa. Concordant conditions, such as stroke and cognitive impairment, are those in which management of one of these conditions is likely to impact management for the other CC.
This review has several limitations. While we aligned with most PRISMA recommended guidelines (Moher et al., 2009), we did not assess for risk of bias within and across studies. We did provide our expert panel with details on study design, sample, measures, analysis, and outcomes in order to inform their ratings of the strength and quality of evidence. Second, we reviewed only one database (PubMed) due to limitations on time and resources to conduct the review. This database was chosen given our focus on the public health implications of addressing both cognitive impairment and co-occurring CCs together. In addition, we recognize the complexity of including function both as a key outcome of interest and a geriatric syndrome, in light of functional impairment being part of the criteria for a dementia diagnosis. While complicated, function (and functional impairments) was viewed as important outcomes of interest given their significant impact on chronic disease management which in turn impacts other outcomes such as mortality and service utilization. Similarly, because Parkinson’s disease and stroke are established causes of dementia with their own significant mortality risk, our finding of increased mortality in these conditions may be expected. Finally, our exclusion criteria, in particular the exclusion of MCI as a potential contributor to other CCs, may have eliminated potentially informative studies, but the expert panel found the lack of an established definition or screening criteria too vague for its evaluation as a contributing factor.
Key findings from this review include the significant impact of cognitive impairment on mortality, service utilization, and functional outcomes for people living with co-occurring CCs, and that there was not strong evidence to support the association between moderate-to-severe cognitive impairment and medication use for co-occurring CCs. In addition, this review identified and prioritized gaps in the evidence to help guide future research including secondary data analyses. The results of this systematic review and the identification of gaps in the literature will strengthen the ability of clinicians, researchers, and policy makers to respond to the growing burden of CCs in older adults attributable to increasing rates of cognitive impairment.
Supplementary Material
Key points.
Living with significant cognitive impairment and co-occurring CCs is an important issue for public health in aging society.
Little is known about how dementia and other significant cognitive impairment impacts morbidity, mortality, and other outcomes for people with multiple CCs.
This systematic review found sufficient evidence that moderate to severe cognitive impairment (including dementia) increased risks of mortality, was associated with prolonged institutional stays, and decreased function in persons with multiple CCs. There was no relationship between significant cognitive impairment and use of cardiovascular or hypertensive medications for persons with these comorbidities.
Further study is needed to better understand how dementia and other significant cognitive impairment influences hospitalizations, disease-specific outcomes, diabetes, chronic pain, CVD, depression, falls, and stroke for people living with multiple CCs.
Acknowledgments
This research was funded by the Centers for Disease Control and Prevention’s (CDC) Healthy Aging Program through the CDC Prevention Research Centers Program, Special Interest Project grant (U48-DP000050) to the University of Washington Health Promotion Research Center. Special thanks to Lucinda L. Bryant, PhD, MSHA, and Catherine Copeland, MPA for their contributions to the literature search and data abstraction, to Angie Deokar for grant management, and to Oejin Shin for her work preparing the manuscript. Please contact the corresponding author for further information about categories not presented or for detailed summary data tables. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC. Please contact the corresponding author for copies of the underlying research materials related to our paper (e.g., detailed summary data tables, article abstraction instruments).
Footnotes
Conflict of Interest
None declared.
Author contributions
M. Snowden led the systematic literature review, including finalizing the design and methods for article screening, abstraction and rating; participated on the expert panel; provided oversight for the updated literature review; and wrote, reviewed and edited the manuscript.
L. Steinman coordinated and conducted the article screening and abstraction for the literature review; prepared summary data tables with key data from included studies; coordinated the rating of the evidence by the expert panel; coordinated the update to the literature review; and wrote, reviewed, and edited the manuscript.
L. Bryant conducted the article screening and abstraction for the literature review; participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature; and reviewed and edited the manuscript.
M. Cherrier participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature, and reviewed and edited the manuscript.
K. Greenlund participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature, and reviewed and edited the manuscript.
K. Leith participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature, and reviewed and edited the manuscript.
C. Levy participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature, and reviewed and edited the manuscript.
R. Logsdon participated on the expert panel that advised on the design of the literature review (including the inclusion and exclusion criteria), reviewed the summary tables of key data from included studies, rated the strength and quality of the evidence, prioritized the gaps in the literature, and reviewed and edited the manuscript.
C. Copeland conducted the article screening and abstraction for the literature review; prepared summary data tables with key data from included studies; and reviewed and edited the manuscript.
M. Vogel conducted the article screening and abstraction for the updated literature review; prepared summary data tables with key data from included studies; and reviewed and edited the manuscript.
L. Anderson advised the literature review design, methods and evidence rating; and wrote, reviewed and edited the manuscript.
D. Atkins advised the research team on the literature review design, methods and evidence rating; and reviewed and edited the manuscript.
J. Bell advised the research team on the literature review design, methods and evidence rating; and reviewed and edited the manuscript.
