Abstract
Background
Lynch syndrome is an autosomal dominant inherited disease caused by germline mutations in mismatch repair genes. Analysis for microsatellite instability (MSI) and immunohistochemistry (IHC) of protein expressions of disease-associated genes is used to screen for Lynch syndrome in endometrial cancer patients. When losses of both MLH1 and PMS2 proteins are observed by IHC, MLH1 promoter methylation analysis is conducted to distinguish Lynch syndrome-associated endometrial cancer from sporadic cancer.
Case presentation
Here we report a woman who developed endometrial cancer at the age of 49 years. She had a family history of colorectal cancer (first-degree relative aged 52 years) and stomach cancer (second-degree relative with the age of onset unknown). No other family history was present, and she failed to meet the Amsterdam II criteria for the diagnosis of Lynch syndrome. Losses of MLH1 and PMS2, but not MSH2 and MSH6, proteins were observed by IHC in endometrial cancer tissues. Because MLH1 promoter hypermethylation was detected in endometrial cancer tissue samples, the epigenetic silencing of MLH1 was suspected as the cause of the protein loss. However, because of the early onset of endometrial cancer and the positive family history, a diagnosis of Lynch syndrome was also suspected. Therefore, we provided her with genetic counseling. After obtaining her consent, MLH1 promoter methylation testing and genetic testing of peripheral blood were performed. MLH1 promoter methylation was not observed in peripheral blood. However, genetic testing revealed a large deletion of exon 5 in MLH1; thus, we diagnosed the presence of Lynch syndrome.
Conclusions
Both MLH1 germline mutation and MLH1 promoter hypermethylation may be observed in endometrial cancer. Therefore, even if MLH1 promoter hypermethylation is detected, a diagnosis of Lynch syndrome cannot be excluded.
Keywords: Lynch syndrome, Endometrial cancer, MLH1 promoter hypermethylation, MLH1 germline mutation, Screening
Background
Lynch syndrome is an autosomal dominant inherited disease caused by germline mutations in mismatch repair genes. MLH1, MSH2, MSH6, PMS2 mutation in this syndrome account for approximately 37, 41, 13, 9%, respectively [1]. It is important to establish a diagnosis for this syndrome because of the associated elevated lifetime risk of developing cancers such as colorectal and endometrial cancers [2]. Amsterdam II [3] and Bethesda [4] criteria have been widely used to screen for Lynch syndrome. However, the sensitivity of both criteria based on family history and clinical background is low [5–7]. Therefore, they have been considered to be insufficient as independent screening tools. To increase the accuracy of screening for Lynch syndrome, microsatellite instability (MSI) and immunohistochemistry (IHC) are used in cases with colorectal cancer, and when losses of MLH1 and PMS2 proteins are detected using IHC, universal screening, including BRAF testing and analysis for MLH1 promoter methylation, is recommended [5, 6]. Similarly, in endometrial cancer, MSI and IHC are useful in screening for Lynch syndrome [7–10], and the mutation site can be estimated using IHC. When losses of MLH1 and PMS2 proteins are observed, both MLH1 germline mutations and epigenetic silencing are conceivable. MLH1 promoter methylation testing is recognized to be useful for distinguishing between the two in the population based screening, because MLH1 promoter hypermethylation is far more responsible for losses of MLH1 and PMS2 protein using IHC than MLH1 germline mutation [7]. However, some reports showed cases with germline mutations among Lynch syndrome patients with colorectal cancer in which although MLH1 and PMS2 proteins were lost by IHC in cancer tissues, MLH1 promoter hypermethylation was observed [11–13]. These reports suggested that it is inappropriate to exclude Lynch syndrome based on a result of MLH1 promoter hypermethylation. Similar assumption is made for Lynch syndrome associated with endometrial cancer. However, to the best of our knowledge, we have been unsuccessful in finding any previous study similar to the present case report.
In this study, we performed genetic analysis of peripheral blood from this endometrial cancer patient with MLH1 and PMS2 protein losses using IHC despite MLH1 promoter hypermethylation because Lynch syndrome was suspected based on family history and clinical factors. As a result, we confirmed the presence of MLH1 germline mutation, and hence, diagnosed this patient with Lynch syndrome.
