ABSTRACT
Streptococcus suis is a major swine and zoonotic pathogen that causes severe infections. Previously, we identified 2 Spx regulators in S. suis, and demonstrated that SpxA1 affects oxidative stress tolerance and virulence. However, the mechanism behind SpxA1 function remains unclear. In this study, we targeted 4 genes that were expressed at significantly reduced levels in the spxA1 mutant, to determine their specific roles in adaptation to oxidative stress and virulence potential. The Δnox strain exhibited impaired growth under oxidative stress conditions, suggesting that NADH oxidase is involved in oxidative stress tolerance. Using murine and pig infection models, we demonstrate for the first time that NADH oxidase is required for virulence in S. suis 2. Furthermore, the enzymatic activity of NADH oxidase has a key role in oxidative stress tolerance and a secondary role in virulence. Collectively, our findings reveal that NADH oxidase plays an important part in SpxA1 function and provide a new insight into the pathogenesis of S. suis 2.
KEYWORDS: NADH oxidase, oxidative stress, Streptococcus suis, virulence
Introduction
Streptococcus suis is a major swine and zoonotic pathogen responsible for severe economic losses in the swine industry and an increasing number of human cases.1 It causes a wide range of diseases in pigs, including meningitis, septicemia and endocarditis.2 S. suis infections in humans lead to meningitis and streptococcal toxic shock-like syndrome (STSLS).2 In total, 33 serotypes (types 1 to 31, 33, and 1/2) of S. suis have been proposed based on capsular polysaccharides,3 of which serotype 2 is the most common cause of infections in humans and pigs worldwide.4 The first human case of S. suis infection was reported in Denmark in 1968.4 By 2012, the total number of S. suis infections in humans was close to 1600 cases, doubling the number published in 2009.5 Remarkably, 2 large outbreaks of S. suis epidemics occurred in China in 1998 and 2005, which resulted in 25 human cases with 14 deaths and 215 human cases with 38 deaths, respectively.6 In addition, S. suis has been reported to be the major cause of adult meningitis in Vietnam, the second in Thailand and the third most common cause of community-acquired bacterial meningitis in Hong Kong.7 Although numerous studies have been performed over the past 40 years, the pathogenesis of S. suis infection is still not entirely known.
During the infection process, bacteria encounter changing environments and host factors. Transcriptional regulators play an important role in response to environmental signals by modulating the expression of related genes. Spx proteins are a group of global regulators found in low-GC content Gram-positive bacteria, and have been shown to be involved in stress responses and virulence.8-10 In previous work, we identified 2 orthologs of the Spx regulator in S. suis, namely SpxA1 and SpxA2, and demonstrated that SpxA1 affects oxidative stress tolerance and virulence.11 Microarray analysis revealed that several genes possibly involved in oxidative stress responses and/or virulence were significantly downregulated in ΔspxA1 compared to the parent strain.11 These included nox (SSUSC84_0648, encoding NADH oxidase), tpx (SSUSC84_1246, encoding thiol peroxidase), copA (SSUSC84_1247, encoding copper-transporting ATPase), and sodA (SSUSC84_1386, encoding superoxide dismutase).11 Genes nox, tpx and sodA have been well studied and reported to be involved in oxidative stress responses and/or virulence in various streptococci and other bacteria,12-29 while copA has been shown to be implicated in copper resistance.30-32 Additionally, an undefined gene, 0350 (SSUSC84_0350, encoding a hypothetical protein) is potentially involved in oxidative stress and/or virulence, as it was downregulated 8.7-fold in ΔspxA1.11 Functional analysis of these SpxA1-regulated genes will be undoubtedly important for the understanding of SpxA1 function and gaining insights into the pathogenesis of S. suis infection. However, of these genes, only sodA has been described in S. suis.26,28 The other 4 genes have not been characterized yet, and their biological function in S. suis remains unclear.
In this work, we examined the roles of the genes 0350, nox, tpx and copA in oxidative stress tolerance and virulence of S. suis 2. The Δnox strain exhibited increased susceptibility to oxidative stress agents and attenuated virulence in murine and pig infection models. Furthermore, the NADH oxidase activity has a key role in oxidative stress tolerance and a secondary role in virulence.