Additional supporting information may be found in the online version of this article at the publisher’s web site.
References
- Aarsland D, Andersen K, Larsen JP, et al. Risk of dementia in Parkinson’s disease a community-based, prospective study. Neurology. 2001a;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001b;16:528–536. doi: 10.1002/gps.389. [DOI] [PubMed] [Google Scholar]
- Abbatecola AM, Bo M, Barbagallo M, et al. Severe hypoglycemia is associated with antidiabetic oral treatment compared with insulin analogs in nursing home patients with type 2 diabetes and dementia: results from the DIMORA study. J Am Med Dir Assoc. 2015;16(4):349.e7–349.e12. doi: 10.1016/j.jamda.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Agüero-Torres H, Fratiglioni L, Guo Z, et al. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88:1452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol. 1999;52:737–743. doi: 10.1016/s0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Agüero-Torres H, Thomas VS, Winblad B, Fratiglioni L. The impact of somatic and cognitive disorders on the functional status of the elderly. J Clin Epidemiol. 2002;55:1007–1012. doi: 10.1016/s0895-4356(02)00461-4. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120:748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One. 2009;4:e5521. doi: 10.1371/journal.pone.0005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (AA) and Centers for Disease Control and Prevention (CDC) The Healthy Brain Initiative: The Public Health Road Map for State and National Partnerships, 2013–2018. Chicago, IL: Alzheimer’s Association [online]; 2013. [Accessed November 24 2013]. Available at http://www.alz.org/publichealth/downloads/2013-roadmap.pdf/ [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th ed., text rev. edn. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andersson M, Hansson O, Minthon L, Ballard CG, Londos E. The period of hypotension following orthostatic challenge is prolonged in dementia with Lewy bodies. Int J Geriatr Psychiatry. 2008;23:192–198. doi: 10.1002/gps.1861. [DOI] [PubMed] [Google Scholar]
- Antonelli-Incalzi R, Corsonello A, Pedone C, et al. Drawing impairment predicts mortality in severe COPD. Chest. 2006;130:1687–1694. doi: 10.1378/chest.130.6.1687. [DOI] [PubMed] [Google Scholar]
- Arfken CL, Lichtenberg PA, Tancer ME. Cognitive impairment and depression predict mortality in medically ill older adults. J Gerontol A Biol Sci Med Sci. 1995;54:152–156. doi: 10.1093/gerona/54.3.m152. [DOI] [PubMed] [Google Scholar]
- Arlt S, Lindner R, Rösler A, von Renteln-Kruse W. Adherence to medication in patients with dementia: predictors and strategies for improvement. Drugs Aging. 2008;25:1033–1047. doi: 10.2165/0002512-200825120-00005. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis. 2008;52:216–226. doi: 10.1053/j.ajkd.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JF, Fitzpatrick AL, Copeland C, et al. Existing data sets to support studies of dementia or significant cognitive impairment and comorbid chronic conditions. Alzheimers Dement. 2015;11:622–38. doi: 10.1016/j.jalz.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Hybels CF, Fillenbaum GG, Pieper CF. Predictors of antidepressant use among older adults: have they changed over time? Am J Psychiatry. 2005;162:705–710. doi: 10.1176/appi.ajp.162.4.705. [DOI] [PubMed] [Google Scholar]
- Boustani M, Schubert C, Sennour Y. The challenge of supporting care for dementia in primary care. Clin Interv Aging. 2007;2:631–636. doi: 10.2147/cia.s1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briss P, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services—methods. Am J Prev Med. 2000;18:35–43. doi: 10.1016/s0749-3797(99)00119-1. [DOI] [PubMed] [Google Scholar]
- Brown SR. Q methodology and qualitative research. Qual Health Res. 1996;6:561–567. [Google Scholar]
- Bruce ML, Hoff RA, Jacobs SC, Leaf PJ. The effects of cognitive impairment on 9-year mortality in a community sample. J Gerontol B Psychol Sci Soc Sci. 1995;50:289–296. doi: 10.1093/geronb/50b.6.p289. [DOI] [PubMed] [Google Scholar]
- Bursi F, Rocca WA, Killian JM, et al. Heart disease and dementia: a population-based study. Am J Epidemiol. 2006;163:135–141. doi: 10.1093/aje/kwj025. [DOI] [PubMed] [Google Scholar]
- Buter TC, van den Hout A, Matthews FE, et al. Dementia and survival in Parkinson disease a 12-year population study. Neurology. 2008;70:1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- Bynum JP, Rabins PV, Weller W, et al. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52:187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- Cacciatore F, Abete P, de Santis D, et al. Mortality and blood pressure in elderly people with and without cognitive impairment. Gerontology. 2005;51:53–61. doi: 10.1159/000081436. [DOI] [PubMed] [Google Scholar]
