Abstract
We report, for the first time, the presence of ungulate malaria parasites in South America. We conducted PCR-based surveys of blood samples of multiple deer species and water buffalo from Brazil and detected Plasmodium sequences from pampas deer (Ozotoceros bezoarticus) samples. Phylogenic analysis revealed that the obtained sequences are closely related to the Plasmodium odocoilei clade 2 sequence from North American white-tailed deer (Odocoileus virginianus). Nucleotide differences suggest that malaria parasites in South American pampas deer and North American P. odocoilei clade 2 branched more recently than the Great American Interchange.
Keywords: Malaria, Pampas deer, South America, Plasmodium odocoilei, Brazil
Graphical abstract
Highlights
-
•
Plasmodium sequence was detected from pampas deer in South America.
-
•
It was most similar to the North American Plasmodium odocoilei clade 2 sequence.
-
•
Estimated divergence time was much more recent than the Great American Interchange.
Plasmodium parasites of even-toed ungulates have been reported from Africa (duiker [Sylvicapra grimmia], marshbuck [Tragelaphus spekii], and goat [Capra aegagrus hircus]; van den Berghe, 1937; reviewed in Garnham, 1966), Asia (water buffalo [Bubalus bubalis] and mouse deer [Tragulus javanicus]; reviewed in Garnham, 1966), and North America (white-tailed deer [Odocoileus virginianus]; Kuttler et al., 1967, Garnham and Kuttler, 1980) based on microscopic observations. In 2016, three groups reported the first molecular analyses of this group of malaria parasites (reviewed in Templeton et al., 2016b). Martinsen et al. (2016) detected two Plasmodium sequences, termed P. odocoilei clade 1 and 2 based on the report by Kuttler et al. (1967), from white-tailed deer and Anopheles mosquitoes in several locations in the United States of America (Martinsen et al., 2016). Boundenga et al. (2016) reported Plasmodium sequences from duiker antelope (Cephalophus spp.) in Africa (Boundenga et al., 2016). Thirdly, Templeton et al. (2016a) detected two distinct Plasmodium sequences from water buffalo in Thailand and Vietnam (Templeton et al., 2016a), which were provisionally called Plasmodium bubalis types I and II based on a report in India (Sheather, 1919); and one sequence from a goat in Zambia, provisionally called Plasmodium caprae based upon a report in Africa (de Mello and Paes, 1923). It was shown that DNA sequences from the three studies formed a monophyletic clade within haemosporidian parasites, distinct from a clade containing other mammalian and avian/reptile Plasmodium parasites (Templeton et al., 2016a, Templeton et al., 2016b).
Based on the divergence of the two groups of Plasmodium sequences detected from white-tailed deer and mosquitoes in North America, Martinsen et al. (2016) suggested that they likely represented distinct species and that the ancestor of these parasites migrated from Siberia to North America with their host deer. This model was based on the estimated divergence time of 2.3–6 million years ago (MYA), consistent with the estimated period ancestral deer crossed the Bering Land Bridge to North America 4.2 to 5.7 MYA (Gilbert et al., 2006). During the Great American Interchange around 3 MYA (Stehli and Webb, 1985, Duarte et al., 2008), deer migrated from North America to South America, and thus it would be expected that Plasmodium parasites co-migrated with their deer hosts, dependent on available mosquito vectors. Water buffalo were introduced to the Brazil Amazon Basin as early as 1895 (Sheikh et al., 2006), thus it is possible that P. bubalis was also introduced to Brazil along with their host. No ungulate Plasmodium parasites have been reported in South America to date, and therefore we sought to determine the occurrence of South American ungulate malaria parasites by conducting PCR-based screening of archived DNA samples obtained from ungulates in Brazil.
