As part of a series of papers on implementing guideline recommendations in the context of preventive health care, 2 of our previous papers discussed communicating harms and benefits, and shared decision making (SDM).1,2 In the latter paper we met John, a 66-year-old smoker, who at his most recent visit to your office wished to focus on quitting smoking. At that time, John was informed of his eligibility for a low-dose chest computed tomography (CT) scan to screen for lung cancer. However, he was not ready to commit to undergoing screening. He now returns after having successfully quit smoking for 6 months to see if lung cancer screening might still be “right” for him.
Navigating to shared and personalized decisions
For clinicians to help their patients navigate a path to the most appropriate decision, they need to understand how much each individual values the potential benefits of an intervention (screening in this case) and how much each individual fears the unanticipated repercussions or harms. While patients who are considering screening are seldom aware of these issues, they are actually critical to reaching a values-informed health decision. This paper will provide suggestions on eliciting insight from patients regarding how each views the benefits and harms associated with screening strategies.
Different kinds of recommendations mean different courses of action
The most common scenario faced by patients and clinicians in the screening context is clinical practice guidelines issuing weak recommendations, sometimes termed conditional recommendations, for or against screening. This approach is central to the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system of guideline development, increasingly used by groups that produce clinical practice guidelines.3 The implication of a weak recommendation is that, while most patients would follow the recommended course of action, many would not.4
Weak recommendations either for or against screening signal that the overall balance between the benefits and harms associated with undergoing or forgoing a screening intervention is close but favours the recommended approach. Uncertainty in the research evidence—a common state of affairs—can also translate into a weak recommendation, as can uncertainty regarding which outcomes are most important to patients.
In the SDM context, it is important to know how patients value the favourable outcome of early detection and early treatment of cancer, for example, compared with the risks of false–positive results such as mislabeling, anxiety, the burden of further testing, or the consequences of an overdiagnosis. Overdiagnosis is defined as the detection of an asymptomatic “abnormality” or “condition” that would ultimately not go on to cause symptoms or death.
What do we mean by values and preferences?
Values is a complex and often loaded term, with ethical, cultural, religious, philosophical, and political implications. In the SDM context specific to screening, it has a more focused meaning referring to how patients “value” the outcomes arising from various options. Patients’ preferences are their most favoured health care options. Values can be inherent to the individual receiving care but are influenced by societal norms and familial expectations. For example, John might be more willing to consider screening because of pressure from his children, or owing to witnessing the situation of a cousin or friend who has recently been diagnosed with advanced lung cancer.
The values of another person are difficult to anticipate and can be influenced by clinicians’ views.5 However, clarifying values can be a rewarding exercise, as it not only ensures the best possible decision but demonstrates to patients that we are genuinely interested in incorporating their views and how they value the outcomes arising from screening options. If a bad outcome occurs (eg, development of cancer after deciding to forgo screening), an SDM approach might make it easier to reconcile those events through an appreciation of having had previous discussions about this as a possible outcome incorporating patients’ informed values and preferences.6
Increasingly, groups such as the Canadian Task Force on Preventive Health Care (CTFPHC) that develop recommendations in their screening guidelines also use findings from systematic reviews of the research to improve their understanding of patient values and preferences when they weigh the benefits and harms of a specific screening intervention. Other strategies employed by the CTFPHC involve independent investigations (eg, focus groups of patients) and engagement activities. While useful, this guidance represents the collective and might not apply to individual patients, hence the importance of eliciting personal perspectives. For example, 2 long-standing smokers with identical risk profiles for lung cancer might opt for entirely different actions once informed of the risks and benefits associated with the low-dose CT screening and nonscreening options. This occurs as a result of the different values they personally place on the outcomes that follow each strategy and the probability of these outcomes (eg, 3 of 1000 persons screened will avoid death from lung cancer while 351 of 1000 who undergo low-dose CT screening will have false-positive findings).7
How do I elicit values through values clarification?