Results
Roles of four genes in oxidative stress tolerance and virulence of S. suis 2
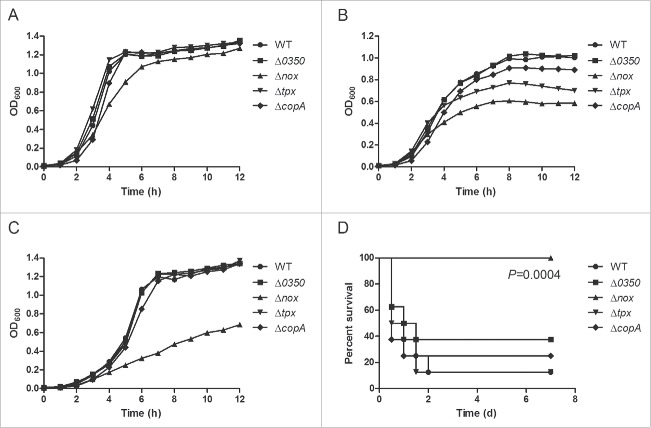
To investigate the roles of the genes 0350, nox, tpx and copA in oxidative stress tolerance, the ability of the mutants (Δ0350, Δnox, Δtpx and ΔcopA) to grow under low- and high-oxygen conditions was examined and compared with the wild-type (WT) strain. Under static conditions (i.e. low oxygen tension), growth of Δnox was slightly reduced compared with the WT strain, while growth of the other 3 mutants was essentially identical to that of the WT strain (Fig. 1A). In contrast, growth of Δnox was severely impaired under vigorous shaking (i.e. high oxygen tension) (Fig. 1B). Δtpx also showed an obvious growth defect under these conditions, while Δ0350 and ΔcopA showed no major difference in growth compared to the WT strain (Fig. 1B). We also evaluated the ability of the WT and mutant strains to grow in the presence of H2O2, and found that Δnox grew poorly compared to the other strains (Fig. 1C). These results suggested that nox is involved in resistance to oxidative stress generated by environmental oxygen and hydrogen peroxide.
Figure 1.
Preliminary research on the role of the genes 0350, nox, tpx and copA in oxidative stress tolerance and virulence of S. suis 2. A, Growth curves of S. suis strains cultured under static conditions, i.e. low-oxygen conditions. B, Growth curves of S. suis strains cultured in a shaking incubator set to 200 rpm, i.e. high-oxygen conditions. C, Growth curves of S. suis strains cultured with 0.5 mM H2O2 under static conditions. Growth curves shown are representative of at least 3 independent experiments. D, Survival curves of mice infected with S. suis strains. Significant difference was observed between the WT and Δnox group (P = 0.0004, the log-rank test).
The roles of the four genes in S. suis virulence were examined using a murine infection model. Mice infected with the Δnox mutant showed no clinical signs and all survived (Fig. 1D). However, mice in other groups developed typical clinical symptoms of S. suis 2 infection, including rough hair coat, lethargy, and swollen eyes. The final survival rates of mice in the Δ0350, Δtpx and ΔcopA groups were 37.5%, 12.5% and 25%, respectively, compared to 12.5% in the WT group (Fig. 1D). The survival rates were significantly lower in the WT-infected mice than in the Δnox-infected mice (P = 0.0004, the log-rank test). The data indicated that nox is required for S. suis infection.
Taken together, these results revealed that nox plays a key role in oxidative stress tolerance and virulence of S. suis 2. Therefore, further research focused on the nox gene, and the complementation strain CΔnox was included in all experiments.
Bioinformatics analysis of S. suis NADH oxidase
In the genome of S. suis 2 strain SC84, the nox gene is annotated to encode NADH oxidase. BlastN analysis using the nox sequence of strain SC84 confirmed the presence of nox in all 23 complete S. suis genomes available in the National Center for Biotechnology Information database as of 31 March 2016 (Table S1). Multiple sequence alignments of NADH oxidase from S. suis and other streptococci revealed that NADH oxidase is highly conserved among streptococcus species (Fig. S1A). Protein homology modeling was performed to predict the structure of S. suis NADH oxidase (Fig. S1B), which may be useful for studying its active sites and the design of therapeutics. The secondary structure of S. suis NADH oxidase is predicted to consist of 11 α-helices, 25 β-sheets, and 5 coils (Fig. S1A).
