Abstract
Background
PARP1 facilitates the recovery of DNA-damaged cells by recruiting DNA damage response molecules such as γH2AX and BRCA1/2, and plays a role in resistance to antitumor therapies. Therefore, PARP inhibition being evaluated as an anti-cancer therapy. However, there are limited studies regrading PARP inhibition in osteosarcoma.
Methods
We evaluated the expression of DNA damage response molecules in 35 human osteosarcomas and investigated the effects of co-treatment of the PARP inhibitor, olaparib, and doxorubicin in osteosarcoma cells.
Results
The expression patterns of PARP1, γH2AX, BRCA1, and BRCA2 were significantly associated with shorter survival of osteosarcoma patients. In osteosarcoma cells, knock-down of PARP1 and treatment of olaparib significantly inhibited proliferation of cells and induced apoptosis. Moreover, the anti-tumor effect was more significant with co-treatment of olaparib and doxorubicin in vitro and in vivo.
Conclusions
This study suggests that combined use of a PARP inhibitor with doxorubicin, a DNA damaging agent, might be effective in the treatment of osteosarcoma patients, especially in the poor-prognostic subgroups of osteosarcoma expressing PARP1, γH2AX, or BRCA1/2.
Keywords: Osteosarcoma, PARP1, Olaparib, Doxorubicin, Prognosis
Background
PARP1 [Poly (ADP-ribose) polymerase 1] is an important DNA repair molecule [1, 2]. When there are stresses inducing DNA damage, PARP1 is activated and detects DNA strand breaks and controls the single strand breaks (SSBs) of DNA as a member of the base excision repair pathway [3, 4]. With low-level of DNA damage, PARP1 promotes cell survival by repairing SSBs of DNA and does not permit their progression to toxic double-strand breaks (DSBs) of DNA [5]. If SSBs of DNA progress to DSBs, PARP1 induces phosphorylation of H2AX (γH2AX) and induces recruitment of BRCA1/2 to repair DSBs, which eventually prevents apoptotic cell death [6, 7]. Therefore, PARP1 has a vital role in the survival of damaged cells, and could confer a survival advantage to cancer cells during anti-cancer therapy targeting apoptosis of cancer cells [8]. Based on the mechanism of PARP1-H2AX-BRCA1/2, recent study has focused on PARP1 as a possible therapeutic target for various human malignant tumors [1, 2], especially in carcinomas have defects in BRCA1/2 [9]. When there are defects in BRCA1/2, inhibition of PARP1 induces accumulation of SSBs which eventually progresses to DSBs that lead to apoptosis [4, 10, 11]. In this context, PARP inhibitors might be promising anticancer drugs in conjunction with DNA repair deficits [3, 4, 6, 7, 12]. Therapeutic efficacy of PARP inhibition has been proposed for human papilloma virus-related squamous cell carcinomas and colorectal carcinomas [13, 14]. Moreover, the expression pattern of PARP1, γH2AX, and BRCA1/2 predicted shorter survival of various human malignant tumors including breast carcinoma and soft tissue sarcomas [15, 16]. The prognostic impact of the expression of PARP1 and PARP1-related molecules suggests the possibility that the expression of these DNA damage response (DDR) molecules may provide resistance to anti-cancer chemotherapy by preventing therapy-related cancer cell death [16].
Osteosarcoma is the most common primary malignant tumor of bone, and the survival of osteosarcoma patients is stagnated despite recent advances in treatment [17]. Therefore, a novel therapeutic approach is needed to improve therapeutic efficacy for osteosarcoma patients [18]. In addition, when considering the recent report that the mutation signature of osteosarcomas is reminiscent of BRCA-deficient tumors [19] and the start of clinical use of PARP inhibitors in human cancers [20], it is important to evaluate the anti-cancer effects of PARP inhibition in osteosarcomas. However, despite extensive assessment of the role of PARP1 as a prognostic marker and therapeutic target of various human malignant tumors, there are limited reports focused on osteosarcoma. Therefore, this study evaluates the expression and prognostic significance of the individual and combined expression patterns of PARP1 and PARP1-related DDR molecules such as γH2AX, BRCA1, and BRCA2 in osteosarcomas. Thereafter, the possibility of therapeutic efficacy of co-treatment of the PARP inhibitor olaparib and doxorubicin was evaluated in osteosarcoma cells.
Methods
Osteosarcoma patients and tissue samples
Thirty-nine primary osteosarcomas of the bone had surgical resection performed of the primary lesion of the osteosarcoma between January 1998 and January 2013 at Chonbuk National University Hospital were evaluated in this study. After de-selecting four cases that were missing tissue blocks, 35 cases of primary osteosarcoma of the bone were subjected to this study, and reviewed based on the 2013 World Health Organization classification of tumors of soft tissue and bone [21]. Clinicopathological information was obtained from medical records, and osteosarcomas were staged according to the guidelines of the American Joint Committee on Cancer [22]. The adjuvant therapies were as follows: chemotherapy only in 20 patients, radiation therapy only in 2 patients, both chemotherapy and radiation therapy in 6 patients, and no adjuvant therapy in 7 patients. The duration of follow-up ranged from 4 to 174 months (median follow-up duration; 44 months). The 5- and 10-year overall survival (OS) rates were 54 and 49%, respectively.
Immunohistochemical staining and scoring in tissue microarray
The expression of PARP1, γH2AX, BRCA1, and BRCA2 in human osteosarcoma tissues were evaluated by immunohistochemical staining of a tissue microarray (TMA). The TMA blocks were constructed by arranging two 3.0 mm cores per one case from the most representative solid area containing well-preserved tumor cells with the highest histological grade and a tumor component consisting of at least 2/3 of TMA core area. Tissue sections from the TMA blocks underwent an antigen retrieval procedure by boiling in Dako Target Retrieval Solution (pH 6.0, DAKO, Glostrup, Denmark) for 20 min using a microwave oven. Thereafter, the following markers were used as primary antibodies: PARP1 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), γH2AX (Ser 139) (1:100, Cell Signaling Technology, Beverly, MA), BRCA1 (1:100, Abcam, Cambridge, MA), and BRCA2 (1:100, Abcam, Cambridge, MA). For negative controls, tissue sections were incubated with antibody diluent (DAKO, Cambridge, UK) without primary antibody. The immunohistochemical staining slides were evaluated without information for the various clinicopathological factors. Scoring for the immunohistochemical staining for PARP1, BRCA1, and BRCA2 were evaluated by the sum of the staining intensity scores (0; no, 1; weak, 2; intermediate, and 3; strong) and the staining area scores (0; 0%, 1; 1%, 2; 2–10%, 3; 11–33%, 4; 34–66%, and 5; 67–100%) in each TMA core [15, 23, 24]. Thereafter, the sum score obtained by adding the scores from two TMA cores ranged from zero to 16 [15, 24]. The sum score for γH2AX was obtained by adding the scores from each TMA core derived by counting the number of γH2AX-positive cells in five 400-magnification fields [15, 25, 26]. The area of one 400-magnification field was 0.238 mm2.
Cell culture, chemicals, and transfection
The human osteosarcoma cell lines, U2OS, SaOS2, and MG63 were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). KHOS/NP cells were kindly provided by Chang-Bae Kong (Department of Orthopedic Surgery, Korea Institute of Radiological and Medical Science) [27]. The cells were cultured in DMEM media containing 10% FBS (Gibco BRL, Gaithersburg, MD), and 10% penicillin/streptomycin (100 U/mL) at 37 °C in a humidified 5% CO2 incubator. The PARP inhibitor, olaparib, was purchased from Santa Cruz Biotechnology (sc-302017, Santa Cruz Biotechnology, Santa Cruz, CA) and doxorubicin was obtained from Sigma (D1515, Sigma, St. Louis, MO). Short interfering RNA (siRNA) for PARP1 and negative control siRNA duplexes were purchased from Santa Cruz biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA). The cells were transfected using Lipofectamine® RNAiMax (Invitrogen, Life technologies, Carlsbad, CA).
Cell proliferation assay, colony forming assay, and soft agar assay
The proliferation of cells was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay (Sigma, St. Louis, MO), colony-forming assay, and soft gar assay. For TTT assay, U2OS (1 × 103), SaOS2 (2 × 103), MG63 (1 × 103), and KHOS/NP (1 × 103) cells were plated onto a 96-well plates and incubated overnight at 37 °C in a humidified incubator containing 5% CO2. The next day, the cells were treated with the indicated concentration of DMSO as a control vehicle and the indicated concentration of olaparib, doxorubicin, or co-treatment with olaparib and doxorubicin for 72 h. Then, the amount of cell proliferation was measured using a Bio-Rad model 680 microtiter plate reader (Bio-Rad, Hercules, CA) at a wavelength of 560 nm. The colony forming assay was performed by culturing U2OS (1 × 103), SaOS2 (2 × 103), MG63 (1 × 103), and KHOS/NP (1 × 103) cells in 24-well culture plates for 7 days. A soft agar assay was performed with U2OS (5 × 102), SaOS2 (1 × 103), and MG63 (5 × 102) cells. The indicated number of tumor cells were mixed with 0.4% agarose in the culture medium and then plated in a 300 mm dish coated with 0.8% agarose in culture medium. After 3 weeks of incubation at 37 °C, cell colonies were stained with 0.01% crystal violet for 1 h. The number of stained cell colonies growing in the soft agar were counted, and the images were captured using a Nikon (TE2000-S, Nikon, Japan) microscope.
