Abstract
Purpose.
To determine the effect of age on the thickness of individual retinal layers, measured with spectral-domain optical coherence tomography (SD-OCT), in a population of healthy Caucasians.
Methods.
One hundred and twenty subjects with an age ranging between 18 and 81 years were examined with SD-OCT. Mean layer thickness was calculated for seven retinal layers, in the fovea (region 1 of the 9 Early Treatment Diabetic Retinopathy Study [ETDRS] regions); in the pericentral ring (ETDRS regions 2 to 5); and the peripheral ring (ETDRS regions 6 to 9) following automated segmentation using the Iowa Reference Algorithm. In addition, mean peripapillary retinal nerve fiber layer (RNFL) thickness was measured. The partial correlation test was performed on each layer to determine the effect of age on layer thickness, while correcting for spherical equivalent, sex, and Topcon image quality factor as confounders, followed by Bonferroni corrections to adjust for multiple testing.
Results.
The thickness of the peripapillary RNFL (R = −0.332; P < 0.001); pericentral ganglion cell layer (R = −0.354, P < 0.001); peripheral inner plexiform layer (R = −0.328, P < 0.001); and foveal outer segment layer (R = −0.381, P < 0.001) decreased significantly with increasing age. Foveal RPE thickness (R = 0.467, P < 0.001) increased significantly with increasing age; other layers showed no significant differences with age.
Conclusions.
Several macular layers and the peripapillary RNFL thickness showed significant changes correlated with age. This should be taken into consideration when analyzing macular layers and the peripapillary RNFL in SD-OCT studies of retinal diseases and glaucoma.
Keywords: SD-OCT, aging, retinal layer thickness
Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography.
Introduction
The introduction of optical coherence tomography (OCT) has made it possible to visualize the human retina noninvasively in vivo with high resolution and to quantify retinal structures, such as total retinal thickness (RT) with high accuracy. Measurement of RT is important in the diagnosis and monitoring of retinal and optic nerve diseases, but to distinguish disease processes from normal age-related changes it is important to know the effect of aging on OCT measurements of the retina. Multiple OCT studies measuring the total RT in healthy subjects have shown a significant decrease in RT with age, in the pericentral and peripheral Early Treatment Diabetic Retinopathy Study (ETDRS) macular regions.1–10
In recent years, several algorithms have been developed for spectral domain OCT (SD-OCT) that allow for automatic measurement of the thickness of individual retinal layers in the macula,11–18 in addition to the segmentation of the retinal nerve fiber layer (RNFL), a tool provided by all commercially available devices.
Employing one of these techniques, the Iowa Reference Algorithm, a thinning of the ganglion cell layer (GCL) has been demonstrated in the pericentral macula and a corresponding loss of RNFL thickness in the peripheral macula in patients with type 1 or type 2 diabetes and no or minimal diabetic retinopathy compared with control subjects.19,20 For studies of changes in retinal layer thickness due to diseases like diabetes mellitus, Alzheimer's disease, glaucoma, or multiple sclerosis, it is essential to include the influence of age as a confounder of individual retinal layer thickness in the analysis. In support of this notion, in a recent study, Ooto et al. have shown changes in individual retinal layer thickness with increasing age in the eyes of a Japanese population, using an automated layer segmentation algorithm.15
There is an increasing interest in the effects of aging in general and parameters of the aging process that can be objectively measured. The eye is of interest in that respect, and several ocular parameters have been defined in a review by Pathai et al.,21 such as the RNFL, but changes in the macular area could be of interest as well.
To collect reference data on the effect of aging on individual retinal layers, in the present pilot study, we used 3D volume scans of the macula and the optic disc made with a SD-OCT (TRC-NW7SF Mark II; Topcon Medical Systems, Inc., Oakland, NJ) in combination with the Iowa Reference Algorithm12,13 to determine the effect of aging on the thickness of seven individual retinal layers in 120 eyes of 120 healthy Caucasian men and women aged between 18 and 81 years.
Subjects and Methods
Subjects
In this prospective, cross-sectional observational study, the subjects were randomly recruited from accompanying persons of patients visiting the ophthalmology outpatient clinic of the Academic Medical Center, Amsterdam, The Netherlands, between January 2012 and January 2013 who fulfilled all inclusion and exclusion criteria. The study adhered to the tenets of the Declaration of Helsinki. Investigative review board approval was obtained at the AMC and all participants gave written informed consent.