Case presentation
Here we report a woman who developed endometrial cancer at the age of 49 years, with no previous history of cancer. She had a family history of a first-degree relative who developed colorectal cancer at the age of 52 years and a second-degree relative who developed stomach cancer at an unknown age of onset. No other relevant family history was revealed, and she did not meet the Amsterdam II criteria for the diagnosis of Lynch syndrome. She visited our hospital with a chief complaint of atypical genital bleeding. A diagnosis of endometrial cancer was made, and we performed hysterectomy and salpingo-oophorectomy and partial omentectomy. Based on the histopathological examination of samples obtained at surgery, a grade 2 endometrioid adenocarcinoma in the endometrium was diagnosed. Similarly, the pathological stage of the disease determined at surgery enabled an International Federation of Gynecology and Obstetrics (2008) stage IA disease to be established.
As a result of our previous IHC analysis to screen for endometrial cancer [10] that revealed losses of MLH1 and PMS2, but not MSH2 and MSH6, proteins in endometrial cancer tissues obtained from this patient (Fig. 1), MLH1 mutation was suspected. We confirmed MLH1 promoter hypermethylation in endometrial cancer tissue samples and suspected epigenetic silencing of MLH1. However, because of the early age of onset of endometrial cancer and presence of family history of Lynch-related malignancies, we suspected her of showing Lynch syndrome; therefore, we offered her genetic counseling. After obtaining consent, MLH1 promoter methylation and genetic testing of peripheral blood were performed. MLH1 promoter methylation was not observed in peripheral blood. However, genetic testing revealed a large deletion in exon 5 of MLH1; therefore, we diagnosed this patient with Lynch syndrome.
Fig. 1.
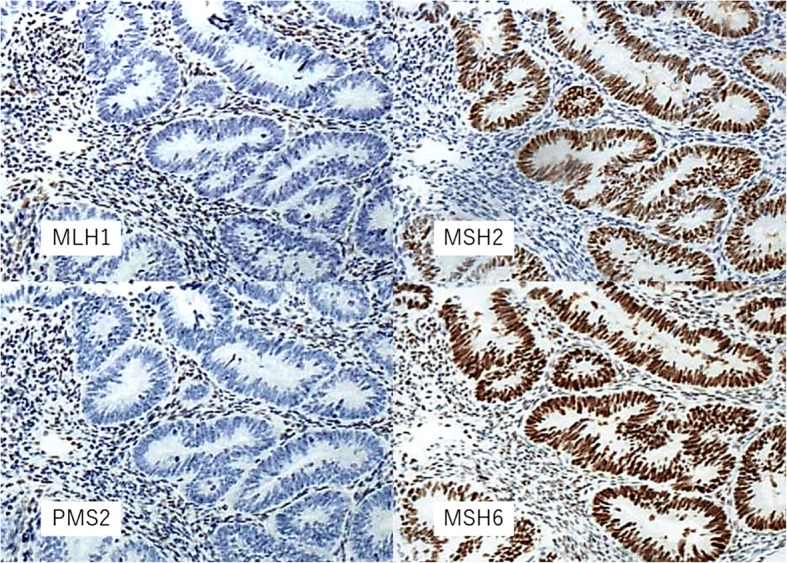
Immunohistochemistry. Losses of MLH1 and PMS2 proteins were detected in the tumor cells. Expressions of MSH2 and MSH6 proteins were detected in the tumor cells. Expressions of MLH1, PMS2, MSH2, MSH6 proteins were detected in the lymphocytes
Discussions and conclusions
This report demonstrated that in endometrial cancer, Lynch syndrome cannot be excluded even if MLH1 promoter hypermethylation is confirmed in cases with MLH1 and PMS2 protein loss detected using IHC.
Buchanan et al. proposed a strategy for screening for Lynch syndrome in endometrial cancer patients using MSI, IHC, and MLH1 promoter methylation. They recommended performing IHC on tumor tissue samples from patients who develop endometrial cancer at or before 60 years of age [7]. MLH1 germline mutation, epimutation [14], or epigenetic silencing can be considered when MLH1 and PMS2 protein losses are detected. Buchanan et al. reported that mismatch repair IHC tumor testing had high sensitivity and poor positive predictive value [7]. Therefore, it is necessary to conduct further examinations because of the poor positive predictive value. BRAF testing in colon cancer is useful to rule out Lynch syndrome [6]. However, the usefulness of BRAF testing to detect endometrial cancer is unclear because BRAF mutation is infrequently found [15]. MLH1 promoter methylation testing is useful for distinguishing between MLH1 germline mutation and epigenetic silencing. MLH1 promoter hypermethylation is far more responsible for losses of MLH1 and PMS2 protein using IHC than MLH1 germline mutation in the population based screening. Thus, cases with MLH1 promoter hypermethylation in cancer tissue are generally classified as sporadic endometrial cancer with abnormal somatic mismatch repair gene [7].