The Δnox strain exhibits reduced tolerance to oxidative stress agents
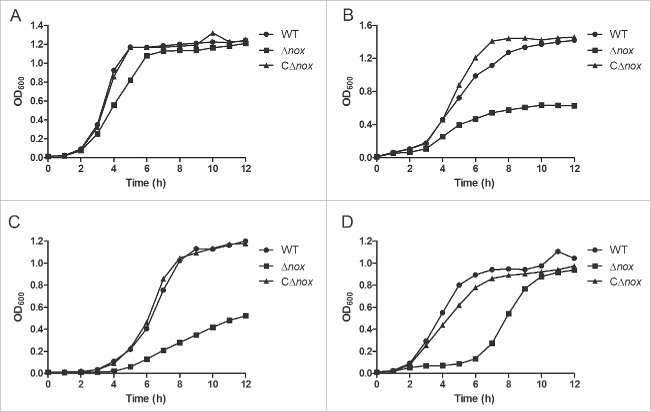
In the preliminary study, we showed that nox is involved in oxidative stress tolerance. To avoid any possible polar effect caused by nox deletion, the same experiments were carried out with the WT, Δnox and CΔnox strains. As expected, Δnox exhibited impaired growth under vigorous shaking or in the presence of H2O2, while CΔnox grew as well as the WT strain under all conditions (Fig. 2A-C). Next, we examined the sensitivity of S. suis strains to SIN-1, which indirectly generates ONOO−.33 In the presence of 2 mM SIN-1, the growth of Δnox was markedly delayed (Fig. 2D). We also evaluated the ability of these strains to grow in the presence of paraquat, which generates intracellular O2− radicals. However, no obvious difference in growth was observed between Δnox and the WT strain (Fig. S2). These results strongly suggested that S. suis NADH oxidase plays a role in oxidative stress tolerance.
Figure 2.
Growth characteristics of the WT, Δnox and CΔnox strains under various conditions. A, Growth under static conditions. B, Growth in a shaking incubator set to 200 rpm. C, Growth with 0.5 mM H2O2 under static conditions. D, Growth with 2 mM SIN-1 under static conditions. Growth curves shown are representative of at least 3 independent experiments.
NADH oxidase contributes to the virulence of S. suis in the murine infection model
To confirm that the lack of nox was responsible for the impaired virulence of Δnox, groups of 10 mice were inoculated intraperitoneally with ∼1.5×108 CFU of the WT, Δnox and CΔnox strains to determine survival rates. As expected, mice in the Δnox group exhibited no clinical signs and the survival rate was 100%, while mice in the WT and CΔnox groups showed severe clinical symptoms, and the survival rates were 20% and 0, respectively (Fig. S3). The survival rates were significantly higher in mice infected with the Δnox mutant than in those infected with the WT strain (P = 0.0003, the log-rank test), and the CΔnox strain (P < 0.0001, the log-rank test). Therefore, the reduced virulence of Δnox was due to the deletion of nox, not a possible polar effect.