- Cankurtaran M, Yavuz BB, Cankurtaran ES, et al. Risk factors and type of dementia: vascular or Alzheimer? Arch Gerontol Geriatr. 2008;47:25–34. doi: 10.1016/j.archger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) and the Alzheimer’s Association (AA) The Healthy Brain Initiative: A National Public Health Road Map to Maintaining Cognitive Health. Chicago, IL: Alzheimer’s Association; 2007. [Accessed December 20 2013]. [on-line]. Available at http://www.cdc.gov/aging/pdf/thehealthybraininitiative.pdf/ [Google Scholar]
- Centers for Medicare and Medicaid Services (CMMS) Chronic Conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Baltimore, MD: 2012. [Accessed November 14 2013]. [online]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/2012Chartbook.pdf/ [Google Scholar]
- Chang SS, Chen S, McAvay GJ, Tinetti ME. Effect of coexisting chronic obstructive pulmonary disease and cognitive impairment on health outcomes in older adults. J Am Geriatr Soc. 2012;60(10):1839–1846. doi: 10.1111/j.1532-5415.2012.04171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry SI, McAvay G, Ning Y, et al. Geriatric impairments and disability: the cardiovascular health study. J Am Geriatr Soc. 2010;58:1686–1692. doi: 10.1111/j.1532-5415.2010.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, March LM, Schwarz J, et al. A multivariate regression model predicted falls in residents living in intermediate hostel care. J Clin Epidemiol. 2005;58:503–508. doi: 10.1016/j.jclinepi.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Chen XL, Liu YH, Chan DK, Shen Q, Van Nguyen H. Characteristics associated with falls among the elderly within aged care wards in a tertiary hospital: a retrospective. Chin Med J (Engl) 2010;123:1668–1672. [PubMed] [Google Scholar]
- Chen R, Hu Z, Wei L, Wilson K. Socioeconomic status and survival among older adults with dementia and depression. Br J Psychiatry. 2014;204(6):436–440. doi: 10.1192/bjp.bp.113.134734. [DOI] [PubMed] [Google Scholar]
- Committee on Quality of HealthCare in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- Covinsky KE, Eng C, Lui LY, Sands LP, Yaffe K. The last 2 years of life: functional trajectories of frail older people. J Am Geriatr Soc. 2003;51(4):492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- Cree M, Soskolne CL, Belseck E, et al. Mortality and institutionalization following hip fracture. J Am Geriatr Soc. 2000;48:283–288. doi: 10.1111/j.1532-5415.2000.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Sano M, Stern Y. Mortality in patients with dementia after ischemic stroke. Neurology. 2002;59:537–543. doi: 10.1212/wnl.59.4.537. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Bagiella E, Sano M, Stern Y. Dementia as a predictor of adverse outcomes following stroke an evaluation of diagnostic methods. Stroke. 1998;29:69–74. doi: 10.1161/01.str.29.1.69. [DOI] [PubMed] [Google Scholar]
- Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, alzheimer’s disease, and vascular dementia in elderly. J Nutr Health Aging. 2010;14:834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- Ebmeier KP, Calder SA, Crawford JR, et al. Parkinson’s disease in Aberdeen: survival after 3.5 years. Acta Neurol Scand. 1990;81:294–299. doi: 10.1111/j.1600-0404.1990.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Gustafson Y, Lundin-Olsson L. Risk factors for falls in people with and without a diagnose of dementia living in residential care facilities: a prospective study. Arch Gerontol Geriatr. 2008;46:293–306. doi: 10.1016/j.archger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS-AIREN criteria. Dementia. 1994;5:189–192. doi: 10.1159/000106721. [DOI] [PubMed] [Google Scholar]
- Feil D, Marmon T, Unützer J. Cognitive impairment, chronic medical illness, and risk of mortality in an elderly cohort. Am J Geriatr Psychiatry. 2003;11:551–560. [PubMed] [Google Scholar]
- Feng L, Scherer SC, Tan BY, et al. Comorbid cognitive impairment and depression is a significant predictor of poor outcomes in hip fracture rehabilitation. Int Psychogeriatr. 2010;22:246–253. doi: 10.1017/S1041610209991487. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer’s disease and vascular dementia. J Neurol Sci. 2005;229:43–49. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Fleming J, Brayne C. Cambridge city over-75s cohort (CC75C) study collaboration. Inability to get up after falling, subsequent time on floor, and summoning help: prospective cohort study in people over 90. BMJ. 2008;337:a2227. doi: 10.1136/bmj.a2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Masaki KH, et al. Associations of symptoms of sleep apnea with cardiovascular disease, cognitive impairment, and mortality among older Japanese-American men. J Am Geriatr Soc. 1999;47:524–528. doi: 10.1111/j.1532-5415.1999.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frederick JT, Steinman LE, Prohaska T, et al. Community-based treatment of late life depression: an expert panel-informed literature review. Am J Prev Med. 2007;33:222–249. doi: 10.1016/j.amepre.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Freels S, Nyenhuis DL, Gorelick PB. Predictors of survival in African American patients with AD, VaD, or stroke without dementia. Neurology. 2002;59:1146–1153. doi: 10.1212/wnl.59.8.1146. [DOI] [PubMed] [Google Scholar]