Inclusion criteria were a history without any ocular disease, diabetes, systemic hypertension, or any other (chronic) autoimmune or infectious disease, such as HIV, multiple sclerosis, and rheumatoid arthritis, that could affect the retina.
Exclusion criteria were refractive errors over S +5.5 or under S −8.5 diopters, visual acuity below 0.1 logMAR, intraocular pressure (IOP) higher than 21 mm Hg, significant media opacities, previous ocular surgery, and a previous diagnosis or any present sign of glaucoma, uveitis, or retinal disease. Visual acuity was measured using a modified ETDRS chart with Sloan letters (Lighthouse, NY) at 4 meters. Best corrected visual acuity (BCVA) was recorded in logMAR units. IOP was measured by air-puff tonometry (computerized tonometer, CT80; Topcon Medical Systems, Inc.). All subjects underwent pupil dilation (0.1% tropicamide) and an ophthalmic examination, including slit-lamp biomicroscopy with a handheld lens, as well as fundus photography to rule out any signs of retinopathy or glaucoma. Only one eye of each participant was randomly selected for OCT examination (3D OCT-1000, Topcon Corp., Tokyo, Japan) and was scanned after pupil dilation.
SD-OCT and Layer Segmentation
OCT images of the subjects were obtained with SD-OCT (Topcon Corp.) using the 3D macular and disc volume scan protocols (6 × 6 × 2.2 mm3), consisting of 128 (y) by 512 (x) by 650 (z) voxels. Only high-quality images with a Topcon image quality factor (QF) >60 were used.
From each 3D macular volume, 11 intraretinal surfaces defining 10 retinal layers were segmented automatically by The Iowa Reference Algorithm (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA. http://www.biomed-imaging.uiowa.edu/downloads/12,13), which uses an extensively validated, robust fully three-dimensional graph search approach. In this study, the highly reflective layer between inner and outer segments, and the outer segments up to the retinal pigment layer were taken together as one layer, the outer segment layer (OSL), ignoring the line ascribed to the cone outer segments. The remaining eight layers were interpreted as follows (from inner to outer surface): (1) RNFL; (2) GCL; (3) inner plexiform layer (IPL); (4) inner nuclear layer (INL); (5) outer plexiform layer (OPL); (6) outer nuclear layer (ONL) + inner segments (IS; photoreceptors); (7) outer segment layer (OSL) (from inner–outer segment transition up to retinal pigment epithelium); (8) retinal pigment epithelium (RPE) (Fig. 1).
Figure 1.
Macular B-scan with intraretinal surfaces as indicated by the colored lines and corresponding retinal layers. (Layer 1) Retinal nerve fiber layer. (Layer 2) Ganglion cell layer. (Layer 3) Inner plexiform layer. (Layer 4) Inner nuclear layer. (Layer 5) Outer plexiform layer. (Layer 6) Outer nuclear layer + inner segments (photoreceptors). (Layer 7) Outer segments (photoreceptors). (Layer 8) Retinal pigment epithelium.
