Abstract
Introduction
Low grade fibromyxoid sarcoma (LGFMS)1 is a rare soft tissue tumor involving deep soft tissues of the extremities and trunk. Abdominal location is extremely uncommon in which the few cases published in the literature are characterized by slow tumoral progression and long recurrence-free intervals.
Methods
We report the first case of an intra-abdominal LGFMS which was discovered incidentally in a 42-year-old woman presenting diffuse peritoneal nodules and hepatic metastasis on CT and MRI scans.
Results
The patient was successfully treated through conservative measures and remained asymptomatic at the 48 month follow-up.
Conclusions
This is the first reported case of LGFMS with peritoneal and hepatic metastases. Despite the discovery of an advance disease at exploration, the patient who refused a major surgical operation presents an uneventful follow-up and long term survival.
Abbreviations: LGFMS, low grade fibromyxoid sarcoma; CT, computed tomography; MRI, magnetic resonance imaging; PC, peritoneal carcinomatosis; EMA, epithelial membrane antigen; FISH, fluorescence in situ hybridization; PCI, peritoneal carcinomatosis index; HSCTGR, hyalinizing spindle cell tumor with giant rosettes; RT-PCR, reverse transcription polymerase chain reaction
Keywords: Low grade fibromyxoid sarcoma, Peritoneal metastases, Hepatic metastases, Prognosis, Treatment
1. Introduction
Low grade fibromyxoid sarcoma (LGFMS) is a rare entity, first described by Evans in 1987 as a malignant tumor having a deceptively benign appearance [1], [2], [3]. It is most commonly found in the trunk and proximal extremities but can occur almost anywhere in the body [1], [2]. There are very few cases of intra-abdominal LGFM reported in the literature, even fewer with diffuse peritoneal nodules [4], [5], [6], [7], [8] and only one case describes a hepatic metastasis in a patient with LGFMS of the extremities [3].
We present the first case of a 42-year-old woman with an incidental discovery of an intra-abdominal LGFMS with peritoneal and hepatic metastases. Diagnosis management and the current follow-up are subsequently discussed.
2. Presentation of case
Following an abdominoplasty in a 42-year-old female patient, resection of a fibro-adipose tissue protruding through a hernial defect revealed a LGFMS with positive resection margins.
The patient was completely asymptomatic and had no relevant medical or surgical history. The physical examination was positive for palpable mobile intra-abdominal masses and nodules in the Douglas pouch.
An abdominal computed tomography (CT), showed a suspicious lesion of 49 mm in segment VIII of the liver and multiple disseminated peritoneal nodules suggesting peritoneal carcinomatosis (PC) (Fig. 1). An abdominal MRI showed non-specific post-operative fatty stranding in the abdominal wall and confirmed the CT scan findings. A whole body 18F-FDG-PET/CT showed only a weak FDG uptake within some peritoneal nodules (Fig. 2).
Fig. 1.

Coronal contrast abdominal CT shows the hepatic metastatic lesions (white arrows) and multiple disseminated peritoneal nodules (black arrows).
Fig. 2.
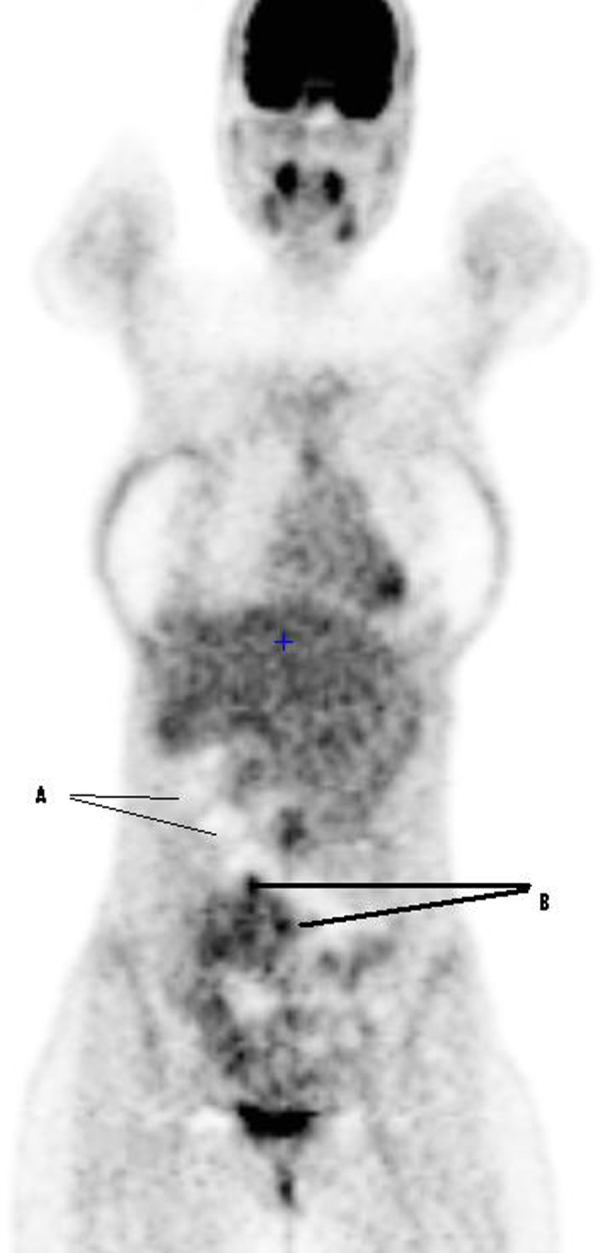
Coronal Pet/CT image. A: nodules without metabolic activity; B: nodules with a weak hypermetabolic activity.