Western blot analysis
The cells were washed twice with phosphate-buffered saline and lysed with PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Korea) containing 1× phosphatase inhibitor cocktails 2, 3 (Sigma, St. Louis, MO). The primary antibodies for PARP1 (Santa Cruz Biotechnology, Santa Cruz, CA), Caspase 3 (Cell Signaling Technology, Beverly, MA), BCL2 (Santa Cruz Biotechnology, Santa Cruz, CA), BAX (Santa Cruz Biotechnology, Santa Cruz, CA), and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used for the western blot analysis.
Flow cytometry analysis to assess apoptosis
Apoptosis was assessed with flow cytometry analysis with staining for FITC-conjugated annexin V and propidium iodide (PI) according to the manufacturer’s directions for the apoptosis detection kit (BD Biosciences, San Jose, CA). Briefly, cells were suspended twice with cold phosphate-buffered saline, pelleted by centrifugation, and re-suspended cells in 100 μL of binding buffer containing 10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl and 2.5 mM CaCl2 at a concentration of 1X106 cell/mL. Next, cells were treated with 5 μL of annexin V-FITC and 5 μL of PI according to the kit protocol and incubated for 15 min at room temperature in the dark. Thereafter, 400 μL buffer was added and samples were analyzed by flow cytometry within 1 h. For each sample, cells were analyzed using a FACStar flow cytometer (Becton-Dickinson, San Jose, CA) and analyzed by FlowJo 7.6.3 software (Tree Star, Ashland, OR).
Orthotopic tumor model
Six-week old male BALB/c nude mice (Orient Bio, Gyonggi-Do, Korea) were used for the orthotopic xenograft model. To establish a tumor in mice, 2.5 × 106 KHOS/NP cells were injected into the marrow space of the right proximal tibia under anesthesia. Two weeks after tumor cell inoculation, mice were randomly distributed into four groups of four mice each. Thereafter, according to the group, normal saline, olaparib (80 mg/kg for 5 days in every week), and/or doxorubicin (4 mg/kg, once a week) were injected intraperitoneally. The control group was injected with normal saline, and the olaparib group was injected with olaparib. The doxorubicin group was injected with doxorubicin, and the combined treatment group was injected with both olaparib and doxorubicin. Body weight and tumor volume were measured twice a week. The tumor volumes were calculated as “length x width x height x 0.52”. The animals were euthanized with sodium pentobarbital at 6 weeks after tumor cell inoculation.
Statistical analysis
The expression of the PARP1, γH2AX, BRCA1, and BRCA2 were grouped as negative or positive by statistical analysis for the best predictive cut-off point to estimate survival of osteosarcoma patients. The cut-off points for the PARP1, γH2AX, BRCA1, and BRCA2 immunostaining were determined by receiver operating characteristic curve analysis. The cut-off points were selected at the points with the highest area of under the curve to estimate the death of patients. The endpoint of follow-up was the date of death of patients or the date of last contact through June 2013. Prognosis was evaluated by analyzing OS and relapse-free survival (RFS). The duration of OS was calculated from the date of diagnosis to the date of death from osteosarcoma or the date of last contact. The patients who were alive at last contact or died from other causes were treated as censored. The duration of RFS was calculated from the date of diagnosis to the date of death from osteosarcoma, the date of relapse, or the date of last contact. The patients who were alive without relapse at last contact or died from other causes were treated as censored for RFS analysis. SPSS statistical software (IBM, version 20.0, CA) was used for the statistical analysis. The correlations between the clinicopathological factors and the expression of the markers used in this study were evaluated by Pearson’s chi-square test. Survival analysis was performed via univariate and multivariate Cox regression hazard analysis and survival curves derived from Kaplan-Meier survival analysis. P values less than 0.05 were considered to be statistically significant.
Results
The expression of PARP1, γH2AX, BRCA1, and BRCA2 are associated with advanced clinical factors of osteosarcoma patients
In human osteosarcoma tissues, the expression of PARP1 and γH2AX were observed in the nuclei of tumor cells. In contrast, BRCA1 and BRCA2 were expressed in both the cytoplasm and the nuclei of the tumor cells (Fig. 1a). However, based on previous reports that the prognostic impact of the expression of PARP1, γH2AX, BRCA1, and/or BRCA2 were associated with their nuclear expression [15, 16], nuclear expression of these markers were used in this study. The cut-off points of the sum score for the PARP1, BRCA1, and BRCA2 immunostaining were 8, 10, and 12, respectively. The immunostaining for PARP1 and γH2AX, BRCA1, and BRCA2 was considered positive if the sum score was equal or greater than 8, 10, and 12, respectively. The expression of γH2AX were considered positive when there were eight or more than γH2AX-positive cells (Fig. 1b). The expression of PARP1, γH2AX, BRCA1, and BRCA2 were grouped as positive in 74% (26 of 35 of cases), 57% (20 of 35 of cases), 49% (17 of 35 of cases), and 46% (16 of 35 of cases) of osteosarcomas, respectively (Table 1). As shown in Table 1, PARP1-positivity was significantly associated with sex of patients (P = 0.038), higher tumor stage (P = 0.024), higher histologic grade (P = 0.008), and the expression of γH2AX (P = 0.001), BRCA1 (P = 0.009), and BRCA2 (P = 0.001). The expression of γH2AX was significantly associated with tumor stage (P = 0.046), latent distance metastasis of osteosarcomas (P = 0.013), and higher histologic grade (P = 0.016). BRCA1-positivity was significantly associated with latent distant metastasis (P = 0.019). BRCA2 expression was significantly associated with sex of patients (P = 0.007), higher tumor stage (P = 0.002), distant metastasis at diagnosis (P = 0.008), and higher histologic grade (P = 0.003).
Fig. 1.
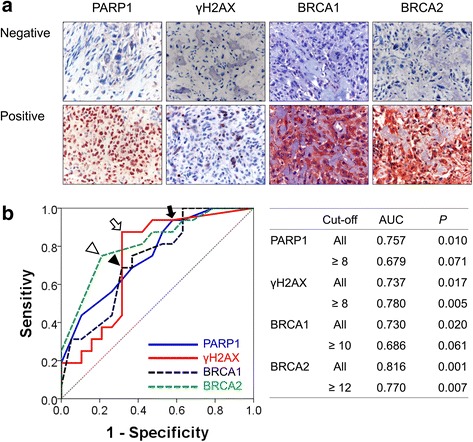
Immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2 in human osteosarcomas. a Immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2 in osteosarcomas. PARP1 and γH2AX are expressed in the nuclei of tumor cells. BRCA1 and BRCA2 are expressed in both the cytoplasm and nuclei of tumor cells. b Statistical analysis for the determination of cut-off points for the immunostaining of PARP1, γH2AX, BRCA1, and BRCA2. The cut-off points for immunohistochemical staining were determined by receiver operator characteristic curve analysis at the highest area under the curve (AUC) value. The cut-off points for PARP1 (arrow), γH2AX (empty arrow), BRCA1 (arrow head), and BRCA2 (empty arrow head) expression were eight, eight, ten, and twelve, respectively
Table 1.