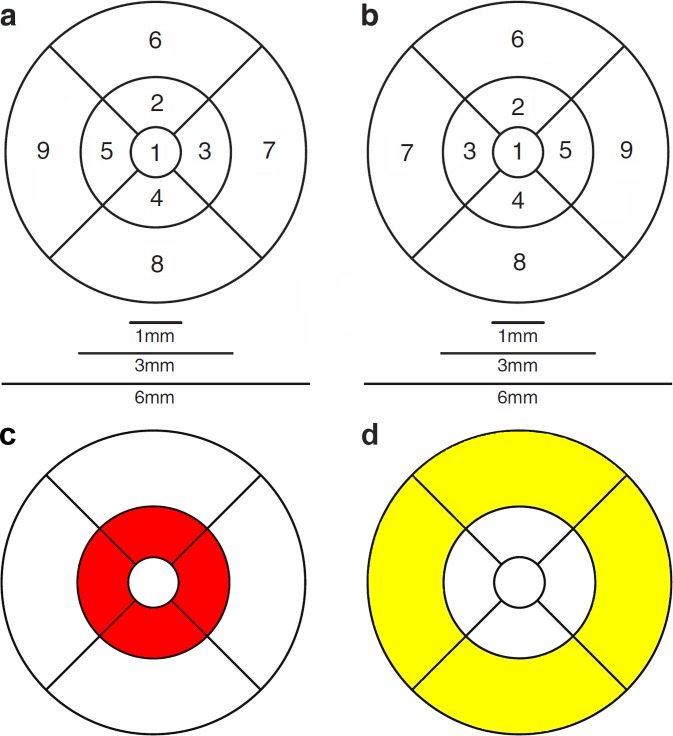
The Iowa Reference Algorithm12,13 allows analysis according to the ETDRS grid, which allows for the calculation of the thickness of all individual retinal layers for each of the nine ETDRS-grid defined regions. In this study for each layer, three retinal areas were defined using this ETDRS grid: the fovea, the central circle with a diameter of 1 mm; the pericentral ring, a donut-shaped ring centered on the fovea with an inner diameter of 1 mm and an outer diameter of 3 mm; and the peripheral ring, with an inner diameter of 3 mm and outer diameter of 6 mm (Fig. 2). Because inner retinal layers are nearly absent in the fovea, only outer retinal layer thicknesses were analyzed in this 1-mm diameter area in the center of the fovea.
Figure 2.
ETDRS grid. Nine subfields of the nine ETDRS regions in each eye. (a) Right eye. (b) Left eye. (c) The four regions around the fovea constitute the pericentral ring (red colored ring). Thickness measurement of the pericentral ring is estimated by averaging the thickness measurements of the four quadrant areas. (d) The four yellow-colored regions constitute the peripheral ring. Thickness measurement of this area is estimated by averaging the thickness measurements of the four quadrant areas.
Thickness measurements of the pericentral and peripheral rings were estimated by averaging the thickness measurements of the four corresponding quadrant areas (segments 2 to 5 for the pericentral ring and segments 6 to 9 for the peripheral ring; Fig. 2). In addition, thickness measurements of the entire area within the ETDRS grid were calculated automatically by the Iowa Reference Algorithm12,13 and this area was defined as “Whole macular region.”
Finally, peripapillary RNFL thickness measurements were acquired from the 3D optic nerve head OCT's using the same Iowa Reference Algorithm.12,13 The peripapillary ring was centered manually if needed, with the center of the ring coinciding with the center of the optic disc.
Statistical Analysis
Statistical analyses were performed with commercial statistical software (IBM SPSS Statistics v. 19 for Windows; SPSS Inc., Chicago, IL). The partial correlation test was used to determine the effect of age on individual layer thickness with spherical equivalent (SE),8,22 Topcon image QF,8,23,24 and sex2,4,5,9,15,25–29 as confounders, since these parameters are known to influence OCT thickness measurements. Bonferroni corrections were applied to counteract the effect of multiple testing with statistical significance set at P < 0.001. Finally, linear regression analysis was performed for the layers that correlated significantly with age.
Results
Demographic and ocular features of the study population are presented in Table 1. There were no significant differences in any of the parameters between men and women.
Table 1.
Demographic and Ocular Features of Included Subjects

Table 2 shows the mean layer thickness measurements (μm) of the individual retinal layers of the subjects in the central fovea (only outer retinal layer thicknesses), pericentral, and peripheral rings (all retinal layers), and the correlation between these layers with age, adjusted for Topcon image QF, SE, and sex. The thickness of the pericentral GCL, peripheral IPL, and foveal OSL decreased significantly with increasing age (Table 2; Figs. 3A–C). Foveal RPE thickness increased significantly with increasing age (Table 2; Fig. 3D); other layers showed no significant differences with age.
Table 2.
Correlations of Age with Thickness of Macular Layers After Adjusting for SE, Topcon Image QF, and Sex

Figure 3.