A biopsy of the liver lesion was positive for liver metastasis of a LGFMS. Initially, the diagnosis has been made exclusively on morphologic criteria including an alternance of collagenized and myxoid areas (Fig. 3). Negative immunostaining for CD117 and KIT ruled out a gastro-intestinal stromal tumor. The FISH test analysis for the FUS gene rearrangement was non-contributory. Afterwards, immunostaining with specific markers including MUC4 and epithelial membrane antigen (EMA) have been performed. EMA was negative but MUC4 was strongly and diffusely expressed (Fig. 4). Serum CA-125 level was normal and serum IgE antibodies for Entameaba histolytica, Entameaba granulosus and Schistosoma mansoni were all negative.
Fig. 3.

Histopathological image of LGFMS characterized by fibrous and myxoid areas and swirling whorled growth patterns with low to moderate cellularity and bland cells with minimal nuclear pleomorphism.
Fig. 4.

MUC4 immunostaining showing strong and diffuse expression.
We opted for an exploratory laparotomy in view of a debulking surgery and central partial hepatectomy. Peri-operatively, the peritoneal carcinomatosis index (PCI) was 25/39, indicating a more advanced peritoneal disease than had been shown on imagery. This would require a very aggressive surgery including: a large small bowel resection, a splenectomy, an omentectomy, a low anterior rectal resection, a partial cystectomy with a partial ureterectomy, a hysterectomy, a douglasectomy, as well as a partial hepatectomy and a protective ileostomy. Despite the fact that complete cytoreductive surgery could be achieved and that PCI score was not a contra-indication for surgery in presence of a low grade disease, we decided to abort surgery since patient agreed to surgery only under the condition that we avoid major imposition on her quality of life. Partial omentectomy was performed for histological examination. Intra-operative histopathological frozen examination of the omentum was positive for the LGFMS, confirmed by final examination.
Postoperatively, the patient confirmed her refusal of such an extensive surgery and also refused any adjuvant palliative treatment proposed after a multi-disciplinary discussion. The patient undergoes regular clinical and radiological follow-ups at 3 month intervals. At 48 months of diagnosis, she is still asymptomatic and in very good general health and the recent abdominal CT scan showed very discrete progression of the peritoneal carcinomatosis with no change at the hepatic and thoracic level.
3. Discussion
LGFMS is a rare type of soft-tissue sarcoma described for the first time in 1987 by Evans, characterized by bland histological features and, paradoxically, a high mitotic rate [1], [2], [3]. During the last 20 years the clinical and histopathological features have been delineated by several series in order to facilitate the diagnosis which was previously often misinterpreted [9].
Cytogenetically, LGFMS is characterized by a t(7;16)(q33; p11) or more rarely t(11;16)(p11; p11) translocation, resulting in FUS-CREB3L2 or FUS-CREB3L1 fusion genes [10], [11], [12]. Recently, MUC4, an epithelial glycoprotein, has been identified as a specific and sensitive marker for LGFMS useful for the differential diagnosis [13]. In our case, MUC4 was not yet available at the moment of diagnosis. But, it has been performed afterwards after the publication of Doyle in 2011 and definitively confirmed the diagnosis of LGFMS.
The radiological appearance of LGFMS is often insufficient to establish the diagnosis. Nevertheless, CT and other imagery tools such as MRI and ultrasound are able to provide important supplementary information used for differential diagnosis and are considered as the exams of choice [14].