- Frytak JR, Henk HJ, Zhao Y, et al. Health service utilization among Alzheimer’s disease patients: evidence from managed care. Alzheimers Dement. 2008;4:361–367. doi: 10.1016/j.jalz.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Antonucci TC, Gagnon M, et al. Depressive symptomatology and cognitive functioning: an epidemiological survey in an elderly community sample in France. Psychol Med. 1992a;22:159–172. doi: 10.1017/s0033291700032815. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Antonucci TC, Dartigues JF. The co-occurrence of depressive symptoms and cognitive impairment in a french community: Are there gender differences. Eur Arch Psychiatry Clin Neurosci. 1992b;242:161–171. doi: 10.1007/BF02191564. [DOI] [PubMed] [Google Scholar]
- Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312:608–611. doi: 10.1136/bmj.312.7031.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch Gen Psychiatry. 2002;59:1046–1052. doi: 10.1001/archpsyc.59.11.1046. [DOI] [PubMed] [Google Scholar]
- García-Lara JM, Aguilar-Navarro S, Gutiérrez-Robledo LM, Avila-Funes JA. The metabolic syndrome, diabetes, and Alzheimer’s disease. Rev Invest Clin. 2010;62:343–349. [PubMed] [Google Scholar]
- Gombojav B, Yi SW, Sull JW, Nam CM, Ohrr H. Combined effects of cognitive impairment and hypertension on total mortality in elderly people: the kangwha cohort study. Gerontology. 2011;57:490–496. doi: 10.1159/000323759. [DOI] [PubMed] [Google Scholar]
- Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhne U, Matschinger H, Angermeyer MC, Riedel-Heller SG. Incident dementia cases and mortality. Results of the Leipzig longitudinal study of the aged (LEILA75+) Dement Geriatr Cogn Disord. 2006;22:185–193. doi: 10.1159/000094786. [DOI] [PubMed] [Google Scholar]
- Gutterman EM, Markowitz JS, Lewis B, Fillit H. Cost of Alzheimer’s disease and related dementia in managed-Medicare. J Am Geriatr Soc. 1999;47:1065–1071. doi: 10.1111/j.1532-5415.1999.tb05228.x. [DOI] [PubMed] [Google Scholar]
- Hajduk AM, Lemon SC, McManus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol. 2013;5(1):407–416. doi: 10.2147/CLEP.S44560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon O, Pequignot R, Seux ML, et al. Relationship between antihypertensive drug therapy and cognitive function in elderly hypertensive patients with memory complaints. J Hypertens. 2006;24:2101–2107. doi: 10.1097/01.hjh.0000244961.69985.05. [DOI] [PubMed] [Google Scholar]
- Harris Y, Gorelick PB, Cohen D, et al. Psychiatric symptoms in dementia associated with stroke: a case-control analysis among predominantly African-American patients. J Natl Med Assoc. 1994;86:697–702. [PMC free article] [PubMed] [Google Scholar]
- Hawkins LA, Kilian S, Firek A, et al. Cognitive impairment and medication adherence in outpatients with heart failure. Hear Lung J Acute Crit Care. 2012;41(6):572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Haydar ZR, Lowe AJ, Kahveci KL, Weatherford W, Finucane T. Differences in end-of-life preferences between congestive heart failure and dementia in a medical house calls program. J Am Geriatr Soc. 2004;52:736–740. doi: 10.1111/j.1532-5415.2004.52210.x. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol. 2001;154:642–648. doi: 10.1093/aje/154.7.642. [DOI] [PubMed] [Google Scholar]
- Hendrie HC, Albert MS, Butters MA, et al. The NIH cognitive and emotional health project: report of the critical evaluation study committee. Alzheimers Dement. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hill JW, Futterman R, Duttagupta S, et al. Alzheimer’s disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- Hobson P, Meara J, Ishihara-Paul L. The estimated life expectancy in a community cohort of Parkinson’s disease patients with and without dementia, compared with the UK population. J Neurol Neurosurg Psychiatry. 2010;81:1093–1098. doi: 10.1136/jnnp.2009.198689. [DOI] [PubMed] [Google Scholar]
- Huang CQ, Dong BR, Zhang YL, et al. Cognitive impairment and hypertension among Chinese nonagenarians and centenarians. Hypertens Res. 2009;32:554–558. doi: 10.1038/hr.2009.72. [DOI] [PubMed] [Google Scholar]
- Huijts M, Van Oostenbrugge RJ, Duits A, et al. Cognitive impairment in heart failure: results from the trial of intensified versus standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. Eur J Heart Fail. 2013;15(6):699–707. doi: 10.1093/eurjhf/hft020. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Cohen A, Ein-Mor E, Maaravi Y, Stessman J. Frailty, cognitive impairment and mortality among the oldest old. J Nutr Heal Aging. 2012;15(8):678–682. doi: 10.1007/s12603-011-0096-3. [DOI] [PubMed] [Google Scholar]