The International Patient Decision Aids Standards define values clarifications as strategies that are intended to help patients evaluate the desirability of options or attributes of options within a specific decision context.8
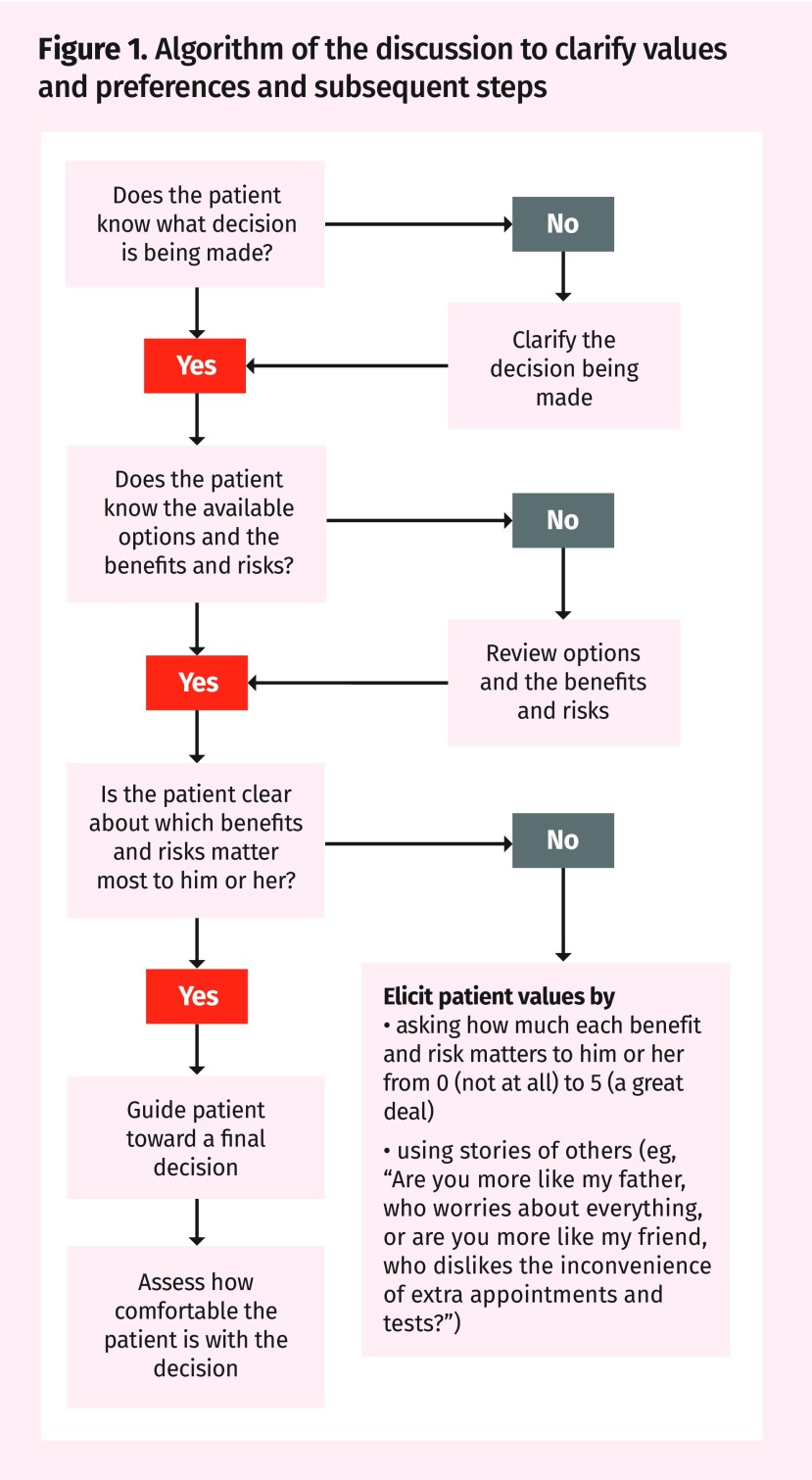
In embarking on a discussion to clarify values and preferences, it is important to make it clear that a decision is being discussed and then to set goals for what the conversation is meant to achieve. Well-informed and strongly opinionated patients with clear preferences are unlikely to alter their firmly held views on screening. Figure 1 presents an algorithm that describes the discussion to clarify values and preferences and subsequent steps. Before clinicians give too much detail on screening, it is important to verify the patient’s understanding and expectations of the benefits and harms of screening. With John, for example, you might say, “Tell me what you expect from lung cancer screening.” It might then be worth assessing the patient’s understanding and expectations of the benefits and harms of screening. The patient will hopefully agree to receive information outlining the likelihood of the outcomes, both beneficial and harmful, associated with each decision. Be prepared to point out how the outcomes of screening versus not screening might be fairly similar and that the pursuit of small benefits might weigh against the often more frequent, although less severe, risks of experiencing some form of harm. This might require detailed descriptions of the risks and tribulations of what could happen if the patient has positive screening results.