However, recently some reports showed cases with germline mutations among colorectal cancer cases in which although MLH1 and PMS2 proteins were lost by IHC, MLH1 promoter hypermethylation was observed in cancer tissues [11–13] (Table 1). These reports suggested that it is inappropriate to exclude Lynch syndrome based on a result of MLH1 promoter hypermethylation. Hagen et al. reported that they confirmed MSH2 germline mutation and somatic MLH1 promoter hypermethylation in a 71-year-old female with confirmed MLH1, MSH2, MSH6, and PMS2 protein losses in the colon cancer tissues using IHC [11]. Raymond et al. also reported confirmed MSH6 germline mutation and somatic MLH1 promoter hypermethylation in a 75-year-old female with losses of MLH1, MSH6, and MSH2 proteins in the colon cancer tissue samples using IHC and concluded that MLH1 promoter hypermethylation does not exclude the diagnosis of Lynch syndrome [12]. Rahner et al. examined MLH1 promoter methylation from 60 carriers of MLH1 germline mutation, 38 carriers of MSH2 germline mutation, and 25 individuals without germline mutation. MLH1 promoter methylation was observed in one carrier each of MLH1 and MSH2 germline mutations. Therefore, they concluded that MLH1 promoter hypermethylation could not exclude the diagnosis of Lynch syndrome [13]. In the National Comprehensive Cancer Network guidelines, when losses of MLH1 and PMS2 proteins are detected using IHC in the presence of somatic BRAF mutations and MLH1 promoter hypermethylation, it is recommended to consider genetic testing in cases of early onset and positive family history of Lynch-associated malignancies [5]. In the present case, sporadic endometrial cancer was suspected based on the results of IHC and MLH1 promoter methylation testing of endometrial cancer tissues. MLH1 promoter methylation was not observed in the peripheral blood; however, a large deletion in exon 5 of this gene was observed. Therefore, we diagnosed the patient with Lynch syndrome. With respect to endometrial cancer, the coexistence of MLH1 germline mutation and MLH1 promoter hypermethylation in the same patient was absent in the range of our literature search. However, similar to colorectal cancer, when screening for Lynch syndrome using endometrial cancer tissue, this is a pitfall that should not be overlooked.
Table 1.
Co-existed MLH1 hypermethylation and mismatch repair genes germline mutations
| IHC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor | MLH1 | MSH2 | MSH6 | PMS2 | MSI | BRAF V600E mutation | MLH1 promoter methylation | Germline mutation | |
| Hagen et al. [11] | CRC | – | – | – | – | N/A | Wild-type | + | MSH2 mutation |
| Raymond et al. [12] | CRC | – | + | – | – | MSI-H | Wild-type | + | MSH6 mutation |
| Rahner et al. [13] | CRC | – | + | + | – | MSI-H | N/A | + | MLH1 mutation |
| Yokoyama et al. | EC | – | + | + | – | N/A | N/A | + | MLH1 mutation |
CRC colorecral cancer, EC uterine endometrial cancer, N/A not available
In summary, we have demonstrated that in endometrial cancer, similar to colorectal cancer, both MLH1 germline mutation and somatic MLH1 promoter hypermethylation may be observed in the same patient and that Lynch syndrome cannot be excluded even if MLH1 promoter hypermethylation is observed. From a cost perspective, it is considered prohibitive to conduct genetic testing for all cases that present with MLH1 promoter hypermethylation. However, it has been suggested that genetic testing should be considered in endometrial cancer patients with MLH1 promoter hypermethylation at least if clinical and family histories are indicative of Lynch syndrome.
Acknowledgments
The authors thank Crimson Interactive Pvt. Ltd. (Ulatus) – http://www.ulatus.jp for their assistance in manuscript translation and editing.
Funding
This research was partially supported by The National Cancer Center Research and Development Fund (25-A-1 and 28-A-1), the Practical Research for Innovative Cancer Control, and the Program for Promoting Practical Applications of Genomic Medicine from the Japan Agency for Medical Research and Development, AMED (15ck0106097 h0102 and 15cK0106168 h0201).
Abbreviations
- IHC
Immunohistochemistry
- MSI
Microsatellite instability
- N/A
Not available
Authors’ contribution
TknY drafted the manuscript and performed the literature review. KT, EF, MS, SOk, YS, and TksY revised the manuscript. KT, NS, KK, SOh, and NT undertook genetic counseling and performed immunohistochemistry and MLH1 methylation testing. SS, KI, and KS performed germline testing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study has been approved by the ethical review board of Shikoku Cancer Center.