Clinicopathologic variables and the expression of PARP1, γH2AX, BRCA1, and BRCA2 in 35 osteosarcomas
| Characteristics | No. | PARP1 | P | γH2AX | P | BRCA1 | P | BRCA2 | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| positive | positive | positive | positive | |||||||
| Age, yr | < 30 | 24 | 16 (67%) | 0.128 | 13 (54%) | 0.599 | 10 (42%) | 0.227 | 11 (46%) | 0.983 |
| ≥ 30 | 11 | 10 (91%) | 7 (64%) | 7 (64%) | 5 (45%) | |||||
| Sex | female | 10 | 5 (50%) | 0.038 | 5 (50%) | 0.589 | 3 (30%) | 0.164 | 1 (10%) | 0.007 |
| male | 25 | 21 (84%) | 15 (60%) | 14 (56%) | 15 (60%) | |||||
| Tumor size, cm | ≤ 8 | 18 | 12 (67%) | 0.289 | 10 (56%) | 0.845 | 7 (39%) | 0.238 | 6 (33%) | 0.130 |
| > 8 | 17 | 14 (82%) | 10 (59%) | 10 (59%) | 10 (59%) | |||||
| Stage | I | 11 | 5 (45%) | 0.024 | 3 (27%) | 0.046 | 3 (27%) | 0.232 | 1 (9%) | 0.002 |
| II | 19 | 16 (84%) | 14 (74%) | 11 (58%) | 10 (53%) | |||||
| III-IV | 5 | 5 (100%) | 3 (60%) | 3 (60%) | 5 (100%) | |||||
| Distant metastasis | absence | 30 | 21 (70%) | 0.155 | 17 (57%) | 0.889 | 14 (47%) | 0.581 | 11 (37%) | 0.008 |
| presence | 5 | 5 (100%) | 3 (60%) | 3 (60%) | 5 (100%) | |||||
| Latent distant metastasis | absence | 25 | 17 (68%) | 0.179 | 11 (44%) | 0.013 | 9 (36%) | 0.019 | 10 (40%) | 0.283 |
| presence | 10 | 9 (90%) | 9 (90%) | 8 (80%) | 6 (60%) | |||||
| Histological grade | low | 11 | 5 (45%) | 0.008 | 3 (27%) | 0.016 | 3 (27%) | 0.088 | 1 (9%) | 0.003 |
| high | 24 | 21 (88%) | 17 (71%) | 14 (58%) | 15 (63%) | |||||
| Histologic type | conventional | 31 | 24 (77%) | 0.238 | 19 (61%) | 0.167 | 16 (52%) | 0.316 | 16 (52%) | 0.051 |
| non-conventional | 4 | 2 (50%) | 1 (25%) | 1 (25%) | ||||||
| BRCA2 | negative | 19 | 10 (53%) | 0.001 | 7 (37%) | 0.008 | 7 (37%) | 0.13 | ||
| positive | 16 | 16 (100%) | 13 (81%) | 10 (63%) | ||||||
| BRCA1 | negative | 18 | 10 (56%) | 0.009 | 7 (39%) | 0.025 | ||||
| positive | 17 | 16 (94%) | 13 (76%) | |||||||
| γH2AX | negative | 15 | 7 (47%) | 0.001 | ||||||
| positive | 20 | 19 (95%) | ||||||||
The expression patterns of PARP1, γH2AX, BRCA1, and BRCA2 are significantly associated with shorter survival of osteosarcoma patients
The factors significantly associated with both OS and RFS of osteosarcoma patients in univariate analysis were tumor size (OS; P = 0.011, RFS; P = 0.024), tumor stage (OS; P = 0.008, RFS; P = 0.014), distant metastasis at diagnosis (OS; P = 0.005, RFS; P = 0.016), histologic grade (OS; P = 0.024, RFS; P = 0.017), and the expression of PARP1 (OS; P = 0.047, RFS; P = 0.039), γH2AX (OS; P = 0.004, RFS; P = 0.003), and BRCA2 (OS; P = 0.005, RFS; P = 0.017). The expression of BRCA1 was significantly associated with shorter RFS and showed borderline significance for OS (OS; P = 0.058, RFS; P = 0.013) (Table 2) (Fig. 2a). In addition, we performed further survival analysis in 30 cases of low-stage (stage I and II) osteosarcomas. In low-stage osteosarcomas, tumor stage (OS; P = 0.036, RFS; P = 0.025), histologic grade (OS; P = 0.036, RFS; P = 0.025), γH2AX expression (OS; P = 0.009, RFS; P = 0.006), and BRCA1 expression (OS; P = 0.026, RFS; P = 0.005) was associated with both OS and RFS (Table 3). BRCA2 expression was associated with OS (OS; P = 0.017, RFS; P = 0.054). The expression of PARP1 showed borderline significance for the survival of low-grade osteosarcoma patients (OS; P = 0.067, RFS; P = 0.055) (Table 3). The prognostic significance of tumor stage and expression of PARP1, γH2AX, BRCA1, and BRCA2 in overall osteosarcomas and low-stage osteosarcomas are shown via Kaplan-Meier survival analysis in Fig. 2.
Table 2.
Univariate Cox proportional hazard regression analysis for overall survival and relapse-free survival in overall osteosarcoma patients
| Characteristics | No. | OS | P | RFS | P |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Age, yr., ≥ 30 (vs. < 30) | 11/35 | 2.632 (0.972–7.127) | 0.057 | 3.252 (1.283–8.242) | 0.013 |
| Sex, male (vs. female) | 25/35 | 1.875 (0.530–6.635) | 0.329 | 2.363 (0.674–8.282) | 0.179 |
| Tumor size, cm, > 8 (vs. ≤ 8) | 17/35 | 4.371 (1.393–13.717) | 0.011 | 3.159 (1.166–8.557) | 0.024 |
| Stage, I | 11/35 | 1 | 0.008 | 1 | 0.014 |
| II | 19/35 | 8.528 (1.098–66.238) | 0.040 | 10.139 (1.323–77.728) | 0.026 |
| III-IV | 5/35 | 28.907 (3.133–266.703) | 0.003 | 26.685 (2.862–248.802) | 0.004 |
| Distant metastasis, presence (vs. absence) | 5/35 | 5.404 (1.661–17.578) | 0.005 | 4.174 (1.298–13.418) | 0.016 |
| Histological grade, high (vs. low) | 24/35 | 10.347 (1.362–78.639) | 0.024 | 11.623 (1.541–87.689) | 0.017 |
| PARP1, positive (vs. negative) | 26/35 | 7.834 (1.030–59.563) | 0.047 | 8.355 (1.109–62.967) | 0.039 |
| γH2AX, positive (vs. negative) | 20/35 | 9.192 (2.052–41.166) | 0.004 | 6.540 (1.858–23.023) | 0.003 |
| BRCA1, positive (vs. negative) | 17/35 | 2.796 (0.967–8.088) | 0.058 | 3.741 (1.318–10.620) | 0.013 |
| BRCA2, positive (vs. negative) | 16/35 | 5.194 (1.652–16.331) | 0.005 | 3.436 (1.253–9.424) | 0.017 |
| CSddrm, score 3–4 (vs. score 0–2) | 18/35 | 3.681 (1.712–7.913) | < 0.001 | 13.249 (2.900–60.537) | < 0.001 |
OS overall survival, RFS relapse-free survival, HR hazard ratio, 95% CI 95% confidence interval, CSddrm the combined score for the immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2
Fig. 2.
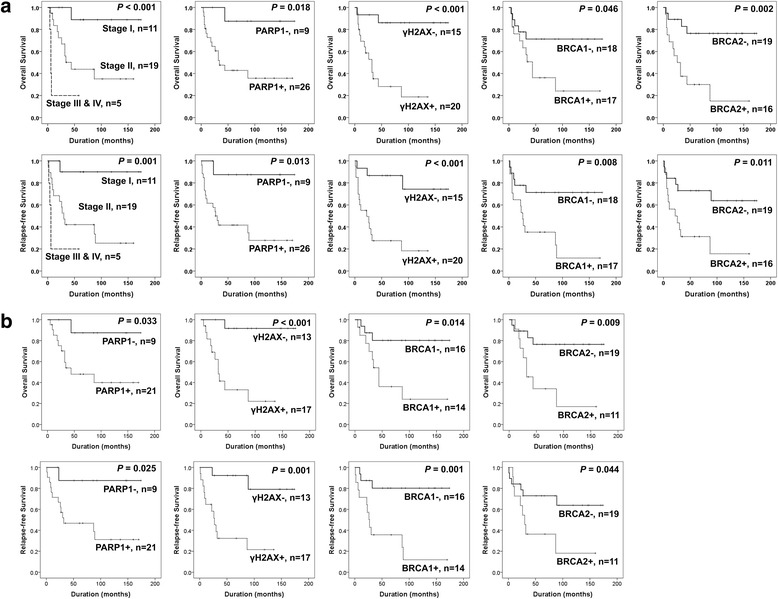
Kaplan-Meier survival analysis in osteosarcomas. a Overall survival and relapse-free survival according to tumor stage and immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2 in 35 osteosarcoma patients. b Overall survival and relapse-free survival according to immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2 in low-stage (stage I and II) osteosarcoma patients
Table 3.