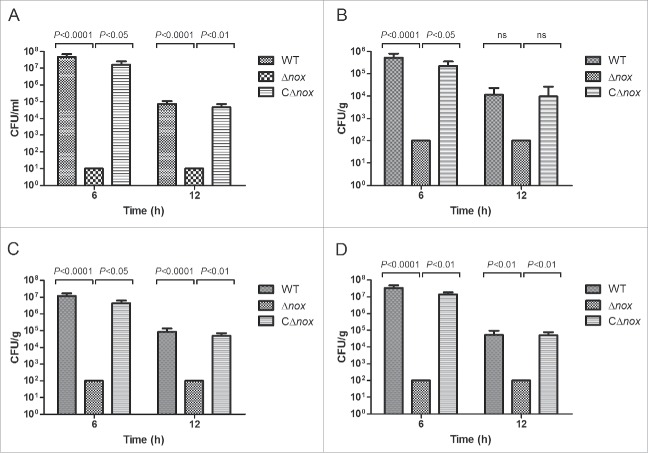
To further investigate the nature of the reduced virulence, bacterial counts of these strains in the blood and organs (brain, liver and spleen) of mice infected with a sublethal dose of bacteria were determined at 6 and 12 h after infection. Bacterial counts in the blood (Fig. 3A), brain (Fig. 3B), liver (Fig. 3C) and spleen (Fig. 3D) of mice in the WT and CΔnox groups reached high levels at 6 h after infection, then decreased to relatively lower levels at 12 h after infection. However, no bacterial cells could be detected at 6 and 12 h after infection in the blood and organs of mice infected with Δnox (Fig. 3). These results indicated that S. suis NADH oxidase is involved in colonization of the blood and organs during animal infection.
Figure 3.
Colonization of various tissues of mice by the WT, Δnox and CΔnox strains. Mice were inoculated intraperitoneally with ∼2 × 107 CFU of the WT, Δnox and CΔnox strains, respectively. Bacterial counts in the blood (A), brain (B), liver (C) and spleen (D) were examined at 6 h and 12 h post infection. The data shown are means with standard deviations for the results from 2 independent experiments. No bacterial cells could be recovered from mice in the Δnox group, and the data shown are the limits of detection. Statistical analyses were performed by a repeated measures test with a Tukey post test. Significant differences were found at 6 h and 12 h between the Δnox group and the WT group, and between the Δnox group and the CΔnox group for all tissues examined, with the exception of brain at 12 h.
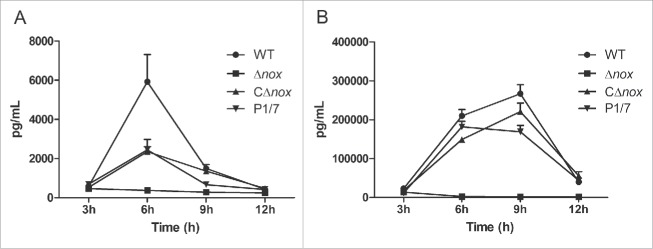
Considering that inflammation plays an important role in S. suis infection,34 we compared the capacity of the strains to induce inflammatory mediators. The production of both TNF-α and MCP-1 was severely reduced in mice infected with the Δnox mutant in comparison to animals infected with the WT and CΔnox strains (Fig. 4). Mice in the WT group reached peak production of TNF-α (Fig. 4A) at 6 h and MCP-1 (Fig. 4B) at 9 h after infection. Serum levels of TNF-α (Fig. 4A) and MCP-1 (Fig. 4B) from CΔnox-infected mice were similar to those from P1/7-infected mice. In contrast, the Δnox mutant triggered a very low production of both TNF-α (Fig. 4A) and MCP-1 (Fig. 4B).
Figure 4.
Time course of production of cytokines in mice infected with S. suis strains. A, Serum levels of TNF-α. Significant differences were found between Δnox and the WT strain, and between Δnox and CΔnox, from 6 h to 12 h (P < 0.01). B, Serum levels of MCP-1. Significant differences were found between the WT and the mutant from 3 h to 12 h, and between the CΔnox strain and the Δnox mutant from 6 h to 12 h (P < 0.01). Data are expressed as means with standard error of the median from 6 mice for each strain at each time point. Statistical analyses were performed using the Mann–Whitney test.
NADH oxidase facilitates the growth of S. suis in blood
To test whether S. suis NADH oxidase plays a role in evasion of innate immune responses, we examined the ability of the WT, Δnox and CΔnox strains to grow in whole blood collected from BALB/c mice. The mean growth factors of Δnox after 1-, 2-, and 3-h of incubation were 0.912, 1.088 and 1.797, respectively, while those of the WT strain were 2.448, 9.387 and 42.053, respectively (Fig. 5). Furthermore, the growth factors of the CΔnox strain were restored relative to the mutant, though they did not reach the level of the WT strain (Fig. 5). Growth of the Δnox mutant in whole blood was significantly slower than that of the WT and CΔnox strains (P < 0.0001, 2-tailed unpaired t test), suggesting that S. suis NADH oxidase has an effect on immune evasion.