Figure 1.
Algorithm of the discussion to clarify values and preferences and subsequent steps
When eliciting patients’ values, you can ask patients to indicate how important it is for them to achieve a benefit or to avoid a harm. For example, in John’s situation, if the CT scan were to have negative results, ask John how important it would be to have “peace of mind” that he does not have cancer. This can be rated on a scale of 0 (not important) to 5 (extremely important). And also ask John how important it is for him to avoid a false alarm, in which the CT scan shows something but further tests, such as a biopsy, reveal there is nothing. Harm outcomes are less emphasized in many guideline recommendations but, in eliciting values and preferences, one should include the emotional strain of knowing that one has positive screening results for a serious illness as well as specifics about timelines for confirmatory tests and next steps. Patients have varying opinions on the value of peace of mind (that is, knowing they have negative screening results for a certain target disease), even if this does not eliminate their risk of developing that condition.
Another option is to use narratives to help patients with value matching. For example, in John’s situation you might say, “Are you more like my father, who worries about cancer and prefers to have every screening test done? Or are you more like my friend, who cannot be bothered with the hassle of extra appointments and tests?”
A third option is patient decision aids. These printed materials, videos, and online interactive programs provide, at a minimum, information on options and their benefits and harms, and help patients clarify their values regarding outcomes of options. Decision aids can be used in preparation for the consultation. Briefer versions of the decision aids are designed for use when discussing the decision during the consultation. Evidence from 105 trials in a Cochrane review shows patient decision aids improve patients’ knowledge, increase patient understanding of the likelihood of benefits and harms, improve patient participation in decision making, and improve the match between patients’ values and the chosen option.9 To find a specific patient decision aid, there is an A-to-Z international inventory.10 The generic Ottawa Personal Decision Guide helps clinicians to guide patients making any health or social decision.11 A search for lung cancer in the A-to-Z international inventory reveals the high-quality lung cancer screening decision aid from the Agency for Healthcare Research and Quality.10,12
The CTFPHC knowledge translation tools and a growing array of high-quality SDM tools available online can be invaluable in guiding the discussion toward clarifying patients’ values and determining their preferences.13
Challenges
Although SDM is gaining increasing acceptance as an essential component of patient-centred care, it can be challenging to communicate the more subtle details around benefits and harms to a spectrum of individuals. The process can be time-consuming and might require more than one visit; it could involve asking patients to review and complete a specific or generic decision support tool, such as the ones noted previously, at home. We also recognize that some patients might experience a change in values or preferences over time or simply prefer their health care providers to make screening decisions for them. However, exploring values and preferences is essential to making many kinds of decisions and the empiric evidence supporting this approach is robust, especially for patients with disadvantages (eg, those with low health literacy).14 Less certain is how much the approach improves patient outcomes and patient and provider experiences in the screening context. Additional insights from the patient perspective are shared in a commentary in this issue (page 10).15
Returning to John
After John uses the CTFPHC 1000-person diagram when reviewing options and outcomes from screening with low-dose CT or not screening, he is surprised to learn about the small chance of benefit. He is also concerned about the time commitment required for serial scanning and the need to perform a biopsy if his screening results are positive, all with risks of complications. With your help, John realizes that he has a somewhat fatalistic attitude on life. He says, “If I am destined to get the big C I do not think it can be avoided; if it is in the cards for me, so be it.” He also notes that he feels well now and will not receive additional reassurance or better sleep if he has normal CT scan findings. While his curiosity about CT screening brought him to the office, he thanks you for the clarifications and says, “I think I will pass on this for now.”