Univariate Cox proportional hazard regression analysis for overall survival and relapse-free survival in stage I and II osteosarcoma patients
| Characteristics | No. | OS | P | RFS | P |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Age, yr., ≥ 30 (vs. < 30) | 8/30 | 1.785 (0.535–5.959) | 0.346 | 2.491 (0.860–7.211) | 0.092 |
| Sex, male (vs. female) | 20/30 | 1.428 (0.383–5.331) | 0.596 | 1.939 (0.532–7.065) | 0.316 |
| Tumor size, cm, > 8 (vs. ≤ 8) | 12/30 | 1.194 (1.037–1.374) | 0.014 | 2.628 (0.901–7.662) | 0.077 |
| Stage, II (vs. I) | 19/30 | 8.998 (1.158–69.931) | 0.036 | 10.325 (1.347–79.152) | 0.025 |
| Histological grade, high (vs. low) | 19/30 | 8.998 (1.158–69.931) | 0.036 | 10.325 (1.347–79.152) | 0.025 |
| PARP1, positive (vs. negative) | 21/30 | 6.780 (0.872–52.737) | 0.067 | 7.310 (0.954–56.000) | 0.055 |
| γH2AX, positive (vs. negative) | 17/30 | 15.788 (2.013–123.838) | 0.009 | 8.414 (1.847–38.322) | 0.006 |
| BRCA1, positive (vs. negative) | 14/30 | 4.428 (1.193–16.439) | 0.026 | 6.323 (1.745–22.916) | 0.005 |
| BRCA2, positive (vs. negative) | 11/30 | 4.372 (1.297–14.734) | 0.017 | 2.898 (0.982–8.554) | 0.054 |
| CSddrm, score 3–4 (vs. score 0–2) | 14/30 | 30.043 (3.724–242.374) | 0.001 | 27.986 (3.501–223.717) | 0.002 |
OS overall survival, RFS relapse-free survival, HR hazard ratio, 95% CI 95% confidence interval, CSddrm the combined score for the immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2
Based on the roles of PARP1, γH2AX, BRCA1, and BRCA2 in DNA damage repair, the combined expression patterns were evaluated in breast carcinoma and soft tissue sarcomas [15, 16]. The combined expression patterns were designated as CSddrm (Combined Score for the expression of DNA damage response molecules PARP1, γH2AX, BRCA1, and BRCA2) [16]. Therefore, this study also investigated the prognostic significance of these four markers in osteosarcomas. The CSddrm was scored from zero to four according to the number of positive markers (negative; 0, positive; 1); for example, CSddrm 3 represents the case where three of four markers were positive. Thereafter, the 35 osteosarcomas were grouped as CSddrm 0–2 (CSddrm 0, 1, or 2) or CSddrm 3–4 (CSddrm 3 or 4) based on receiver operator characteristic curve analysis (Fig. 3a). In univariate analysis, CSddrm was significantly associated with shorter OS (Log-rank, P < 0.001) and RFS (Log-rank, P < 0.001) (Table 2) (Fig. 3b). In low-stage osteosarcomas, CSddrm was significantly associated with shorter OS (Log-rank, P < 0.001) and RFS (Log-rank, P < 0.001) (Table 3) (Fig. 3c).
Fig. 3.
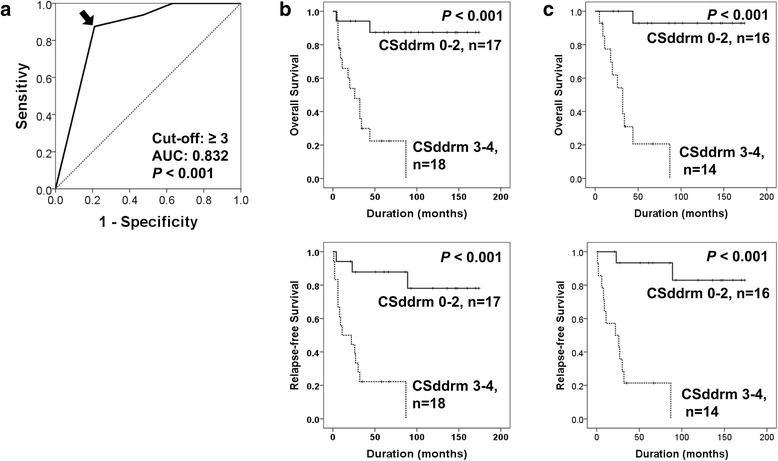
Survival analysis according to the combined expression pattern of PARP1, γH2AX, BRCA1, and BRCA2 in osteosarcomas. a Receiver operator characteristic curve analysis for the CSddrm (Combined Scores for the DNA damage response molecules PARP1, γH2AX, BRCA1, and BRCA2). CSddrm ranged from zero to four according to the number of positive markers, and the cut-off point (arrow) was three. CSddrm 3–4 includes the cases positive for three or four markers. b Kaplan-Meier survival analysis for the overall survival and relapse-free survival in the CSddrm 0–2 and CSddrm 3–4 sub-groups of osteosarcoma. c Kaplan-Meier survival analysis in low-stage (stage I and II) osteosarcomas according to the CSddrm subgroups of osteosarcoma
The variables included in multivariate analysis for the 35 osteosarcomas were the factors significantly associated with OS or RFS: age, tumor size, tumor stage, distant metastasis, histologic grade, and the expression of PARP1, γH2AX, BRCA1, and BRCA2. Multivariate analysis revealed tumor stage and γH2AX-positivity as independent indicators of poor prognosis of OS and RFS (Table 4). The expression of γH2AX predicted a 12.239-fold [95% confidence interval (95% CI); 2.118–70.722, P = 0.005]) greater risk of death and a 5.212-fold (95% CI; 1.372–19.800, P = 0.015) greater risk of relapse or death. In addition, multivariate analysis performed with inclusion of CSddrm instead of the individual expression patterns of PARP1, γH2AX, BRCA1, and BRCA2 revealed CSddrm as an independent indicators of poor prognosis of OS and RFS (multivariate analysis Model 2 in Table 4). The expression of combined expression of PARP1, γH2AX, BRCA1, and BRCA2 predicted an 11.791 -fold (95% CI; 2.427–57.294, P = 0.002) greater risk of death and an 11.312-fold (95% CI; 2.513–50.9130, P = 0.002) greater risk of relapse or death. In low-stage osteosarecomas, γH2AX-positivity (OS; P = 0.005, RFS; P = 0.029, multivariate analysis Model 1 in Table 5) and CSddrm (OS; P = 0.001, RFS; P = 0.002, multivariate analysis Model 2 in Table 5) were independent indicators of poor prognosis of both OS and RFS. Tumor size was an independent indicator of poor prognosis of OS (P = 0.022) and BRCA1 expression predicted poor prognosis of RFS (P = 0.046) (Table 5).
Table 4.
Multivariate Cox proportional hazard regression analysis for overall survival and relapse-free survival in overall osteosarcoma patients
| Characteristics | OS | P | RFS | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Multivariate analysis Model 1a | ||||
| Tumor size, cm, > 8 (vs. ≤ 8) | 3.528 (0.937–13.284) | 0.062 | ||
| Age, yr., ≥ 30 (vs. < 30) | 2.404 (0.918–6.301) | 0.074 | ||
| Stage, I | 1 | 0.046 | 1 | 0.026 |
| II | 2.958 (0.333–26.295) | 0.331 | 5.204 (0.641–42.213) | 0.123 |
| III-IV | 1.631 (1.348–205.244) | 0.028 | 21.071 (1.989–223.276) | 0.011 |
| γH2AX, positive (vs. negative) | 12.239 (2.118–70.722) | 0.005 | 5.212 (1.372–19.800) | 0.015 |
| Multivariate analysis Model 2b | ||||
| Tumor size, cm, > 8 (vs. ≤ 8) | 3.158 (0.961–10.383) | 0.058 | ||
| Age, yr., ≥ 30 (vs. < 30) | 2.565 (0.993–6.625) | 0.520 | ||
| CSddrm, score 3–4 (vs. score 0–2) | 11.791 (2.427–57.294) | 0.002 | 11.312 (2.513–50.913) | 0.002 |
OS overall survival, RFS relapse-free survival, HR hazard ratio, 95% CI 95% confidence interval, CSddrm the combined score for the immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2
aVariables considered in multivariate analysis Model 1 were age, tumor size, tumor stage, distant metastasis, histologic grade, and the expression of PARP1, γH2AX, BRCA1, and BRCA2
bVariables considered in multivariate analysis Model 2 were age, tumor size, tumor stage, distant metastasis, histologic grade, and CSddrm
Table 5.