Figure 5.
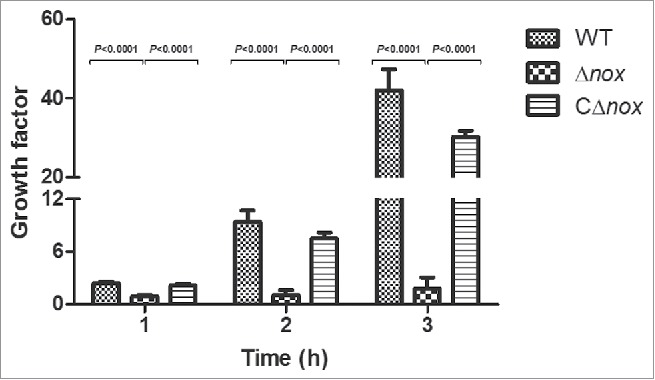
Growth factors of the WT, Δnox and CΔnox strains in mouse blood. The WT, Δnox and CΔnox strains were adjusted to 1 × 105 CFU/mL. Bacterial suspensions (50 μL) were combined with fresh whole blood (450 μL), and the mixtures were incubated at 37°C for 3 h with end-to-end rotation. The growth factor was defined as the ratio of CFU in each sample after incubation over the CFU in the corresponding inoculum. The data shown are means with standard deviations for the results from 3 independent experiments carried out in duplicate. The two-tailed unpaired t test was used for statistical analysis. Significant differences were observed at 1, 2 and 3 h between the Δnox group and the WT group, and between the Δnox group and the CΔnox group (P < 0.0001).
The Δnox mutant is attenuated in the pig model of infection
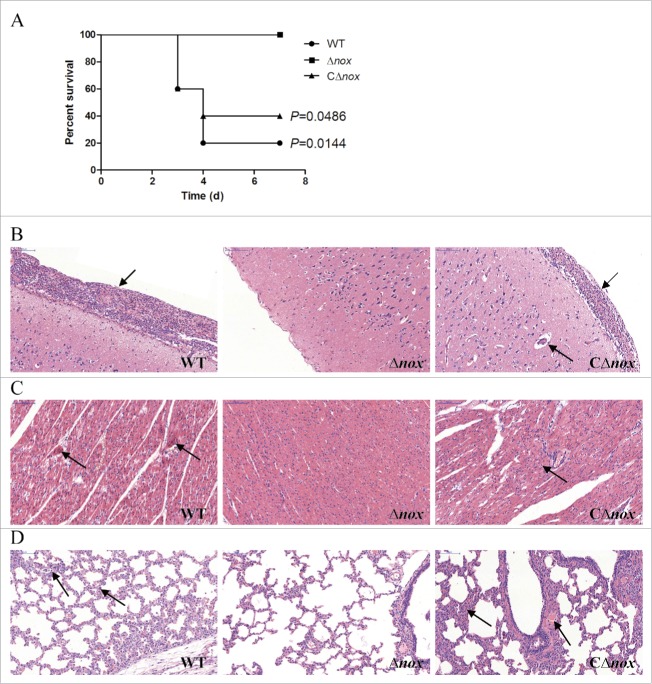
To confirm the observed impaired virulence of the Δnox mutant, we conducted a trial in pigs, which are the natural hosts of infection. Animals in the control group did not present any clinical signs during the test. Conversely, all pigs infected with the WT strain developed most of the typical symptoms, including depression, prostration, swollen joints and shaking within 24 h. Two of them died or were sacrificed for ethical reasons at day 3 post-infection and 2 others at day 4 post-infection. In contrast, animals in the Δnox group showed no signs of infection and all survived until the end of the trial. In the CΔnox group, 3 of 5 pigs presented severe clinical signs and died within 3 to 4 days, while another 2 showed mild symptoms and survived. Significant differences in survival rates were found between the Δnox group and the WT group (P = 0.0144, the log-rank test), and between the Δnox group and the CΔnox group (P = 0.0486, the log-rank test) (Fig. 6A).