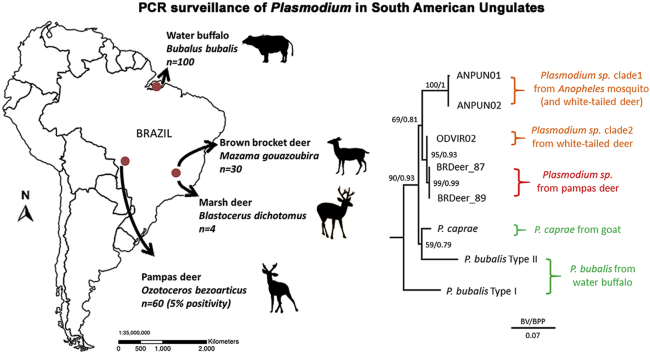
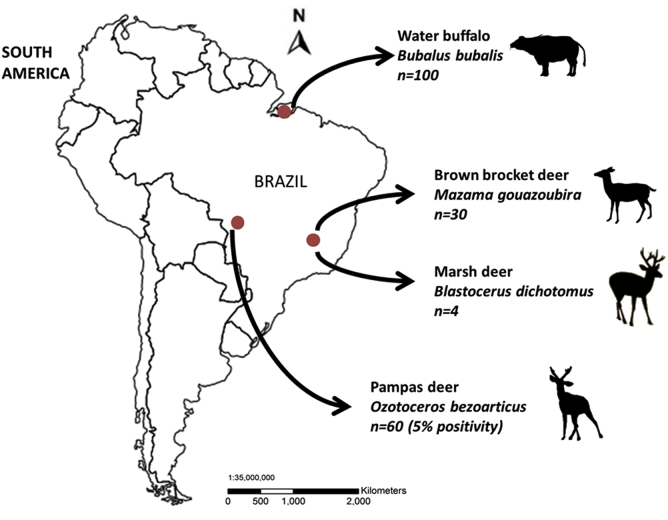
A total of 194 DNA samples were examined from the following animals: 60 free-living pampas deer (Ozotoceros bezoarticus) in the Pantanal region, 30 free-living or captive brown brocket deer (Mazama gouazoubira) and 4 captive marsh deer (Blastocerus dichotomus) in Minas Gerais state, and 100 Asian water buffalo (Bubalus bubalis) in Para state in the Amazon region (Fig. 1). All DNA samples were originally collected for a study to detect tick-borne pathogens, under the approval of the Ethics Committee on Animal Experimentation and the Brazilian Institute for Environment and Natural Renewable Resources (Silveira et al., 2011, Silveira et al., 2012, Silveira et al., 2013, Silveira et al., 2014, Silveira et al., 2016). Nested PCR targeting Plasmodium mitochondrial cytochrome b (cytb) (Templeton et al., 2016a) revealed 3 pampas deer samples were positive. PCR products were directly sequenced and 747 to 773 bp nucleotide sequences were determined. Sequences from two deer (BRDeer_87 and BRDeer_88) were identical and the sequence from deer BRDeer_89 showed one nucleotide difference from the first sequence. BLASTN search of the NCBI non-redundant nucleotide collection database with the 747 bp BRDeer_87 nucleotide sequence as a query hit P. caprae (LC090215.1) with 98% identity and P. bubalis (LC090214.1 and LC090213.1) with 96% identity, indicating that the PCR products amplified from pampas deer samples were derived from Plasmodium species.
Fig. 1.
Map of Brazil depicting the sampling sites of deer and water buffalo with respective sample size.
Sixty pampas deer (Ozotoceros bezoarticus) in the Pantanal region, 30 brown brocket deer (Mazama gouazoubira) and 4 marsh deer (Blastocerus dichotomus) in Minas Gerais state, and 100 water buffalo (Bubalus bubalis) in Para state in the Amazon region were analyzed. Note that only 3 pampas deer samples were positive (5% positivity).