Multivariate Cox proportional hazard regression analysis for overall survival and relapse-free survival in stage I and II osteosarcoma patients
| Characteristics | OS | P | RFS | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Multivariate analysis Model 1a | ||||
| Tumor size, cm, > 8 (vs. ≤ 8) | 4.617 (1.251–17.038) | 0.022 | 3.188 (0.982–10.349) | 0.054 |
| BRCA1, positive (vs. negative) | 4.257 (1.024–17.696) | 0.046 | ||
| γH2AX, positive (vs. negative) | 19.634 (2.412–159.853) | 0.005 | 6.366 (1.203–33.695) | 0.029 |
| Multivariate analysis Model 2b | ||||
| CSddrm, score 3–4 (vs. score 0–2) | 30.043 (3.724–242.374) | 0.001 | 27.986 (3.501–223.717) | 0.002 |
OS overall survival, RFS relapse-free survival, HR hazard ratio, 95% CI 95% confidence interval, CSddrm the combined score for the immunohistochemical expression of PARP1, γH2AX, BRCA1, and BRCA2
aVariables considered in multivariate analysis Model 1 were tumor size, tumor stage, histologic grade, and the expression of PARP1, γH2AX, BRCA1, and BRCA2
bVariables considered in multivariate analysis Model 2 were tumor size, tumor stage, histologic grade, and CSddrm
Co-treatment of PARP inhibitor olaparib and doxorubicin inhibited proliferation of osteosarcoma cells
Because the individual and combined expression patterns of PARP1, γH2AX, BRCA1, and BRCA2 were significantly associated with advanced clinicopathologic factors and survival of osteosarcoma patients, we evaluated the effects of PARP inhibition on the survival of osteosarcoma cells. The treatment of olaparib, a PARP inhibitor, and doxorubicin, genotoxic chemotherapeutic agent commonly used for the treatment of osteosarcoma, significantly inhibited the proliferation of U2OS, SaOS2, MG63, and KHOS/NP osteosarcoma cells in a dose- and time-dependent manner (Fig. 4). Based on the assumption that PARP inhibition makes tumor cells susceptible to genotoxic agents, we evaluated the effects of a combined treatment of olaparib and doxorubicin on the survival of osteosarcoma cells. Co-treatment of 10 μM olaparib and 0.2 μM doxorubicin significantly inhibited proliferation of U2OS, SaOS2, MG63, and KHOS/NP cells as indicated by an MTT and colony-forming assay (Fig. 5a). A soft-agar proliferation assay also showed a synergistic effect of combining olaparib and doxorubicin in inhibiting the proliferation of osteosarcoma cells (Fig. 5b). Moreover, individual and co-treatment of olaparib and doxorubicin significantly inhibited in vivo growth of KHOS/NP osteosarcoma cells (Fig. 6).
Fig. 4.
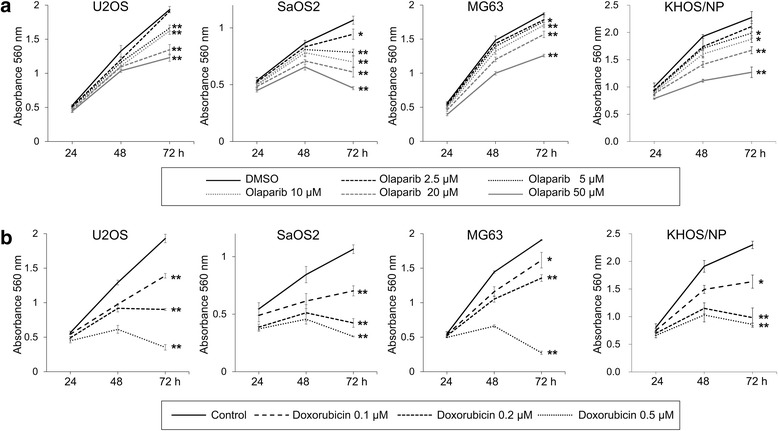
The effect of the PARP inhibitor, olaparib, and doxorubicin on the proliferation of osteosarcoma cells. Treatment with the PARP inhibitor, olaparib (a), and doxorubicin (b) significantly inhibited the growth of U2OS, SaOS2, MG63, and KHOS/NP osteosarcoma cells in a dose- and time-dependent manner. At the end of treatment, the cell viability was measured by an MTT assay. *, versus control, P < 0.05; **, versus control, P < 0.001
Fig. 5.
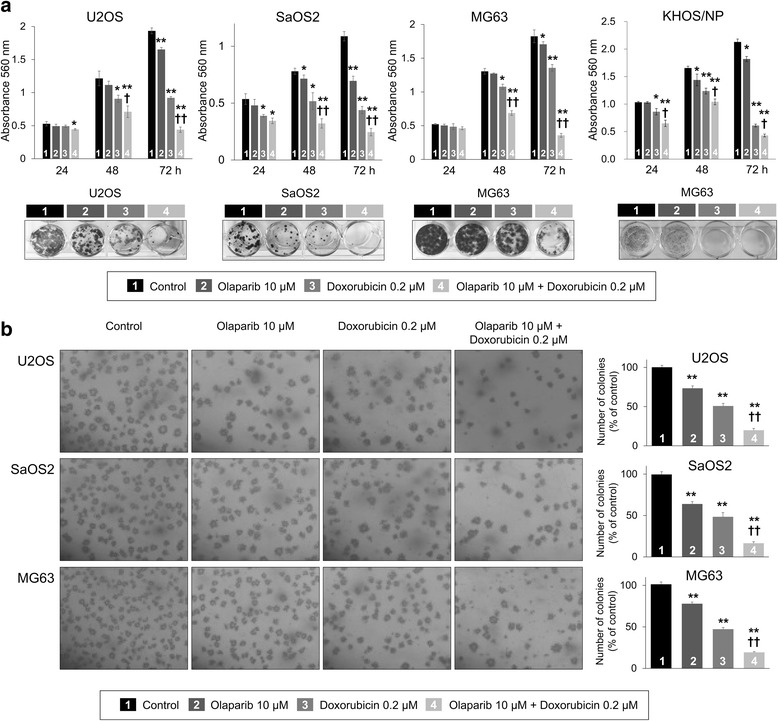
The effect of co-treatment of olaparib and doxorubicin on the proliferation of osteosarcoma cells. a Co-treatment of the PARP inhibitor, olaparib, and doxorubicin significantly inhibited the growth of U2OS, SaOS2, MG63, and KHOS/NP cells compared with individual treatment of olaparib or doxorubicin in MTT and colony forming assay. b Soft agar assay showed significantly decreased proliferation of U2OS, SaOS2, and MG63 cells with co-treatment of 10 μM of olaparib and 0.2 μM of doxorubicin (magnification, × 100). *, versus control, P < 0.05; **, versus control, P < 0.001; †, versus doxorubicin, P < 0.05; ††, versus doxorubicin, P < 0.001
Fig. 6.
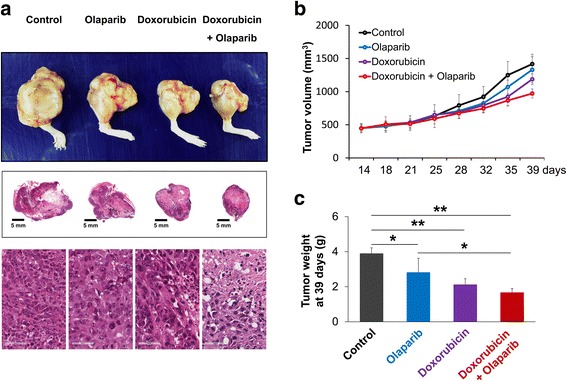
Co-treatment of olaparib and doxorubicin inhibited growth of KHOS/NP osteosarcoma cells in mice. a The gross and histologic findings of KHOS/NP osteosarcoma around the knee of mice. The tumor volume (b) and tumor weight (c) were significantly decreased with treatment of 10 μM olaparib or 0.2 μM doxorubicin. Especially, tumor growth was significantly decreased with co-treatment of 10 μM olaparib and 0.2 μM doxorubicin in mice. *, P < 0.05; **, P < 0.001
Anti-proliferative effect of co-treatment of olaparib and doxorubicin mediated by induction of apoptosis of osteosarcoma cells
Cell proliferation assays in Fig. 5, especially in the colony-forming assay, suggest that co-treatment of olaparib and doxorubicin induces death of osteosarcoma cells. In addition, flow cytometry analysis with annexin V and PI staining demonstrate a significant increase of apoptosis of U2OS, SaOS2, and MG63 osteosarcoma cells with co-treatment with olaparib and doxorubicin (Fig. 7a). Increased apoptosis of osteosarcoma cells by co-treatment of olaparib and doxorubicin was associated with increased expression of cleaved PARP1, cleaved caspase 3, and BAX, and decreased expression of BCL2 (Fig. 7b).
Fig. 7.