Scatterplots of simple linear regression between: age and mean pericentral GCL thickness (A), mean peripheral IPL thickness (B), mean foveal OSL thickness (C), mean foveal RPE thickness (D), and mean peripapillary RNFL thickness (E); and between mean pericentral GCL thickness and mean peripapillary RNFL thickness (F). Formulas: (A) PCR GCL (μm) = 55,456 − 0,103 · Age (y), (B) PR IPL (μm) = 39,104 − 0,046 · Age (y), (C) foveal OSL (μm) = 52,775 − 0,088 · Age (y), (D) foveal RPE (μm) = 15,192 + 0,066 · Age (y), (E) PP RNFL (μm) = 105,465 − 0,133 · Age (y), (F) PP RNFL (μm) = 42,575 + 1,118 · PCR GCL (μm). PCR, pericentral; GCL, ganglion cell layer; PR, peripheral; IPL, inner plexiform layer; OSL, outer segment layer; RPE, retinal pigment epithelium; PP, peripapillary; RNFL, retinal nerve fiber layer.
Mean peripapillary RNFL thickness decreased significantly with age (R = −0.332, P < 0.001; partial correlation test; adjusted for spherical equivalent, sex, and Topcon image quality factor; Fig. 3E). There was a significant positive correlation between mean peripapillary RNFL thickness and mean pericentral GCL thickness (R = 0.553, P < 0.001; Pearson correlation coefficient; Fig. 3F).
Discussion
The purpose of this study was to evaluate the effect of age on individual retinal layer thickness and peripapillary RNFL thickness, calculated from 3D-volume scans made with a SD-OCT (Topcon Medical Systems, Inc.), using the Iowa Reference Algorithm.12,13 The data were adjusted for confounders SE,8,22 Topcon image QF,8,23,24 and sex,2,4,5,9,15,25–29 since these factors are known to influence OCT thickness measurements.
The present study demonstrated a significant decrease in peripapillary RNFL thickness, pericentral GCL thickness, peripheral IPL thickness, and foveal OSL thickness with increasing age, while foveal RPE thickness correlated positively with age. Regarding the topographic distribution of the changes in retinal layer thickness over time, we postulated that the effect of age on the neuroretina would be most probably a diffuse loss of neural tissue over time, and would include all cells of the retina. A minute percentage loss of cells/tissue would be best measured in those areas where a certain cell type in a certain retinal layer is thickest. For that reason, changes in RNFL can be best measured in a ring around the optic nerve and changes in the ganglion cell layer in the pericentral area. The same could be true for the changes in RPE thickness and OSL in the fovea, but another explanation for these central changes can be the excessive metabolic strain that accumulates over the years in this most central part of the retina. The inner plexiform layer in the peripheral area reflects perhaps the loss of the pericentral GCL and the connections with the bipolar cells.
Using the findings of this study (Fig. 3) one can estimate that, over a period of 20 years, an individual will lose approximately 2.66 μm of peripapillary RNFL, 2.06 μm of pericentral GCL, 0.92 μm of peripheral IPL and 1.76 μm of foveal OSL, while the foveal RPE will increase with 1.32 μm. However, these numbers are just an impression of the theoretical speed of age related changes based on the found linear relationship between thickness measurements and age (Fig. 3). These figures are hypothetical, and can only be demonstrated with a longitudinal study (perhaps changes do not occur early in life, but only from a certain age, and this would be obscured in our analysis).
The mean OCT-based thickness data of the layers acquired in this study (Table 2) are similar to those reported in other SD-OCT studies,8,11,14,15,30 with some small differences that can be attributed to differences in study populations, the OCT devices used, and the algorithms to calculate the thickness of the individual layers.
The differences of the thickness of the individual retinal layers with age observed in the present study are mostly in concordance with previous studies,6,10,15,31 although Ooto et al.15 report a thickening of the OSL while we described a thinning with aging and the RPE was not included in other segmentation algorithms. Ooto et al.15 use a different definition of the OSL, compared with the present study, and mention in their discussion that RPE and OS tip lines were difficult to identify independently in some subjects, which may have led to an underestimation of OSL thickness in their study. Because of this ambiguity in the definition of the OS tips, we defined the layer between the IS/OS transition and RPE as representing the OSL.
Histological studies support our results: the GCL and their axons (the RNFL) are vulnerable to loss during aging32,33 and there is a decrease in cone pigment (contained within several hundreds of infolded plasma membrane discs of the outer segments) with age,33,34 which indicates a loss and displacement of photoreceptors with age.35 Several structural changes occur as the RPE ages, including loss of melanin granules, increase in the density of residual bodies and accumulation of lipofuscin, accumulation of basal deposits on or within Bruch's membrane, formation of drusen, and thickening of Bruch's membrane.36 This can all lead to a thickening of the RPE with older age on OCT measurements, either real or due to increased reflectivity leading to optical “pseudothickening.”