Figure 6.
Role of NADH oxidase in S. suis 2 virulence in the pig infection model. A, Survival curves of pigs infected with S. suis strains. Animals inoculated with PBS are not shown for simplicity. P = 0.0144 for comparison of the Δnox group with the WT group, and P = 0.0486 for comparison of the Δnox group with the CΔnox group (the log-rank test). B, Pathological examination of brain tissues of the infected pigs. C, Pathological examination of heart tissues of the infected pigs. D, Pathological examination of lungs tissues of the infected pigs. Arrowheads show the pathological changes. Representative images are shown for each group. Bars, 100 μm.
Histopathological studies were carried out to examine the pathological changes in brain, heart and lungs of the infected pigs. The meninges of pigs in the WT group were severely thickened and a mass of inflammatory cells could be observed (Fig. 6B). Similar pathological alterations occurred in the meninges of CΔnox-infected pigs, while the meninges of Δnox-infected pigs showed no obvious changes (Fig. 6B). In the heart of pigs inoculated with the WT strain, parts of myocardial fibers arranged disorderly, accompanied by myocardial cells edema and degeneration (Fig. 6C). By contrast, there were no obvious changes in the heart of Δnox-infected pigs (Fig. 6C). Additionally, disordered arrangement of myocardial fibers and disappearance of transverse striations were observed in the heart of CΔnox-infected pigs (Fig. 6C). Pathological characteristics in the lungs of pigs in the WT and CΔnox groups were also quite distinct from those of pigs infected with the Δnox mutant. Pigs challenged with the WT and CΔnox strains exhibited thickened alveolar walls, expansive capillaries, serous effusion and infiltration of inflammatory cells (Fig. 6D). However, animals in the Δnox group displayed no obvious pathological changes (Fig. 6D).
Taken together, the experimental infection on pigs also indicated that NADH oxidase contributes significantly to the virulence of S. suis 2.
The NADH oxidase activity plays a key role in oxidative stress tolerance and a secondary role in virulence
To detect the enzymatic activity of NADH oxidase, we prepared recombinant NADH oxidase (rNox) by cloning the nox gene into pET-30a vector and introducing the resultant plasmid into E. coli BL21 (DE3) strain for expression. As seen in Figure 7A, purified rNox corresponded to an active form with a high activity (6258.2 U/mg) compared with NADH oxidase expressed in engineered Saccharomyces cerevisiae strains.35 We also generated 2 inactive forms of NADH oxidase with point mutations of the putative active sites (H11A and C44A). Both inactive forms displayed significantly reduced activity compared to the WT NADH oxidase (Fig. 7A).
Figure 7.
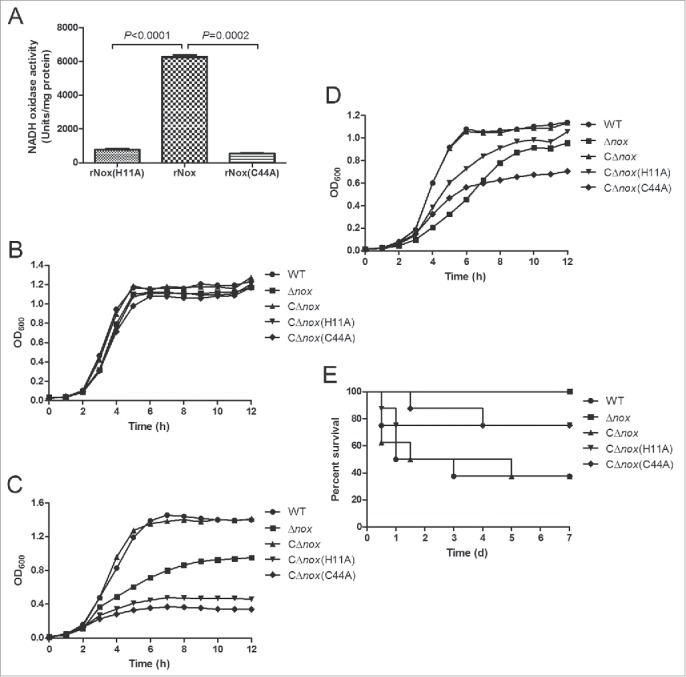
Role of the NADH oxidase activity in oxidative stress tolerance and virulence of S. suis 2. A, NADH oxidase activities of purified rNox, rNox(H11A) and rNox(C44A). The data shown are means with standard deviations of 3 independent experiments. Statistical analyses were performed using the 2-tailed paired t test. B, Growth curves of S. suis strains cultured under static conditions. C, Growth curves of S. suis strains cultured in a shaking incubator set to 200 rpm. D, Growth curves of S. suis strains cultured with 0.5 mM H2O2 under static conditions. Growth curves shown are representative of at least 3 independent experiments. E, Survival curves of mice infected with S. suis strains. P = 0.0082 for comparison of the CΔnox group with the Δnox group, P = 0.1624 for comparison of the CΔnox group with the CΔnox(H11A) group, and P = 0.1147 for comparison of the CΔnox group with the CΔnox(C44A) group (the log-rank test).