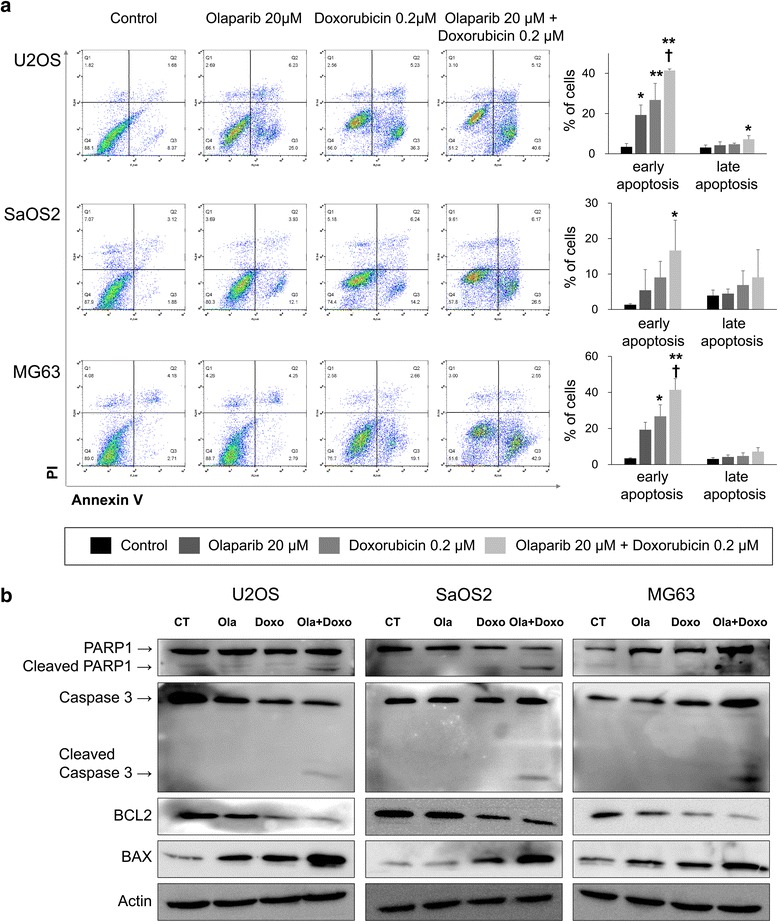
Co-treatment of olaparib and doxorubicin increases apoptosis of osteosarcoma cells. a Flow cytometry analysis with annexin V and propidium iodide staining in U2OS, SaOS2, and MG63 cells showed a significant increase of apoptotic population (early apoptosis; annexin V+/PI−, late apoptosis; annexin V+/PI+) with co-treatment of 10 μM olaparib and 0.2 μM doxorubicin. Flow cytometry analysis was performed 72 h after treatment of olaparib and/or doxorubicin. b The expression of cleaved PARP1, cleaved caspase 3, and BAX increased and the expression of BCL2 was decreased with co-treatment of olaparib and doxorubicin as demonstrated by western blot analysis. *, versus control, P < 0.05; **, versus control, P < 0.001; †, versus doxorubicin, P < 0.05
Knock-down of PARP1 potentiates the anti-cancer effects of doxorubicin in osteosarcoma cells
As was shown with the co-treatment of olaparib and doxorubicin, the knock-down of PARP1 with siRNA for PARP1 also potentiates the anti-cancer effects of doxorubicin in osteosarcoma cells (Fig. 8). Individual knock-down of PARP1 significantly inhibited proliferation of U2OS, SaOS2, and MG63 osteosarcoma cells (Fig. 8a). In addition, co-treatment of siRNA for PARP1 and doxorubicin significantly inhibited the proliferation of osteosarcoma cells compared with individual treatment of siRNA for PARP1 or doxorubicin (Fig. 8a). Moreover, co-treatment of siRNA for PARP1 and doxorubicin stimulated apoptotic signaling of osteosarcoma cells as indicated by an increase of cleaved PARP1 and BAX and a decrease of BCL2 expression (Fig. 8b).
Fig. 8.
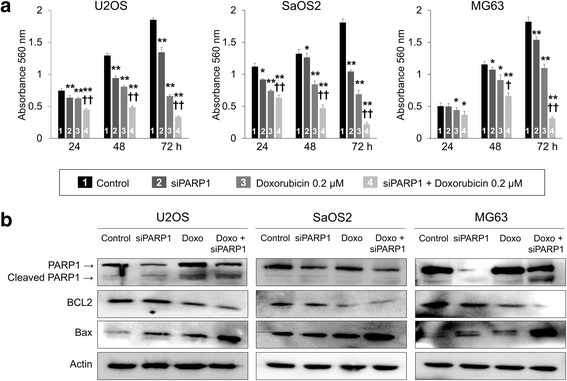
The knock-down of PARP1 potentiates the anti-cancer effect of olaparib in osteosarcoma cells. a The knock-down of PARP1 with siRNA for PARP1 and doxorubicin significantly inhibited the growth of U2OS, SaOS2, and MG63 cells. Moreover, the knock-down of PARP1 potentiated the anti-proliferative effect of doxorubicin in osteosarcoma cells as indicated by an MTT assay. b The expression of cleaved PARP1 and BAX increased, and the expression of BCL2 decreased with co-treatment of siRNA for PARP1 and with doxorubicin as indicated by western blot analysis. *, versus control, P < 0.05; **, versus control, P < 0.001; †, versus doxorubicin, P < 0.05; ††, versus doxorubicin, P < 0.001
Discussion
In this study, we have shown that PARP1-targetted therapy might be helpful in the treatment of osteosarcoma patients. In osteosarcoma tissue samples, the expression of PARP1 and the molecules related with the response of PARP1 to DNA damage were significantly associated with shorter survival of osteosarcoma patients. Moreover, inhibition of PARP1 with either olaparib or siRNA induced apoptosis of osteosarcoma cells and potentiated the cytotoxic effects of doxorubicin. Therefore, regarding the treatment of osteosarcoma patients, the expression of PARP1 might be helpful in the estimation of prognosis and potentially the selection of osteosarcoma patients for anti-PARP therapy.
In clinical tissue samples, the expression of PARP1, γH2AX, BRCA1, and BRCA2 were closely associated with each other and their expression patterns were correlated with advanced clinical indicators of osteosarcoma such as higher stage, latent distant metastasis, and higher histological grade. Moreover, individual expression of PARP1, γH2AX, and BRCA2 were significantly associated with shorter OS and RFS. Especially, γH2AX-positivity and the combined expression patterns of DDR molecules designated as CSddrm were independent indicators of poor prognosis of OS and RFS in both of overall and low-stage osteosarcoma patients. In line with these results in osteosarcomas, the expression pattern of the DDR molecules PARP1, γH2AX, BRCA1, and/or BRCA2 have been associated with the progression of various cancers, such as gastric cancer [28], breast cancer [29], ovarian cancer [28, 30], glioblastoma [31], and chordoma [32]. These findings suggest that PARP1, γH2AX, BRCA1, and BRCA2 are widely involved in the progression of various types of human malignant tumors and might be effective as a prognostic markers.
Poor prognosis of cancer patients expressing PARP1-related DDR molecules might be related with their role in DNA damage. When there is DNA damage, SSBs drive PARP1 to sense DNA damage; PARP1 then binds to strand breaks and recruits DNA repair factors [33–35]. Therefore, when there are no DDR molecules such as γH2AX and BRCA1/2, PARP inhibitors could result in accumulation of DSBs. Accumulation of DSBs eventually leads to synthetic lethality with defective homologous recognitions, and these persistent DSBs induce cell death [36]. Therefore, PARP inhibitors have been proposed as new therapeutic strategies for human malignant tumors carrying mutations in BRCA1/2 genes [4, 37, 38]. The effectiveness of PARP inhibition in BRCA1/2-mutated breast and ovarian cancers are under extensive evaluation [39]. In our study, as predicted, the expression of the PARP1-H2AX pathway and preservation of BRCA1/2 expression was associated with shorter survival of osteosarcoma patients. These findings suggest that responsiveness of DDR pathways are involved in the resistance of cancer cells to therapeutic agents which induce DNA damage. In this respect, osteosarcomas which preserve the PARP-H2AX-BRCA1/2 pathway might be resistant to genotoxic therapies and could be a therapeutic targets of PARP inhibition. Supportively, single uses of the PARP inhibitor olaparib and knock-down of PARP1 with siRNA significantly inhibited proliferation of osteosarcoma cells in a dose- and time-dependent manner. In addition, anti-cancer therapy which combine PARP inhibition and genotoxic chemotherapeutic agents according to the expression of PARP1, γH2AX, BRCA1, and BRCA2 has been suggested for breast carcinomas [37] and Ewing sarcomas [38, 40]. Olaparib suppressed BRCA1 protein level and induced hypersensitivity to radiation in lymphoblastoid cells [41]. In addition, it has recently been reported that PARP inhibitors are effective for the treatment of BRCA1/2-mutated Ewing sarcoma [38]. However, because of the limited studies on the PARP inhibition of sarcoma, we evaluated the combined effects of a PARP inhibitor and doxorubicin in osteosarcoma cells and showed that the combination of the PARP inhibitor olaparib and doxorubicin was more effective than olaparib or doxorubicin monotherapy. The synergistic effect of the combined use of these two drugs was associated with increased apoptosis of cells as indicated by flow cytometry analysis and western blotting, which show increased expression of cleaved PARP1, cleaved caspase 3, and BAX and decreased expression of BCL2. Therefore, in agreement with findings that PARP inhibition could be a promising therapeutic modality for the treatment of human cancers [42], the result of this study present the possibility that PARP inhibition could be an effective treatment of osteosarcomas by affecting the survival of cancer cells. Therefore, with respect to the treatment of sarcomas, this study has shown potential therapeutic efficacy of the PARP inhibitor olaparib for osteosarcomas.