In the present study, there is a significant positive correlation between pericentral GCL thickness and peripapillary RNFL thickness (Fig. 3F). Since the RNFL consists of axons of the GCL, it is feasible that a thinner GCL would indeed lead to a thinner RNFL.
This is also described in other studies where the GCL(-IPL) thickness correlated with peripapillary RNFL thickness.26,27,37
Limitations of the present study are the relatively small sample size (n = 120 compared with larger numbers in other studies) and the fact that it was based on cross-sectional data rather than longitudinal data. Another pitfall is that the subjects were not objectively checked for systemic diseases such as diabetes or hypertension, but that rather self-reported health information was used.
In conclusion, this study indicates that changes in the thickness of several retinal layers occur with increasing age and this should be taken into consideration while interpreting retinal layer and RNFL thickness data in studies concerned with the effects of disease on the retina. The age-related changes of the retina may also be of use as a simple method to provide an objective parameter for aging in general, or aging in the course of systemic diseases.21
Acknowledgments
Disclosure: N. Demirkaya, None; H.W. van Dijk, None; S.M. van Schuppen, None; M.D. Abràmoff, None; M.K. Garvin, None; M. Sonka, None; R.O. Schlingemann, None; F.D. Verbraak, None
References
- 1.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. 2003;87:899–901. doi: 10.1136/bjo.87.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan XR, Liang YB, Friedman DS, et al. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. 2010;117:1585–1594. doi: 10.1016/j.ophtha.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson U, Alm A. Macular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCT. 2009;93:1448–1452. doi: 10.1136/bjo.2007.131094. [DOI] [PubMed] [Google Scholar]
- 4.Kim NR, Kim JH, Lee J, Lee ES, Seong GJ, Kim CY. Determinants of perimacular inner retinal layer thickness in normal eyes measured by Fourier-domain optical coherence tomography. 2011;52:3413–3418. doi: 10.1167/iovs.10-6648. [DOI] [PubMed] [Google Scholar]
- 5.Liu T, Hu AY, Kaines A, Yu F, Schwartz SD, Hubschman JP. A pilot study of normative data for macular thickness and volume measurements using Cirrus high-definition optical coherence tomography. 2011;31:1944–1950. doi: 10.1097/IAE.0b013e31820d3f13. [DOI] [PubMed] [Google Scholar]
- 6.Manassakorn A, Chaidaroon W, Ausayakhun S, Aupapong S, Wattananikorn S. Normative database of retinal nerve fiber layer and macular retinal thickness in a Thai population. 2008;52:450–456. doi: 10.1007/s10384-008-0538-6. [DOI] [PubMed] [Google Scholar]
- 7.Neuville JM, Bronson-Castain K, Bearse MA, Jr, et al. OCT reveals regional differences in macular thickness with age. 2009;86:E810–E816. doi: 10.1097/OPX.0b013e3181adff59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao HL, Kumar AU, Babu JG, Kumar A, Senthil S, Garudadri CS. Predictors of normal optic nerve head, retinal nerve fiber layer, and macular parameters measured by spectral domain optical coherence tomography. 2011;52:1103–1110. doi: 10.1167/iovs.10-5997. [DOI] [PubMed] [Google Scholar]
- 9.Song WK, Lee SC, Lee ES, Kim CY, Kim SS. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. 2010;51:3913–3918. doi: 10.1167/iovs.09-4189. [DOI] [PubMed] [Google Scholar]
- 10.Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. 2009;116:1119–1124. doi: 10.1016/j.ophtha.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagci AM, Shahidi M, Ansari R, Blair M, Blair NP, Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. 2008;146:679–687. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvin MK, Abramoff MD, Kardon R, Russell SR, Wu X, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. 2008;27:1495–1505. doi: 10.1109/TMI.2008.923966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvin MK, Abramoff MD, Wu X, Russell SR, Burns TL, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. 2009;28:1436–1447. doi: 10.1109/TMI.2009.2016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loduca AL, Zhang C, Zelkha R, Shahidi M. Thickness mapping of retinal layers by spectral-domain optical coherence tomography. 2010;150:849–855. doi: 10.1016/j.ajo.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooto S, Hangai M, Tomidokoro A, et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. 2011;52:8769–8779. doi: 10.1167/iovs.11-8388. [DOI] [PubMed] [Google Scholar]