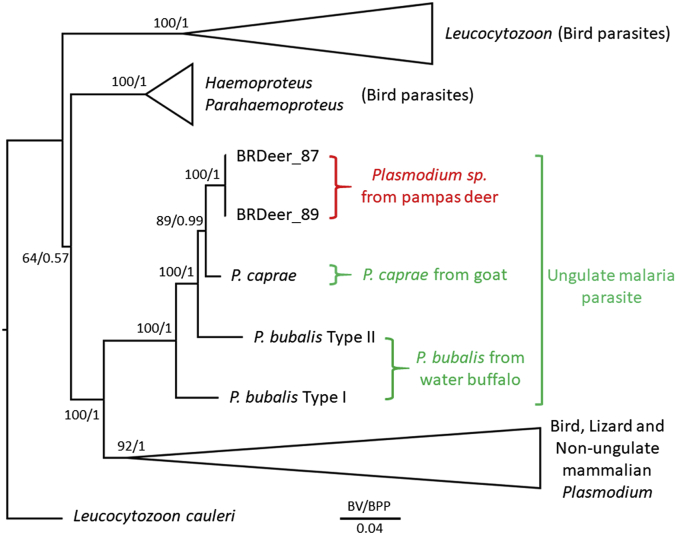
For in-depth phylogenetic analysis, we attempted to determine the whole mitochondrial DNA (mtDNA) sequences (∼6 kb) for these samples; however, likely due to the low amount of parasite DNA, only ∼3.4 kb of the mtDNA sequence containing the entire cytb and cytochrome oxidase I (cox1) sequences were determined for BRDeer_87 and BRDeer_89. A phylogenetic tree was constructed by the maximum likelihood (ML) method with related haemosporidian parasites for which sequences corresponding to the ∼3.4 kb mtDNA region were available. Model analysis using IQ-TREE ver. 1.5.5 indicated that the GTR + I + G model was superior to other models by both Akaike and Bayesian information criterion (Trifinopoulos et al., 2016). Following model analysis, ML analysis was conducted using IQ-TREE ver. 1.5.5 with 1000 replicates of ultrafast bootstrap analysis. Bayesian posterior probabilities (BPP) were also obtained using MrBayes ver. 3.2 with Metropolis-coupled Markov chain Monte Carlo runs, consisting of one cold and four heated chains with a chain temperature of 0.1, for 10,000,000 generations (Ronquist et al., 2012). Log-likelihood scores and trees with branch lengths were sampled every 1000 generations and the first 2,500,000 generations were excluded as burn-in, and the remaining trees were summarized to obtain BPP. The tree was visualized by FigTree ver1.4.3. The sequences from Brazilian pampas deer localized within the ungulate malaria parasite clade with the highest ML bootstrap support and BPP (100 and 1.00, respectively, Fig. 2). The tree also indicated that the pampas deer Plasmodium sequences form one clade with P. caprae and P. bubalis Type II sequences (100/1.00) apart from the P. bubalis Type I sequence, which also confirmed a phylogenetic relationship reported by Templeton et al. (2016b). P. odocoilei sequences from white-tailed deer were not included in this analysis, due to the lack of sequence information for the corresponding mtDNA region.
Fig. 2.
Phylogenetic relationships of Plasmodium sequences from Brazilian pampas deer within Haemosporidia.
The tree was constructed using ∼3.4 kb of partial mitochondrial nucleotide sequences by the maximum likelihood (ML) method based on the GTR + I + G model. Bootstrap values (BV) for ML with 1000 replicates of ultrafast bootstrap analysis and Bayesian posterior probability (BPP) are indicated for each internal branch. The compositions of collapsed clades are Leucocytozoon (L. fringillinarium, L. majoris, and L. sabrasezi); Haemoproteus and Parahaemoproteus (Haemoproteus sp. jb1.JA27, Haemoproteus sp. jb2.SEW5141, and Parahaemoproteus vireonis); and bird, lizard and non-ungulate mammalian Plasmodium (P. gallinaceum, P. relictum, P. juxtinucleare, P. lutzi, P. floridense, P. mexicanum, P. falciparum, P. vivax, P. malariae, P. ovale, P. coatneyi, P. cynomolgi, P. fieldi, P. gonderi, P. inui, P. knowlesi, P. fragile, P. simiovale, P. simium, P. hylobati, P. reichenowi, P. billicollinsi, P. billbrayi, P. berghei, P. chabaudi, P. vinckei, and P. yoelii). Mitochondrial DNA sequences (including cytb and cox1) used in this study were listed in the Supplementary Table S1 of Templeton et al. (2016a). Nucleotide sequences of Plasmodium sp. in the North American white-tailed deer was based on Table S4 of Martinsen et al. (2016). Nucleotide positions containing indels or undetermined nucleotides, or those where the alignment was not clearly made were excluded. Nucleotide positions corresponding to the P. falciparum mtDNA (NC_002375.1) 974–1502, 1509–1576, 1578–1628, 1637–1678, 1698–1760, 1762–1769, 1774–1800, 1806–1831, 1834–1867, 1870–1909, 1914–2031, 2050–3474, and 3486–4444 were used. The length for the substitutions/site (0.04) is indicated.