This study has also shown that the expression of PARP1, γH2AX, BRCA1/2 are significantly associated with RFS of osteosarcoma patients. These findings suggest that PARP1, γH2AX, BRCA1/2 might play a role in the progression of osteosarcoma by inducing resistance to therapy. A recent report also has shown that PARP1-mediated chemo-resistance is associated with the expression of snail, the master gene involved in the epithelial to mesenchymal transition of cancer cells [43]. In addition, the expression of PARP1 affected the functional localization of the tumor-suppressor FOXO3 [28]. Inhibition of PARP1 induced nuclear localization of FOXO3, which had an anti-proliferative effect on gastric cancer cells [28]. These findings suggest that depletion of PARP1 might be helpful for the treatment of human malignant tumors by suppressing the expression of oncogenic snail [44] and inducing the tumor-suppressor FOXO3 [28].
Our results suggest that the expression of DDR molecules is useful for the selection of osteosarcoma patients that could potentially benefit from of anti-PARP1-γH2AX-BRCA1/2 therapy because these subgroups of osteosarcoma patients have a shorter survival rate even in low-stage osteosarcomas. Moreover, as this study has demonstrated a synergistic effect of combining olaparib and doxorubicin, combining PARP inhibition with a conventional genotoxic anti-cancer agents might be beneficial in the treatment of osteosarcoma patients. However, at present, PARP inhibitors are approved for use in some cancers which have responded to conventional therapies [20]. Therefore, regarding the application of PARP inhibition, our results suggest that more cases of cancers might benefit from PARP inhibition therapy because a substantial number of cases are included in the poor-prognostic group by expressing PARP1/γH2AX/BRCA1/2. In this study, 74% (26/35), 57% (20/35), 49% (17/35), and 46% (16/35) of cases were included in the poor-prognostic group by expressing PARP1, γH2AX, BRCA1, and BRCA2, respectively. In our previous study of breast carcinoma and soft-tissue sarcomas, 51 (98/192) % and 56 (63/112) % were also included in the poor prognostic group expressing PARP1, respectively [15, 16]. Moreover, when we performed addition analysis on the combined expression patterns of PARP1, γH2AX, BRCA1, and BRCA2, 51% (18/35) of osteosarcomas were included in the poor-prognostic group.
Conclusions
In conclusion, this study demonstrated that the individual and combined expression patterns of PARP1, γH2AX, BRCA1, and BRCA2 might be useful for the prediction of survival of osteosarcoma patients. Furthermore, combined use of the PARP inhibitor olaparib and doxorubicin synergistically inhibited proliferation of various types of osteosarcoma cells by inhibiting proliferation and activating apoptosis. Doxorubicin is a very effective conventional chemotherapeutic agent for osteosarcomas. However, despite its anti-cancer efficacy, use of doxorubicin is limited by its significant toxicity at doses during treatment of osteosarcomas. Therefore, the synergistic effect of the PARP inhibitor olaparib might serve to reduce the therapeutic dose of doxorubicin and decrease of the risk of doxorubicin toxicity. However, further study is needed on the clinical use of the combination of PARP inhibition and conventional genotoxic agents. Based on this rationale, this study might also be helpful for the selection of osteosarcoma patients that could potentially benefit from anti-PARP therapy, especially those in the poor-prognostic subgroup of osteosarcomas expressing PARP1, γH2AX, or BRCA1/2.
Acknowledgments
We thank CB Kong (Department of Orthopedic Surgery, Korea Institute of Radiological and Medical Science) for providing KHOS/NP cells; DB Leveson-Gower who provided medical writing services.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (grant numbers 2017R1A2B2005730 and 2017R1E1A1A01074533).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 95% CI
95% confidence interval
- CSddrm
Combined Score for the expression of DNA damage response molecules PARP1, γH2AX, BRCA1, and BRCA2
- DDR
DNA damage response
- DSBs
Double-strand breaks
- HR
Hazard ratio
- OS
Overall survival
- PARP1
Poly (ADP-ribose) polymerase 1
- PI
Propidium iodide
- RFS
Relapse-free survival
- siRNA
Short interfering RNA
- SSB
Single strand break
- TMA
Tissue microarray
- γH2AX
Phosphorylated H2AX
Authors’ contributions
HJP, JSB, KMK, YJM, SHP, SHH, UKH, ZZ, HSP, BHP, WSM, JRK and KYJ participated in the study design. HJP, JSB, KMK, YJM, SHP, SHH, UKH, ZZ, JRK and KYJ performed experiment. HJP, JSB, KMK, YJM, HSP, BHP, WSM, JRK and KYJ were involved in data collection and data interpretation. HJP, JSB, KMK, YJM, JRK and KYJ participated in the statistical analyses. HJP, JSB, KMK, YJM, SHP, SHH, HSP, BHP, WSM, JRK and KYJ wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study obtained institutional review board approval form Chonbuk National University Hospital (IRB number, CUH 2016–04–012-001) and was performed according to Declaration of Helsinki. Based on the retrospective and anonymous character of the study the approval contained a waiver for written informed consent. All animal experiments were performed with approval of the institutional animal care and use committee of Chonbuk National University (approval number: CBNU 2017–0080).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Hye Jeong Park and Jun Sang Bae contributed equally to this work.
Contributor Information
Hye Jeong Park, Email: kellypark0814@gmail.com.
Jun Sang Bae, Email: jsbae78@jbnu.ac.kr.
Kyoung Min Kim, Email: kmkim@jbnu.ac.kr.
Young Jae Moon, Email: yj-lunar@daum.net.
See-Hyoung Park, Email: imsesame@gmail.com.
Sang Hoon Ha, Email: sanghoon@jbnu.ac.kr.
Usama Khamis Hussein, Email: usamahussein@jbnu.ac.kr.
Zhongkai Zhang, Email: porkchop@iambad.in.
Ho Sung Park, Email: hspark@jbnu.ac.kr.
Byung-Hyun Park, Email: bhpark@jbnu.ac.kr.
Woo Sung Moon, Email: mws@jbnu.ac.kr.
Jung Ryul Kim, Email: jrkeem@jbnu.ac.kr.
Kyu Yun Jang, Email: kyjang@chonbuk.ac.kr.