- Janzing J, Teunisse R, Bouwens P, van’t Hof M, Zitman F. The course of depression in elderly subjects with and without dementia. J Affect Disord. 2000;57:49–54. doi: 10.1016/s0165-0327(99)00060-9. [DOI] [PubMed] [Google Scholar]
- Kammoun S, Gold G, Bouras C, et al. Immediate causes of death of demented and non-demented elderly. Acta Neurol Scand. 2000;102:96–99. doi: 10.1034/j.1600-0404.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- Katsoulis M, Kyrozis A, Trichopoulou A, et al. Cognitive impairment and cancer mortality: a biological or health care explanation? Cancer Causes Control. 2014;25(11):1565–1570. doi: 10.1007/s10552-014-0460-9. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Brenner DE, Speck CE, et al. Causes of death associated with Alzheimer disease: variation by level of cognitive impairment before death. J Am Geriatr Soc. 1994;42:723–726. doi: 10.1111/j.1532-5415.1994.tb06531.x. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Zhao Y, Weir S, Kramer MS, Ash AS. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care. 2008;46:839–846. doi: 10.1097/MLR.0b013e318178940b. [DOI] [PubMed] [Google Scholar]
- Lang PO, Heitz D, Hédelin G, et al. Early markers of prolonged hospital stays in older people: a prospective, multicenter study of 908 inpatients in French acute hospitals. J Am Geriatr Soc. 2006;54:1031–1039. doi: 10.1111/j.1532-5415.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Zheng L, Weiner MW, et al. Association of depressed mood and mortality in older adults with and without cognitive impairment in a prospective naturalistic study. Am J Psychiatry. 2010;167:589–597. doi: 10.1176/appi.ajp.2009.09020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sirois MJ, Moore L, et al. Return to the ED and hospitalisation following minor injuries among older persons treated in the emergency department: predictors among independent seniors within 6 months. Age Ageing. 2015;44(4):624–629. doi: 10.1093/ageing/afv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- Liebetrau M, Steen B, Skoog I. Stroke in 85-year-olds: prevalence, incidence, risk factors, and relation to mortality and dementia. Stroke. 2003;34:2617–2622. doi: 10.1161/01.STR.0000094420.80781.A9. [DOI] [PubMed] [Google Scholar]
- Llibre JDJ, López AM, Valhuerdi A, Guerra M, Llibre-Guerra JJ, Sánchez YY, Bosch R, Zayas T, Moreno C. Frailty, dependency and mortality predictors in a cohort of Cuban older adults, 2003–2011. MEDICC Rev. 2014;16(1):24–30. doi: 10.37757/MR2014.V16.N1.6. Available at http://www.ncbi.nlm.nih.gov/pubmed/24487672. [DOI] [PubMed] [Google Scholar]
- Llinàs-Regla J, López-Pousa S, Vilalta-Franch J, Garre-Olmo J, Román GC. Mortality after a diagnosis of dementia in a population aged 75 and over in Spain. Neuroepidemiology. 2007;31:80–88. doi: 10.1159/000144088. [DOI] [PubMed] [Google Scholar]
- Lo RY, Tanner CM, Albers KB, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009;66:1353–1358. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- Lopponen M, Raiha I, Isoaho R, et al. Dementia associates with undermedication of cardiovascular diseases in the elderly: a population-based study. Dement Geriatr Cogn Disord. 2005;22:132–141. doi: 10.1159/000093739. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Toone L, Tschanz J, et al. Population-based study of medical comorbidity in early dementia and “cognitive impairment, no dementia (CIND)”: association with functional and cognitive impairment: the cache county study. Am J Geriatr Psychiatry. 2005;13:656–664. doi: 10.1176/appi.ajgp.13.8.656. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Samus QM, Baker A, et al. Effect of dementia and treatment of dementia on time to discharge from assisted living facilities: the Maryland assisted living study. J Am Geriatr Soc. 2007;55:1031–1037. doi: 10.1111/j.1532-5415.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Machlin SR, Soni A. Health care expenditures for adults with multiple treated chronic conditions: estimates from the medical expenditure panel survey. Prev Chronic Dis. 2009;10:E63. doi: 10.5888/pcd10.120172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaziner J, Zimmerman S, Gruber-Baldini AL, et al. Mortality and adverse health events in newly admitted nursing home residents with and without dementia. J Am Geriatr Soc. 2005;53:1858–1866. doi: 10.1111/j.1532-5415.2005.53551.x. [DOI] [PubMed] [Google Scholar]