Consent for publication
Written informed consent to publish the data was obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takanori Yokoyama, Phone: +81-89-999-1111, Email: tayokoyama@shikoku-cc.go.jp.
Kazuhiro Takehara, Email: katakehara@shikoku-cc.go.jp.
Nao Sugimoto, Email: naokamae@saitama-med.ac.jp.
Keika Kaneko, Email: kekaneko@shikoku-cc.go.jp.
Etsuko Fujimoto, Email: etfujimoto@shikoku-cc.go.jp.
Mika Okazawa-Sakai, Email: misakai@shikoku-cc.go.jp.
Shinichi Okame, Email: shookame@shikoku-cc.go.jp.
Yuko Shiroyama, Email: y-shiroyama73182@pref.hiroshima.lg.jp.
Takashi Yokoyama, Email: tyokoyam@shikoku-cc.go.jp.
Norihiro Teramoto, Email: nteramot@shikoku-cc.go.jp.
Shozo Ohsumi, Email: sosumi@shikoku-cc.go.jp.
Shinya Saito, Email: labo-g1@tochigi-cc.jp.
Kazuho Imai, Email: kazimai@tochigi-cc.jp.
Kokichi Sugano, Email: ksugano@tochigi-cc.jp.
References
- 1.Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. EPICOLON Consortium. Identification of lynch syndrome among patients with colorectal cancer. JAMA 2012 ;308:1555–1565. [DOI] [PMC free article] [PubMed]
- 2.Bonadona V, Bonaïti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. French Cancer genetics network. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 3.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, lynch syndrome) proposed by the international collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/S0016-5085(99)70510-X. [DOI] [PubMed] [Google Scholar]
- 4.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Provenzale D, Gupta S, Ahnen DJ, Bray T, Cannon JA, Cooper G, et al. Genetic/familial high-risk assessment: colorectal version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2016;14:1010–1030. doi: 10.6004/jnccn.2016.0108. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the US multi-society task force on colorectal Cancer. Dis Colon rectum. 2014;57:1025–1048. doi: 10.1097/DCR.000000000000000. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014;32:90–100. doi: 10.1200/JCO.2013.51.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for lynch syndrome screening in endometrial cancers from GOG210: an NRG oncology and gynecologic oncology group study. J Clin Oncol. 2015;33:4301–4308. doi: 10.1200/JCO.2015.63.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon JS, Scott JL, Gilks CB, Daniels MS, Sun CC, Lu KH. Testing women with endometrial cancer to detect lynch syndrome. J Clin Oncol. 2011;29:2247–2252. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takehara K, Komatsu M, Okame S, Shiroyama Y, Yokoyama T, Tanaka S, et al. Evaluation of DNA mismatch repair protein expression as a preliminary screening for lynch syndrome in young Japanese women with endometrial cancer. Gynecol Obstet. 2016;6:2161–0932. doi: 10.4172/2161-0932.1000400. [DOI] [Google Scholar]
- 11.Hagen CE, Lefferts J, Hornick JL, Srivastava A. “null pattern” of immunoreactivity in a lynch syndrome-associated colon cancer due to germline MSH2 mutation and somatic MLH1 hypermethylation. Am J Surg Pathol. 2011;35:1902–1905. doi: 10.1097/PAS.0b013e318237c6ab. [DOI] [PubMed] [Google Scholar]
- 12.Raymond VM, Morris AM, Hafez KS, Greenson JK. MLH1 promotor hypermethylation does not rule out a diagnosis of lynch syndrome: a case report. Familial Cancer. 2015;14:77–80. doi: 10.1007/s10689-014-9753-0. [DOI] [PubMed] [Google Scholar]
- 13.Rahner N, Friedrichs N, Steinke V, Aretz S, Friedl W, Buettner R, et al. Coexisting somatic promoter hypermethylation and pathogenic MLH1 germline mutation in lynch syndrome. J Pathol. 2008;214:10–16. doi: 10.1002/path.2263. [DOI] [PubMed] [Google Scholar]
- 14.Hitchins MP, Ward RL. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet. 2009;46:793–802. doi: 10.1136/jmg.2009.068122. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf AM, Spurdle AB. Endometrial tumour BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Familial Cancer. 2014;13:1–12. doi: 10.1007/s10689-013-9671-6. [DOI] [PubMed] [Google Scholar]