KEY POINTS
▸ As many screening recommendations highlight the close balance between benefits and harms, eliciting patients’ values helps weigh benefits and harms against each other to reach an optimal decision.
▸ In the context of shared decision making, values pertain to the importance patients place on the potential beneficial and harmful outcomes that can result from a screening intervention or test. Patients’ preferences are their most favoured health care options.
▸ For screening decisions, clarifying values focuses on determining patients’ desire to diagnose disease early, as well as their understanding and aversion to the risks and implications of false-positive test results and overdiagnosis.
▸ Clarifying patients’ values often helps inform their preferred options but can also be challenging for patients who prefer not to be involved in decision making.
Footnotes
Competing interests
All authors have completed the International Committee of Medical Journal Editors’ Unified Competing Interest form (available on request from the corresponding author) and declare that they have no competing interests. For additional information on the Canadian Task Force on Preventive Health Care conflicts of interest, please visit https://canadiantaskforce.ca.
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de janvier 2018 à la page e13.
References
- 1.Bell NR, Grad R, Dickinson JA, Singh H, Moore AE, Kasperavicius D, et al. Better decision making in preventive health screening. Balancing benefits and harms. Can Fam Physician. 2017;63:521–4. (Eng), 525–8 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 2.Grad R, Légaré F, Bell NR, Dickinson JA, Singh H, Moore AE, et al. Shared decision making in preventive health care. What it is; what it is not. Can Fam Physician. 2017;63:682–4. (Eng), e377–80 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 3.Bell NR, Connor Gorber S, Tonelli M, Pottie K, Singh H, Joffres M, et al. From ABCs to GRADE. Canadian Task Force on Preventive Health Care’s new rating system for clinical practice guidelines. Can Fam Physician. 2013;59:1282–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–51. doi: 10.1136/bmj.39493.646875.AE. Erratum in: BMJ 2008;336(7658):0–b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. doi: 10.1136/bmj.e6572. [DOI] [PubMed] [Google Scholar]
- 6.Gattellari M, Ward JE. Men’s reactions to disclosed and undisclosed opportunistic PSA screening for prostate cancer. Med J Aust. 2005;182(8):386–9. doi: 10.5694/j.1326-5377.2005.tb06756.x. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Task Force on Preventive Health Care . Clinician FAQ. Lung cancer screening. Calgary, AB: University of Calgary; 2016. Available from: https://canadiantaskforce.ca/wp-content/uploads/2016/05/ctfphclung-cancerclinician-faqfinalv2-1.pdf. Accessed 2017 Nov 10. [Google Scholar]
- 8.Fagerlin A, Pignone M, Abhyankar P, Col N, Feldman-Stewart D, Gavaruzzi T, et al. Clarifying values: an updated review. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S8. doi: 10.1186/1472-6947-13-S2-S8. Epub 2013 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottawa Hospital Research Institute . Patient decision aids. A to Z inventory of decision aids. Ottawa, ON: Ottawa Hospital Research Institute; 2014. Available from: https://decisionaid.ohri.ca/AZinvent.php. Accessed 2017 Nov 10. [Google Scholar]
- 11.Ottawa Hospital Research Institute . Ottawa personal decision guide. Ottawa, ON: University of Ottawa; 2015. Available from: https://decisionaid.ohri.ca/docs/das/OPDG.pdf. Accessed 2017 Nov 10. [Google Scholar]
- 12.Agency for Healthcare Research and Quality . Lung cancer screening tools. Is lung cancer screening right for me? Rockville, MD: Agency for Healthcare Research and Quality; 2016. Available from: https://effectivehealthcare.ahrq.gov/decision-aids/lung-cancer-screening/patient. Accessed 2017 Nov 10. [Google Scholar]
- 13.Moore AE, Straus SE, Kasperavicius D, Bell NR, Dickinson JA, Grad R, et al. Knowledge translation tools in preventive health care. Can Fam Physician. 2017;63:853–8. (Eng), e466–72 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 14.Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9(4):e94670. doi: 10.1371/journal.pone.0094670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telfer C, Kasperavicius D. Patient perspectives. Exploring patient values and preferences. Can Fam Physician. 2018;64:10–1. (Eng), 13–5 (Fr). [PMC free article] [PubMed] [Google Scholar]