References
- 1.Sukhanova MV, Abrakhi S, Joshi V, Pastre D, Kutuzov MM, Anarbaev RO, Curmi PA, Hamon L, Lavrik OI. Single molecule detection of PARP1 and PARP2 interaction with DNA strand breaks and their poly(ADP-ribosyl)ation using high-resolution AFM imaging. Nucleic Acids Res. 2016;44(6):e60. doi: 10.1093/nar/gkv1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LA, Sharpless NE. PARP and cancer--if it's broke, don't fix it. N Engl J Med. 2011;364(3):277–279. doi: 10.1056/NEJMe1012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engert F, Kovac M, Baumhoer D, Nathrath M, Fulda S. Osteosarcoma cells with genetic signatures of BRCAness are susceptible to the PARP inhibitor talazoparib alone or in combination with chemotherapeutics. Oncotarget. 2016;8(30):48794–48806. doi: 10.18632/oncotarget.10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HJ, Yoon C, Schmidt B, Park DJ, Zhang AY, Erkizan HV, Toretsky JA, Kirsch DG, Yoon SS. Combining PARP-1 inhibition and radiation in Ewing sarcoma results in lethal DNA damage. Mol Cancer Ther. 2013;12(11):2591–2600. doi: 10.1158/1535-7163.MCT-13-0338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Luijsterburg MS, de Krijger I, Wiegant WW, Shah RG, Smeenk G, de Groot AJ, Pines A, Vertegaal AC, Jacobs JJ, Shah GM, et al. PARP1 links CHD2-mediated chromatin expansion and H3.3 deposition to DNA repair by non-homologous end-joining. Mol Cell. 2016;61(4):547–562. doi: 10.1016/j.molcel.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oei AL, Vriend LE, van Leeuwen CM, Rodermond HM, Ten Cate R, Westermann AM, Stalpers LJ, Crezee J, Kanaar R, Kok HP, et al. Sensitizing thermochemotherapy with a PARP1-inhibitor. Oncotarget. 2016;8(10):16303–16312. doi: 10.18632/oncotarget.11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JR, Shin KS, Choi CY, Kang SJ. PARP1 regulates the protein stability and proapoptotic function of HIPK2. Cell Death Dis. 2016;7(10):e2438. doi: 10.1038/cddis.2016.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meehan RS, Chen AP. New treatment option for ovarian cancer: PARP inhibitors. Gynecol Oncol Res Pract. 2016;3:3. doi: 10.1186/s40661-016-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 10.Talhaoui I, Lebedeva NA, Zarkovic G, Saint-Pierre C, Kutuzov MM, Sukhanova MV, Matkarimov BT, Gasparutto D, Saparbaev MK, Lavrik OI, et al. Poly(ADP-ribose) polymerases covalently modify strand break termini in DNA fragments in vitro. Nucleic Acids Res. 2016;44(19):9279–9295. doi: 10.1093/nar/gkw675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh R, Roy S, Kamyab J, Dantzer F, Franco S. Common and unique genetic interactions of the poly(ADP-ribose) polymerases PARP1 and PARP2 with DNA double-strand break repair pathways. DNA Repair (Amst) 2016;45:56–62. doi: 10.1016/j.dnarep.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogiwara H, Ui A, Shiotani B, Zou L, Yasui A, Kohno T. Curcumin suppresses multiple DNA damage response pathways and has potency as a sensitizer to PARP inhibitor. Carcinogenesis. 2013;34(11):2486–2497. doi: 10.1093/carcin/bgt240. [DOI] [PubMed] [Google Scholar]
- 13.Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, Yang ES. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015;6(29):26995–27007. doi: 10.18632/oncotarget.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Chen Z, Cui Y, Qin C, He Y, Song X. Combined olaparib and oxaliplatin inhibits tumor proliferation and induces G2/M arrest and gamma-H2AX foci formation in colorectal cancer. Onco Targets Ther. 2015;8:3047–3054. doi: 10.2147/OTT.S89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SH, Noh SJ, Kim KM, Bae JS, Kwon KS, Jung SH, Kim JR, Lee H, Chung MJ, Moon WS, et al. Expression of DNA damage response molecules PARP1, gammaH2AX, BRCA1, and BRCA2 predicts poor survival of breast carcinoma patients. Transl Oncol. 2015;8(4):239–249. doi: 10.1016/j.tranon.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KM, Moon YJ, Park SH, Park HJ, Wang SI, Park HS, Lee H, Kwon KS, Moon WS, Lee DG, et al. Individual and combined expression of DNA damage response molecules PARP1, gammaH2AX, BRCA1, and BRCA2 predict shorter survival of soft tissue sarcoma patients. PLoS One. 2016;11(9):e0163193. doi: 10.1371/journal.pone.0163193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill J, Ahluwalia MK, Geller D, Gorlick R. New targets and approaches in osteosarcoma. Pharmacol Ther. 2013;137(1):89–99. doi: 10.1016/j.pharmthera.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Roth M, Barris DM, Piperdi S, Kuo V, Everts S, Geller D, Houghton P, Kolb EA, Hawthorne T, Gill J, et al. Targeting glycoprotein NMB with antibody-drug conjugate, Glembatumumab Vedotin, for the treatment of osteosarcoma. Pediatr Blood Cancer. 2016;63(1):32–38. doi: 10.1002/pbc.25688. [DOI] [PubMed] [Google Scholar]
- 19.Kovac M, Blattmann C, Ribi S, Smida J, Mueller NS, Engert F, Castro-Giner F, Weischenfeldt J, Kovacova M, Krieg A, et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun. 2015;6:8940. doi: 10.1038/ncomms9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchtel KM, Vogel Postula KJ, Weiss S, Williams C, Pineda M, Weissman SM. FDA approval of PARP inhibitors and the impact on genetic counseling and genetic testing practices. J Genet Couns. 2018;27(1):131–139. doi: 10.1007/s10897-017-0130-7. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of tumours of soft tissue and bone. 4. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 22.Edge S, Cancer AJCo. AJCC cancer staging handbook: from the AJCC cancer staging manual: Springer; 2010.
- 23.Allred D, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–168. [PubMed] [Google Scholar]
- 24.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Yu TK, Kim KM, Park HS, Lee J-H, Moon WS. Expression of SIRT1 and DBC1 is associated with poor prognosis of soft tissue sarcomas. PLoS One. 2013;8(9):e74738. doi: 10.1371/journal.pone.0074738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8(12):e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, Moon WS, Lee DG, Jang KY. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol. 2013;6(3):282–289. doi: 10.1593/tlo.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EH, Kim MS, Lee KH, Koh JS, Jung WG, Kong CB. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget. 2016;7(43):70869–70880. doi: 10.18632/oncotarget.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho D, Park H, Park SH, Kim K, Chung M, Moon W, Kang M, Jang K. The expression of DBC1/CCAR2 is associated with poor prognosis of ovarian carcinoma. J Ovarian Res. 2015;8:2. doi: 10.1186/s13048-015-0129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojo F, Garcia-Parra J, Zazo S, Tusquets I, Ferrer-Lozano J, Menendez S, Eroles P, Chamizo C, Servitja S, Ramirez-Merino N, et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol. 2012;23(5):1156–1164. doi: 10.1093/annonc/mdr361. [DOI] [PubMed] [Google Scholar]
- 30.Gan A, Green AR, Nolan CC, Martin S, Deen S. Poly(adenosine diphosphate-ribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum Pathol. 2013;44(8):1638–1647. doi: 10.1016/j.humpath.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Galia A, Calogero AE, Condorelli R, Fraggetta F, La Corte A, Ridolfo F, Bosco P, Castiglione R, Salemi M. PARP-1 protein expression in glioblastoma multiforme. Eur J Histochem. 2012;56(1):e9. doi: 10.4081/ejh.2012.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Lv T, Liu Y, Huang X, Qiu Z, Li J. PARP1 is a novel independent prognostic factor for the poor prognosis of chordoma. Cancer Biomark. 2016;16(4):633–639. doi: 10.3233/CBM-160605. [DOI] [PubMed] [Google Scholar]
- 33.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342(Pt 2):249–268. doi: 10.1042/bj3420249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13(7):411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 35.Ko HL, Ren EC. Functional aspects of PARP1 in DNA repair and transcription. Biomol Ther. 2012;2(4):524–548. doi: 10.3390/biom2040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortusewicz O, Ame JC, Schreiber V, Leonhardt H. Feedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cells. Nucleic Acids Res. 2007;35(22):7665–7675. doi: 10.1093/nar/gkm933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avila-Arroyo S, Nunez GS, Garcia-Fernandez LF, Galmarini CM. Synergistic effect of Trabectedin and Olaparib combination regimen in breast Cancer cell lines. J Breast Cancer. 2015;18(4):329–338. doi: 10.4048/jbc.2015.18.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill SJ, Travers J, Pshenichnaya I, Kogera FA, Barthorpe S, Mironenko T, Richardson L, Benes CH, Stratton MR, McDermott U, et al. Combinations of PARP inhibitors with Temozolomide drive PARP1 trapping and apoptosis in Ewing's sarcoma. PLoS One. 2015;10(10):e0140988. doi: 10.1371/journal.pone.0140988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess M, Puhalla S. BRCA 1/2-mutation related and sporadic breast and ovarian cancers: more alike than different. Front Oncol. 2014;4:19. doi: 10.3389/fonc.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MA, Reynolds CP, Kang MH, Kolb EA, Gorlick R, Carol H, Lock RB, Keir ST, Maris JM, Billups CA, et al. Synergistic activity of PARP inhibition by talazoparib (BMN 673) with temozolomide in pediatric cancer models in the pediatric preclinical testing program. Clin Cancer Res. 2015;21(4):819–832. doi: 10.1158/1078-0432.CCR-14-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourton EC, Ahorner PA, Plowman PN, Zahir SA, Al-Ali H, Parris CN. The PARP-1 inhibitor Olaparib suppresses BRCA1 protein levels, increases apoptosis and causes radiation hypersensitivity in BRCA1(+/−) lymphoblastoid cells. J Cancer. 2017;8(19):4048–4056. doi: 10.7150/jca.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariano G, Ricciardi MR, Trisciuoglio D, Zampieri M, Ciccarone F, Guastafierro T, Calabrese R, Valentini E, Tafuri A, Del Bufalo D, et al. PARP inhibitor ABT-888 affects response of MDA-MB-231 cells to doxorubicin treatment, targeting snail expression. Oncotarget. 2015;6(17):15008–15021. doi: 10.18632/oncotarget.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun M, Guo X, Qian X, Wang H, Yang C, Brinkman KL, Serrano-Gonzalez M, Jope RS, Zhou B, Engler DA, et al. Activation of the ATM-snail pathway promotes breast cancer metastasis. J Mol Cell Biol. 2012;4(5):304–315. doi: 10.1093/jmcb/mjs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