Although, several studies with insufficient follow-up have suggested a relatively good prognosis of LGFMS, the rate of local recurrences and late metastasis are proven to be relatively high [2], [3]. In 2011, Evans has demonstrated a high mitotic rate corresponding to 63% of local recurrences and 45% of metastases in a series with long term follow-up [3]. The metastases developed in intervals from 0 to 45 years after the primary diagnosis with typical metastatic sites in the lungs, pleura, chest wall and bone [3].
The golden standard treatment is a complete surgical resection of any resectable lesion. The rate of local recurrence proved to be clearly lower in specimens with negative resection margins [3], [12]. Surgical resection is also considered as a treatment of choice in cases of local recurrence or localized metastasis. Radiotherapy and chemotherapy could be used for metastatic or non resectable lesions, however clinical and histological responses seem quite poor [3].
We present a case of diffuse intra-abdominal LGFMS with peritoneal and hepatic metastases present at the moment of diagnosis. Although, LGFMS could be seen in more atypical locations like the retroperitoneum, chest wall or thoracic cavity, the intra-abdominal location is extremely rare. Only 6 cases had been previously published in the literature (Table 1).
Table 1.
Studies reporting cases of LGFS.
| Age/sex | Site of LGFMS | Treatment | Recurrence/follow up (mo) | |
|---|---|---|---|---|
| Harish et al. [4] | 37 years/M | Falciform ligament | Surgery | No/12 |
| Koishi et al. [5] | 38 years/F | Omentum | Surgery | No/36 |
| Park et al. [6] | 43 years/M | Colon | Surgery | No/24 |
| Laurini et al. [7] | 53 years/F | Small intestine | Surgery | No/2 |
| 71 years/M | Small intestine | Surgery | No/9 | |
| 52 years/F | Small intestine | Surgery | Yes/84 | |
| 69 years/F | Small intestine | Surgery | Unknown | |
| Evans [3] | 33 years/M | Omentum, mesentery, and peritoneum | Surgery (partial excision followed by 2nd partial excision at 1year) | Yes/60 |
| Chemoradiotherapy adjuvant | ||||
| Alevizopoulos et al. [8] | 48 years/M | Renal pelvis | Surgery | No/7 |
Recently, Evans reported a case of diffuse LGFMS of the omentum, mesentery and peritoneum in a 33-year-old-man, treated by partial excision (omentectomy). At 1 year he underwent another partial excision, followed by chemoradiotherapy of unknown protocol. The patient died due to tumor progression at 5 years after diagnosis [3].
In 2003, Harish et al. published a case of LGFMS of the falciform ligament in a 37-year-old man mistaken for a liver haemangioma on the CT scan. He was treated by a complete surgical excision and was recurrence-free at 1 year [4].
In 2003, Yoshiko et al. reported the LGFMS subtype with giant rosettes cell of omentum in a 38-year-old woman, consulted for pain and abdominal mass sensations. CT and MRI revealed a large multilobular tumor of the right adnexae. The patient underwent a surgical excision and the histology confirmed the diagnosis of LGFMS. The patient remains recurrence-free without any adjuvant treatment at 3 years [5].
A first case of LGFMS of the colon was published by Park et al. in 2007 in 43-year-old man who was explored surgically because of an obstructive mass of a hepatic flexure of the colon, discovered by colonofibroscopic examination without evidence of cancer by multiple colonoscopic biopsies. The treatment consisted of right hemicolectomy with right nephrectomy; the patient was recurrence-free without any other treatment at 2 years [6].
In 2011, four new cases of intestinal LGFMS were reported by Laurini et al. [7]. The patients, 3 females and 1 male ranging from 52 to 71 years presented a small bowel obstruction and underwent surgical exploration. One patient was lost to follow-up, and two patients were alive without any evidence of disease at 2 and 9 months after surgery. Three patients died of LGFMS at 7 years after diagnosis. No adjuvant treatment was delivered.
Finally in 2012, Alevizopoulos et al. reported a LGFMS of the renal pelvis in a 48-year-old man. The CT scan and ureteroscopy showed a renal pelvic tumoral mass on the left kidney. The radical nephro-ureterectomy was performed and the patient was free of local recurrence at the 7 month follow-up [8].
The only reported case of liver metastasis was published by Evans in 2011 [3]. A 38-year-old woman with LGFMS in the lower extremities, who had undergone surgical excision with probable positive margins, presented a local recurrence and lung, sternal, vertebral and liver metastases at 15 years. One lung and thoracic spine metastases were resected, respectively; followed by chemo-radiotherapy. The patient died from a tumor at 18.5 year follow-up.