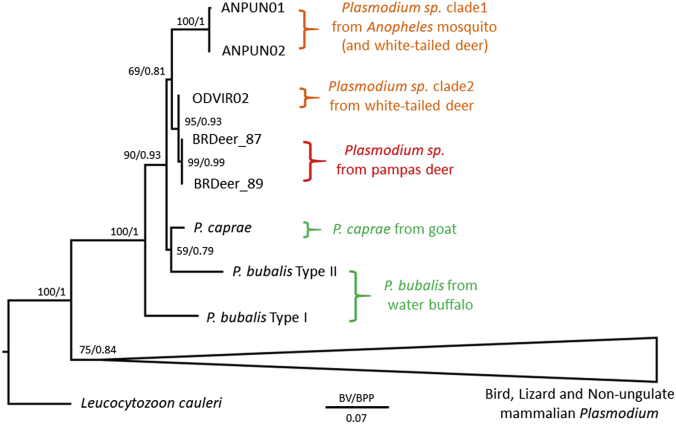
We next examined the relationship of the Plasmodium sequences from Brazilian pampas deer with North American P. odocoilei sequences. Partial cytb (607 bp) and cox1 (490 bp) sequence regions available for North American P. odocoilei clade 1 (KU133755 and KU133748 for cytb, KU133758 and KU133757 for cox1) and clade 2 (KU133751 and KU133759, respectively) were concatenated (total 1097 bp) and phylogenetic analysis was conducted with related haemosporidian parasites for which the corresponding cytb and cox1 sequences were available. The resulting tree indicated that the 2 sequences from Brazilian pampas deer form one clade with all reported P. odocoilei-type sequences (with ML/BPP of 69/0.81, respectively), and were monophyletic with the P. odocoilei clade 2 with strong support (95/0.93, respectively) (Fig. 3). The difference between Plasmodium sequences from Brazilian pampas deer and P. odocoilei clade 2 sequence (ODVIR02) were 1 nucleotides per 607 bp for cytb and 6 nucleotides per 982 bp for cox1. When a divergence rate of 0.5–1.3% My−1, proposed for Plasmodium cytb, was adopted (Ricklefs and Outlaw, 2010, Pacheco et al., 2011) the Plasmodium in Brazilian pampas deer and P. odocoilei clade 2 were estimated to have diverged approximately 0.3–0.9 MYA (0.1–0.3 MYA by cytb and 0.5–1.2 MYA by cox1). This divergence estimate is much more recent than the Great American Interchange, which occurred around 3 MYA (Stehli and Webb, 1985, Duarte et al., 2008). Ancestors of South American pampas deer and North American white-tailed deer are believed to have separated in North America about 5 MYA (Pitra et al., 2004, Gilbert et al., 2006), and then the ancestor of pampas deer migrated to South America and expanded, whereas this lineage became extinct in North America. One evolutionary scenario of Plasmodium in Brazilian pampas deer is that P. odocoilei clades 1 and 2 evolved in North American deer and clade 2 recently migrated to South America (less than 0.9 MYA) and infected local pampas deer. If this is the case, clade 1 P. odocoilei might have also migrated to South America and awaits discovery. An alternative hypothesis is as follows, the estimated divergence time of P. odocoilei clades 1 and 2 was 2.3–6 MYA, and thus divergence of P. odocoilei clades 1 and 2 could have occurred during the Great American Interchange (∼3 MYA). Thus, it is possible that the Plasmodium parasite in the ancestor of pampas deer migrated to South America with its host during the Great American Interchange and via geographic isolation diverged to form the clade 2 P. odocoilei malaria parasite in South America and clade 1 P. odocoilei in North America. Following this divergence the clade 2 P. odocoilei then migrated back to North America.
Fig. 3.
Phylogenetic relationship of Plasmodium sequences from Brazilian pampas deer within ungulate Plasmodium spp.