- Marder K, Leung D, Tang M, et al. Are demented patients with Parkinson’s disease accurately reflected in prevalence surveys? a survival analysis. Neurology. 1991;41:1240–1240. doi: 10.1212/wnl.41.8.1240. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- McCormick WC, Kukull WA, van Belle G, et al. Symptom patterns and comorbidity in the early stages of Alzheimer’s disease. J Am Geriatr Soc. 1994;42:517–521. doi: 10.1111/j.1532-5415.1994.tb04974.x. [DOI] [PubMed] [Google Scholar]
- McCormick WC, Hardy J, Kukull WA, et al. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49:1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- McGuire LC, Ford ES, Ajani UA. The impact of cognitive functioning on mortality and the development of functional disability in older adults with diabetes: the second longitudinal study on aging. BMC Geriatr. 2006;6:8. doi: 10.1186/1471-2318-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meerman L, van de Lisdonk EH, Koopmans RT, Zielhuis GA, Olde Rikkert MG. Prognosis and vascular co-morbidity in dementia a historical cohort study in general practice. J Nutr Health Aging. 2008;12:145–150. doi: 10.1007/BF02982568. [DOI] [PubMed] [Google Scholar]
- Melkas S, Oksala NK, Jokinen H, et al. Poststroke dementia predicts poor survival in long-term follow-up: influence of prestroke cognitive decline and previous stroke. J Neurol Neurosurg Psychiatry. 2009;80:865–870. doi: 10.1136/jnnp.2008.166603. [DOI] [PubMed] [Google Scholar]
- Melton LJ3rd, Leibson CL, Achenbach SJ, et al. Fracture risk after the diagnosis of Parkinson’s disease: influence of concomitant dementia. Mov Disord. 2006;21:1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- Mercer SW, Gunn J, Bower P, Wyke S, Guthrie B. Managing patients with mental and physical multimorbidity. BMJ. 2012;345:e5559. doi: 10.1136/bmj.e5559. [DOI] [PubMed] [Google Scholar]
- Mignardot J-B, Beauchet O, Annweiler C, Cornu C, Deschamps T. Postural sway, falls, and cognitive status: a cross-sectional study among older adults. J Alzheimers Dis. 2014;41:431–439. doi: 10.3233/JAD-132657. [DOI] [PubMed] [Google Scholar]
- Millán-Calenti JC, Maseda A, Rochette S, et al. Mental and psychological conditions, medical comorbidity and functional limitation: differential associations in older adults with cognitive impairment, depressive symptoms and co-existence of both. Int J Geriatr Psychiatry. 2011;26:1071–1079. doi: 10.1002/gps.2646. [DOI] [PubMed] [Google Scholar]
- Mitchell SL, Rockwood K. The association between parkinsonism, Alzheimer’s disease, and mortality: a comprehensive approach. J Am Geriatr Soc. 2000;48:422–425. doi: 10.1111/j.1532-5415.2000.tb04701.x. [DOI] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Petersen RC, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28:1962–1967. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Rubin EH, Morris EJ, Mandel SA. Senile dementia of the Alzheimer’s type: an important risk factor for serious falls. J Gerontol. 1987;42:412–417. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- Murao K, Leys D, Jacquin A, et al. Thrombolytic therapy for stroke in patients with pre-existing cognitive impairment. Cerebrovasc Dis. 2014;37(1):98. [Google Scholar]
- National Academy on an Aging Society. [Accessed May 13 2014];Alzheimer’s Disease and Dementia: A Growing Challenge. 2000 Number 11 [online]. Available at http://www.agingsociety.org/agingsociety/pdf/alzheimers.pdf/
- National Institutes of Health (NIH) [Accessed November 24 2013];Cognitive and Emotional Health Project: The Healthy Brain. 2001 Workshop [online]. Available at http://trans.nih.gov/cehp/HBPemot.htm/
- Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Cognitive impairment and mortality in older Mexican Americans. J Am Geriatr Soc. 2003;51(2):178–183. doi: 10.1046/j.1532-5415.2003.51055.x. [DOI] [PubMed] [Google Scholar]
- Nikolova R, Demers L, Béland F, Giroux F. Transitions in the functional status of disabled community-living older adults over a 3-year follow-up period. Arch Gerontol Geriatr. 2011;52:12–7. doi: 10.1016/j.archger.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes: a systematic review. Am J Prev Med. 2002;22(4):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- O’Donnell M, Teo K, Gao P, et al. Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J. 2012;33(14):1777–1786. doi: 10.1093/eurheartj/ehs053. [DOI] [PubMed] [Google Scholar]
- Oksala NK, Jokinen H, Melkas S, et al. Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Psychiatry. 2009;80:1230–1235. doi: 10.1136/jnnp.2009.174573. [DOI] [PubMed] [Google Scholar]
- Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009;57:1816–1824. doi: 10.1111/j.1532-5415.2009.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashos SA, Maraganore DM, O’Brien PC, Rocca WA. Medical services utilization and prognosis in Parkinson disease: a population-based study. Mayo Clin Proc. 2002;77:918–925. doi: 10.4065/77.9.918. [DOI] [PubMed] [Google Scholar]