- 16.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. 2010;18:19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajic V, Povazay B, Hermann B, et al. Robust segmentation of intraretinal layers in the normal human fovea using a novel statistical model based on texture and shape analysis. 2010;18:14730–14744. doi: 10.1364/OE.18.014730. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Reisman CA, Wang Z, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. 2010;18:21293–21307. doi: 10.1364/OE.18.021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. 2010;51:3660–3665. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijk HW, Verbraak FD, Kok PH, et al. Early neurodegeneration in the retina of type 2 diabetic patients. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathai S, Shiels PG, Lawn SD, Cook C, Gilbert C. The eye as a model of ageing in translational research - Molecular, epigenetic and clinical aspects. 2012;12:490–508. doi: 10.1016/j.arr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Lim MC, Hoh ST, Foster PJ, et al. Use of optical coherence tomography to assess variations in macular retinal thickness in myopia. 2005;46:974–978. doi: 10.1167/iovs.04-0828. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Liu X, Wu Z, Sadda S. Image quality affects macular and retinal nerve fiber layer thickness measurements on fourier-domain optical coherence tomography. 2011;42:216–221. doi: 10.3928/15428877-20110324-01. [DOI] [PubMed] [Google Scholar]
- 24.Samarawickrama C, Pai A, Huynh SC, Burlutsky G, Wong TY, Mitchell P. Influence of OCT signal strength on macular, optic nerve head, and retinal nerve fiber layer parameters. 2010;51:4471–4475. doi: 10.1167/iovs.09-3892. [DOI] [PubMed] [Google Scholar]
- 25.Kashani AH, Zimmer-Galler IE, Shah SM, et al. Retinal thickness analysis by race, gender, and age using Stratus OCT. 2010;149:496–502. doi: 10.1016/j.ajo.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh VT, Tham YC, Cheung CY, et al. Determinants of ganglion cell-inner plexiform layer thickness measured by high-definition optical coherence tomography. 2012;53:5853–5859. doi: 10.1167/iovs.12-10414. [DOI] [PubMed] [Google Scholar]
- 27.Mwanza JC, Durbin MK, Budenz DL, et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency domain optical coherence tomography. 2011;52:7872–7879. doi: 10.1167/iovs.11-7896. [DOI] [PubMed] [Google Scholar]
- 28.Wagner-Schuman M, Dubis AM, Nordgren RN, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. 2011;52:625–634. doi: 10.1167/iovs.10-5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wexler A, Sand T, Elsas TB. Macular thickness measurements in healthy Norwegian volunteers: an optical coherence tomography study. 2010;10:13. doi: 10.1186/1471-2415-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendschneider D, Tornow RP, Horn FK, et al. Retinal nerve fiber layer thickness in normals measured by spectral domain OCT. 2010;19:475–482. doi: 10.1097/IJG.0b013e3181c4b0c7. [DOI] [PubMed] [Google Scholar]
- 31.Budenz DL, Anderson DR, Varma R, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. 2007;114:1046–1052. doi: 10.1016/j.ophtha.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. 1992;33:1–17. [PubMed] [Google Scholar]
- 33.Nag TC, Wadhwa S. Ultrastructure of the human retina in aging and various pathological states. 2012;43:759–781. doi: 10.1016/j.micron.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Kilbride PE, Hutman LP, Fishman M, Read JS. Foveal cone pigment density difference in the aging human eye. 1986;26:321–325. doi: 10.1016/0042-6989(86)90029-5. [DOI] [PubMed] [Google Scholar]
- 35.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. 2002;1:381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 36.Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. 2008;2:413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K, Kwon YH, Garvin MK, Niemeijer M, Sonka M, Abramoff MD. Distribution of damage to the entire retinal ganglion cell pathway: quantified using spectral-domain optical coherence tomography analysis in patients with glaucoma. 2012;130:1118–1126. doi: 10.1001/archophthalmol.2012.669. [DOI] [PMC free article] [PubMed] [Google Scholar]