The tree was constructed using concatenated partial nucleotide sequences of cytb and cox1 by maximum likelihood (ML) method based on the GTR + I + G model. Bootstrap values (BV) for ML with 1000 replicates of ultrafast bootstrap analysis and Bayesian posterior probability (BPP) are indicated for each internal branch. The compositions of collapsed clades are described in Fig. 2 legend. The length for the substitutions/site (0.07) is indicated.
Water buffalo in the Brazilian Amazon are infected with Theileria parasites that harbor similar sequences with Asian Theileria parasites such as T. buffali, T. orientalis or T. sinensis (Silveira et al., 2016). Thus we expected to detect P. bubalis in these water buffalo samples; however, this was not the case and further studies will be necessary to clarify if P. bubalis was introduced to South America.
In conclusion, this is the first report of ungulate malaria parasites in South America. Plasmodium DNA sequences originating from Brazilian pampas deer form a monophyletic group with P. odocoilei clade 2 that infects white-tailed deer in North America. The estimated diverged time of 0.3–0.9 MYA is much more recent than the Great American Interchange. Because our data is solely based on the DNA information, morphological investigations should be performed in the future to confirm that the obtained sequences were derived from Plasmodium parasites actively infecting the pampas deer. Comprehensive population genetic surveys for Plasmodium species infecting South American deer would provide information to clarify the evolutionary history of this group of parasites, which may in-turn shed new light on the migration history of their host deer in American continents.
Acknowledgements
We thank Thomas Templeton for critical reading of the manuscript. This study was supported in part by JSPS Grants-in-Aids for Scientific Research (B), No 16H05807 to OK and MA, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, No 309202/2013-2 to EMB, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil) No 25242 and Universidade Federal de Minas Gerais (UFMG, Brazil) No 23853. The authors are grateful to IBAMA (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis) and to EMBRAPA-Pantanal (Brazilian Enterprise for Agricultural Research). This work was partly conducted at the Joint Usage/Research Center on Tropical Disease, Institute of Tropical Medicine, Nagasaki University.
Footnotes
Nucleotide sequences determined from this study were deposited to DDBJ/ENA/GenBank with the accession numbers: LC326033 (BRDeer_87), LC326034 (BRDeer_89) and LC326035 (BRdeer_88).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.01.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Boundenga L., Makanga B., Ollomo B., Gilabert A., Rougeron V., Mve-Ondo B., Arnathau C., Durand P., Moukodoum N.D., Okouga A.P., Delicat-Loembet L., Yacka-Mouele L., Rahola N., Leroy E., Ba C.T., Renaud F., Prugnolle F., Paupy C. Haemosporidian parasites of antelopes and other vertebrates from Gabon, Central Africa. PLoS One. 2016;11:e0148958. doi: 10.1371/journal.pone.0148958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello F., Paes S. Sur une plasmodiae du sang des chèvres. C.r. Séanc. Soc. Biol. 1923;88:829–830. [Google Scholar]
- Duarte J.M., González S., Maldonado J.E. The surprising evolutionary history of South American deer. Mol. Phylogenet. Evol. 2008;49:17–22. doi: 10.1016/j.ympev.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Garnham P.C.C. Blackwell Scientific Publications; 1966. Malaria Parasites and Other Haemosporidia. [Google Scholar]
- Garnham P.C., Kuttler K.L. A malaria parasite of the white-tailed deer (Odocoileus virginianus) and its relation with known species of Plasmodium in other ungulates. Proc. R. Soc. Lond. B Biol. Sci. 1980;206:395–402. doi: 10.1098/rspb.1980.0003. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Ropiquet A., Hassanin A. Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): systematics, morphology, and biogeography. Mol. Phylogenet. Evol. 2006;40:101–117. doi: 10.1016/j.ympev.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Kuttler K.L., Robinson R.M., Rogers W.P. Exacerbation of latent erythrocytic infections in deer following splenectomy. Can. J. Comp. Med. Vet. Sci. 1967;31:317–319. [PMC free article] [PubMed] [Google Scholar]