- Park J-I, Park TW, Yang J-C, Chung S-K. Factors associated with depression among elderly Koreans: the role of chronic illness, subjective health status, and cognitive impairment. Psychogeriatrics. 2016;16(1):62–69. doi: 10.1111/psyg.12160. [DOI] [PubMed] [Google Scholar]
- Prince M, Guerchet M, Prina M Alzheimer’s Disease International. [Accessed February 4 2015];Policy Brief for Heads of Government: The Global Impact of Dementia 2013 – 2050. 2013 Available at http://www.alz.co.uk/research/GlobalImpactDementia2013.pdf/
- Prinz N, Stingl J, Dapp A, et al. High rate of hypoglycemia in 6770 type 2 diabetes patients with comorbid dementia: a multicenter cohort study on 215,932 patients from the German / Austrian diabetes registry. Diabetes Res Clin Pract. 2015;2:73–81. doi: 10.1016/j.diabres.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Provencher V, Sirois MJ, Ouellet MC, et al. Decline in activities of daily living after a visit to a Canadian emergency department for minor injuries in independent older adults: are frail older adults with cognitive impairment at greater risk? J Am Geriatr Soc. 2015;63(5):860–8. doi: 10.1111/jgs.13389. [DOI] [PubMed] [Google Scholar]
- Punthakee Z, Miller ME, Launer LJ, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35:787–793. doi: 10.2337/dc11-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CC, Gruber-Baldini AL, Port CL, et al. The role of nursing home admission and dementia status on care for diabetes mellitus. J Am Geriatr Soc. 2009;57:1628–1633. doi: 10.1111/j.1532-5415.2009.02382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastas S, Verkkoniemi A, Polvikoski T, et al. Atrial fibrillation, stroke, and cognition a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38:1454–1460. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- Robb C, Boulware D, Overcash J, Extermann M. Patterns of care and survival in cancer patients with cognitive impairment. Crit Rev Oncol Hematol. 2010;74:218–224. doi: 10.1016/j.critrevonc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Roe CM, Fitzpatrick AL, Xiong C, et al. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74:106–112. doi: 10.1212/WNL.0b013e3181c91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook PN, Cameron ID, Chen JS, et al. Influence of fall related factors and bone strength on fracture risk in the frail elderly. Osteoporos Int. 2007;18:603–610. doi: 10.1007/s00198-006-0290-z. [DOI] [PubMed] [Google Scholar]
- Sanyal J, Banerjee TK, Rao VR. Dementia and cognitive impairment in patients with Parkinson’s disease from India: a 7-year prospective study. Am J Alzheimers Dis Other Demen. 2014;29(7):630–636. doi: 10.1177/1533317514531442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuvée-Moreau J, Kurz X, Dresse A. The economic impact of dementia in Belgium: results of the national dementia economic study (NADES) Acta Neurol Belg. 2002;102:104–113. [PubMed] [Google Scholar]
- Sinclair AJ, Girling AJ, Bayer AJ. Cognitive dysfunction in older subjects with diabetes mellitus: impact on diabetes self-management and use of care services. All Wales Research into Elderly (AWARE) Study. Diabetes Res Clin Pract. 2000;50:203–212. doi: 10.1016/s0168-8227(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Smith GE, Kokmen E, O’Brien PC. Risk factors for nursing home placement in a population-based dementia cohort. J Am Geriatr Soc. 2000;48:519–525. doi: 10.1111/j.1532-5415.2000.tb04998.x. [DOI] [PubMed] [Google Scholar]
- Snowden MB, Atkins DC, Steinman LE, et al. Longitudinal association of dementia and depression. Am J Geriatr Psychiatry. 2015;23(9):897–905. doi: 10.1016/j.jagp.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M, Steinman L, Mochan K, et al. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59:704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- Sousa RM, Ferri CP, Acosta D, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–1830. doi: 10.1016/S0140-6736(09)61829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John PD, Montgomery P. Does a single-item measure of depression predict mortality? Can Fam Physician. 2009;55:e1–e5. [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Xue QL, Masaki K, et al. Change in blood pressure and incident dementia a 32-year prospective study. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott DJ, Spilg E, Campbell AM, et al. Haemostasis in ischaemic stroke and vascular dementia. Blood Coagul Fibrinolysis. 2001;12:651–657. doi: 10.1097/00001721-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Stump TE, Callahan CM, Hendrie HC. Cognitive impairment and mortality in older primary care patients. J Am Geriatr Soc. 2001;49:934–940. doi: 10.1046/j.1532-5415.2001.49184.x. [DOI] [PubMed] [Google Scholar]