4. Conclusion
LGFMS is characterized by a high rate of local recurrences and late metastasis despite a deceptively bland histological appearance. Our case has shown an indolent course of LGFMS even in the presence of diffuse intra-abdominal disease and hepatic metastasis, characteristic for an advanced disease. We highlight the importance to regularly discuss the individual treatment options with the patients. Since there are no established standardized multi-modality approaches for the curative treatment, very aggressive surgery with adjuvant chemotherapy is used. This may significantly impair the quality of life of patients in whom the disease may show a very slow, innocuous evolution without any treatment as reported in the present case. Further studies are needed in order to establish a more standardized multi-modality approach to this rare and poorly understood entity.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
None.
Ethical approval
Not applicable.
No EC approbation has been asked for this study because it is only the report of a clinical case.
Authors’ contributions
Jana Konecna: writing the manuscript (literature review), Gabriel Liberale: correction of the manuscript and supervision, Johnny Haddad: collaborating in the writing of the case report, Nicolas de Saint-Aubain: writing and images for pathological part, Issam El Nakadi: supervision.
Consent
The patient give her inform consent for the publication of this study.
Guarantor
Liberale Gabriel, M.D. and Ph.D. student.
Footnotes
LGFMS low grade fibromyxoid sarcoma.
References
- 1.Evans H.L. Low-grade fibromyxoid sarcoma, a report of 12 cases. Am. J. Surg. Pathol. 1993;17(June (6)):595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Folpe A.L., Lane K.L., Paull G. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes. Am. J. Surg. Pathol. 2000;24(October (10)):1353–1360. doi: 10.1097/00000478-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Evans H.L. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am. J. Surg. Pathol. 2011;35(October (10)):1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]
- 4.Harish K., Ashok A.C., Alva N.K. Low grade fibromyxoid sarcoma of the falciform ligament: a case report. BMC Surg. 2003;3(September):7. doi: 10.1186/1471-2482-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koishi A., Gomibuchi H., Inoue J. Hyalinizing spindle cell tumor with giant rosettes of the omentum. J. Obstet. Gynaecol. Res. 2003;29(December (6)):388–391. doi: 10.1111/j.1341-8076.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 6.Park I.J., Kim H.C., Yu C.S. Low-grade fibromyxoid sarcoma of the colon. Dig. Liver Dis. 2007;39(March (3)):274–277. doi: 10.1016/j.dld.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Laurini J.A., Zhang L., Goldblum J.R. Low-grade fibromyxoid sarcoma of the small intestine: report of 4 cases with molecular cytogenetic confirmation. Am. J. Surg. Pathol. 2011;35(July (7)):1069–1073. doi: 10.1097/PAS.0b013e31821bc17a. [DOI] [PubMed] [Google Scholar]
- 8.Alevizopoulos A., Mygdalis V., Tyritzis S. Low-grade fibromyxoid sarcoma of the renal pelvis: first report. Case Rep. Nephrol. Urol. 2012;2(July (2)):87–91. doi: 10.1159/000341191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillou L., Benhattar J., Gengler C. Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French sarcoma group. Am. J. Surg. Pathol. 2007;31(September (9)):1387–1402. doi: 10.1097/PAS.0b013e3180321959. [DOI] [PubMed] [Google Scholar]
- 10.Panagopoulos I., Storlazzi C.T., Fletcher C.D. The chimeric FUS/CREB3l2 gene is specific for low-grade fibromyxoid sarcoma. Genes Chromosomes Cancer. 2004;40(3):218–228. doi: 10.1002/gcc.20037. [DOI] [PubMed] [Google Scholar]
- 11.Mentzel T., Calonje E., Wadden C. Myxofibrosarcoma, clinicopathological analysis of 75 cases with emphasis on the low-grade variant. Am. J. Surg. Pathol. 1996;20(4):391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Goodlad J.R., Mentzel T., Fletcher C.D.M. Low grade fibromyxoid sarcoma, clinicopathological analysis of eleven new cases in support of a distinct entity. Histopathology. 1995;26:229–237. doi: 10.1111/j.1365-2559.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 13.Doyle L.A., Moller E., Dal Cin P. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am. J. Surg. Pathol. 2011;35(May (5)):733–741. doi: 10.1097/PAS.0b013e318210c268. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.Y., Kim M.Y., Hwang Y.J. Low-grade fibromyxoid sarcoma: CT, sonography, and MR findings in 3 cases. J. Thorac. Imaging. 2005;20(November (4)):294–297. doi: 10.1097/01.rti.0000171420.81428.16. [DOI] [PubMed] [Google Scholar]