- Martinsen E.S., McInerney N., Brightman H., Ferebee K., Walsh T., McShea W.J., Forrester T.D., Ware L., Joyner P.H., Perkins S.L., Latch E.K., Yabsley M.J., Schall J.J., Fleischer R.C. Hidden in plain sight: cryptic and endemic malaria parasites in North American white-tailed deer (Odocoileus virginianus) Sci Adv. 2016;2:e1501486. doi: 10.1126/sciadv.1501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M.A., Battistuzzi F.U., Junge R.E., Cornejo O.E., Williams C.V., Landau I., Rabetafika L., Snounou G., Jones-Engel L., Escalante A.A. Timing the origin of human malarias: the lemur puzzle. BMC Evol. Biol. 2011;11:299. doi: 10.1186/1471-2148-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitra C., Fickel J., Meijaard E., Groves P.C. Evolution and phylogeny of old world deer. Mol. Phylogenet. Evol. 2004;33:880–895. doi: 10.1016/j.ympev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E., Outlaw D.C. A molecular clock for malaria parasites. Science. 2010;329:226–229. doi: 10.1126/science.1188954. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheather A.L. A malaria parasite in the blood of a buffalo. J. Comp. Pathol. Therapeut. 1919;32:223–226. [Google Scholar]
- Sheikh P.A., Merry F.D., McGrath D.G. Water buffalo and cattle ranching in the lower Amazon basin: comparisons and conflicts. Agric. Syst. 2006;87:313–330. [Google Scholar]
- Silveira J.A., de Oliveira C.H., Silvestre B.T., Albernaz T.T., Leite R.C., Barbosa J.D., Oliveira C.M., Ribeiro M.F. Molecular assays reveal the presence of Theileria spp. and Babesia spp. in Asian water buffaloes (Bubalus bubalis, Linnaeus, 1758) in the Amazon region of Brazil. Ticks Tick Borne Dis. 2016;7:1017–1023. doi: 10.1016/j.ttbdis.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Silveira J.A., Rabelo E.M., Lacerda A.C., Borges P.A., Tomás W.M., Pellegrin A.O., Tomich R.G., Ribeiro M.F. Molecular detection and identification of hemoparasites in pampas deer (Ozotoceros bezoarticus Linnaeus, 1758) from the Pantanal Brazil. Ticks Tick Borne Dis. 2013;4:341–345. doi: 10.1016/j.ttbdis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Silveira J.A., Rabelo E.M., Lima P.C., Chaves B.N., Ribeiro M.F. Post-mortem hemoparasite detection in free-living Brazilian brown brocket deer (Mazama gouazoubira, Fischer 1814) Rev. Bras. Parasitol. Vet. 2014;23:206–215. doi: 10.1590/s1984-29612014035. [DOI] [PubMed] [Google Scholar]
- Silveira J.A., Rabelo E.M., Ribeiro M.F. Detection of Theileria and Babesia in brown brocket deer (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus) in the State of Minas Gerais, Brazil. Vet. Parasitol. 2011;177:61–66. doi: 10.1016/j.vetpar.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Silveira J.A., Rabelo E.M., Ribeiro M.F. Molecular detection of tick-borne pathogens of the family Anaplasmataceae in Brazilian brown brocket deer (Mazama gouazoubira, Fischer, 1814) and marsh deer (Blastocerus dichotomus, Illiger, 1815) Transbound. Emerg. Dis. 2012;59:353–360. doi: 10.1111/j.1865-1682.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- Stehli F.G., Webb S.D. Plenum Press; New York: 1985. The Great American Biotic Interchange. Topics in Geobiology. [Google Scholar]
- Templeton T.J., Asada M., Jiratanh M., Ishikawa S.A., Tiawsirisup S., Sivakumar T., Namangala B., Takeda M., Mohkaew K., Ngamjituea S., Inoue N., Sugimoto C., Inagaki Y., Suzuki Y., Yokoyama N., Kaewthamasorn M., Kaneko O. Ungulate malaria parasites. Sci. Rep. 2016;6:23230. doi: 10.1038/srep23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton T.J., Martinsen E., Kaewthamasorn M., Kaneko O. The rediscovery of malaria parasites of ungulates. Parasitology. 2016;143:1501–1508. doi: 10.1017/S0031182016001141. [DOI] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L.T., von Haeseler A., Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe L. Plasmodium limnotragi n. sp., d’une antelope Limnotragus spekei. Bull. Soc. Pathol. Exot. Fil. 1937;30:272–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.