- Suehs BT, Davis CD, Alvir J, et al. The clinical and economic burden of newly diagnosed Alzheimer’s disease in a medicare advantage population. Am J Alzheimers Dis Other Demen. 2013;28:384–92. doi: 10.1177/1533317513488911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemichi TK, Paik M, Bagiella E, et al. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25:1915–1919. doi: 10.1161/01.str.25.10.1915. [DOI] [PubMed] [Google Scholar]
- Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff. 2010;29:1–7. doi: 10.1377/hlthaff.2009.0474. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran C, Skoog I, et al. Dementia: the leading predictor of death in a defined elderly population the cache county study. Neurology. 2004;62:1156–1162. doi: 10.1212/01.wnl.0000118210.12660.c2. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Multiple Chronic Conditions: A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC: 2010. [Accessed November 24 2013]. [online]. Available at http://www.hhs.gov/ash/initiatives/mcc/mcc_framework.pdf/ [Google Scholar]
- U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion (US DHHS ODPHP). Healthy People 2020. Washington, DC: 2010. [Accessed December 20 2013]. [online]. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/default.aspx/ [Google Scholar]
- van Asch IF, Nuyen J, Veerbeek MA, et al. The diagnosis of depression and use of antidepressants in nursing home residents with and without dementia. Int J Geriatr Psychiatry. 2013;28(3):312–8. doi: 10.1002/gps.3830. [DOI] [PubMed] [Google Scholar]
- Van Iersel MB, Verbeek ALM, Bloem BR, et al. Frail elderly patients with dementia go too fast. J Neurol Neurosurg Psychiatry. 2006;77:874–876. doi: 10.1136/jnnp.2005.084418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik SA, Jantzi M, Poss J, et al. Factors associated with pharmacologic treatment of osteoporosis in an older home care population. J Gerontol A Biol Sci Med Sci. 2007;62:872–878. doi: 10.1093/gerona/62.8.872. [DOI] [PubMed] [Google Scholar]
- Vinyoles E, De la Figuera M, Gonzalez-Segura D. Cognitive function and blood pressure control in hypertensive patients over 60 years of age: COGNIPRES study. Curr Med Res Opin. 2008;24:3331–3339. doi: 10.1185/03007990802538724. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Liu Z, et al. A five-year community-based longitudinal survival study of dementia in Beijing, China: a 10/66 Dementia Research Group population-based study. Int Psychogeriatr. 2010a;22:761–768. doi: 10.1017/S1041610210000669. [DOI] [PubMed] [Google Scholar]
- Wang J, Chang LH, Eberly LE, Virnig BA, Kane RL. Cognition moderates the relationship between facility characteristics, personal impairments, and nursing home residents’ activities of daily living. J Am Geriatr Soc. 2010b;58:2275–2283. doi: 10.1111/j.1532-5415.2010.03173.x. [DOI] [PubMed] [Google Scholar]
- Wang JK, Su TP, Chou P. Sex differences in prevalence and risk indicators of geriatric depression: the Shih-Pai community-based survey. J Formos Med Assoc. 2010c;109:345–353. doi: 10.1016/S0929-6646(10)60062-9. [DOI] [PubMed] [Google Scholar]
- Warchol-Celinska E, Styczynska M, Prejbisz A, et al. Hypertension in patients with Alzheimer’s disease-prevalence, characteristics, and impact on clinical outcome. Experience of one neurology center in Poland. J Am Soc Hypertens. 2015;9:711–24. doi: 10.1016/j.jash.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Warshaw G. Introduction: advances and challenges in care of older people with chronic illness. Generations. 2006;30:5–10. [Google Scholar]
- Welmerink DB, Longstreth WT, Lyles MF, Fitzpatrick AL. Cognition and the risk of hospitalization for serious falls in the elderly: results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65:1242–1249. doi: 10.1093/gerona/glq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A, Prince M. World Alzheimer Report 2010: The Global Economic Impact of Dementia. Alzheimer’s Disease International; London: 2010. [Google Scholar]
- Zekry D, Herrmann FR, Grandjean R, et al. Demented versus non-demented very old inpatients: the same comorbidities but poorer functional and nutritional status. Age Ageing. 2008;37:83–89. doi: 10.1093/ageing/afm132. [DOI] [PubMed] [Google Scholar]
- Zekry D, Herrmann FR, Grandjean R, et al. Does dementia predict adverse hospitalization outcomes? A prospective study in aged inpatients. Int J Geriatr Psychiatry. 2009;24:283–291. doi: 10.1002/gps.2104. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8:108. doi: 10.1186/1472-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Fratiglioni L, Guo Z, et al. Association of stroke with dementia, cognitive impairment, and functional disability in the very old a population-based study. Stroke. 1998;29:2094–2099. doi: 10.1161/01.str.29.10.2094. [DOI] [PubMed] [Google Scholar]
- Zivin K, Kabeto MU, Kales HC, Langa KM. The effect of depression and cognitive impairment on enrollment in Medicare Part D. J Am Geriatr Soc. 2009;57:1433–1440. doi: 10.1111/j.1532-5415.2009.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.