In addition, we constructed 2 complementation strains of Δnox with the inactive NADH oxidase, i.e. CΔnox(H11A) and CΔnox(C44A). These two strains exhibited severely impaired growth under vigorous shaking or in the presence of H2O2, while their growth was almost identical to that of the WT and CΔnox strains under static conditions (Fig. 7B-D). Moreover, the survival rates were lower in mice infected with CΔnox than in those infected with CΔnox(H11A) (P = 0.1624, the log-rank test) and CΔnox(C44A) (P = 0.1147, the log-rank test) (Fig. 7E). Although the differences are not significant, it is quite obvious that the complementation strains of Δnox with the inactive forms of NADH oxidase are attenuated compared with CΔnox in the murine model of infection.
Overall, these results revealed that the enzymatic activity of NADH oxidase plays a key role in oxidative stress tolerance and a secondary role in virulence.
Discussion
Reactive oxygen species (ROS) generated by host phagocytes possess antimicrobial activity against a large number of pathogens.36 The ability to survive oxidative stress is a key virulence-related trait in various bacteria.37 Evidence is increasing that transcriptional regulation by Spx is important for low-GC Gram-positive bacteria to cope with oxidative stress.8-11,38,39 We have recently shown that SpxA1 modulates oxidative stress tolerance and virulence in S. suis, and that a large number of genes are regulated by SpxA1.11 In order to better understand the mechanism behind SpxA1 function, we targeted 4 genes (0350, nox, tpx and copA) that were expressed at significantly reduced levels in ΔspxA1,11 to determine their specific roles in adaptation to oxidative stress and virulence potential.
In the preliminary study, the WT and mutant strains were cultured under different conditions to test the effect of each gene on oxidative stress tolerance. Genes nox and tpx are involved in oxidative stress tolerance, while genes 0350 and copA have no major role in adaptation to the tested oxidative stresses. A murine infection model was adopted to evaluate the role of these genes in S. suis virulence. The data revealed that nox is required for infection. These results, coupled with previous study,11 indicate that nox plays an important part in SpxA1 function. This is not surprising, as S. suis NADH oxidase shows considerable identity to its orthologs from various streptococcal species, which have been shown to be involved in oxidative stress response and virulence.12-15,29
The Δnox mutant exhibits reduced resistance to oxidative stress generated by environmental oxygen and hydrogen peroxide. This result is in agreement with the observed effects of spxA1 deletion in S. suis and nox2 deletion in Group B Streptococcus.11,13 In addition, nox has a role in tolerance to ONOO−, which is indirectly generated by SIN-1 and represents a variant of highly reactive and bactericidal species.33 Unlike the Group B Streptococcus nox2 mutant that is hypersensitive to paraquat,13 no paraquat tolerance phenotype was associated with nox deletion in S. suis. In S. pneumoniae and Streptococcus mutans, NADH oxidase acts to reduce diatomic oxygen to water through the oxidation of NADH to NAD+, thus preventing formation of ROS.12,40 We therefore reasoned that NADH oxidase protects S. suis against oxidative stress via a similar mechanism.
NADH oxidase contributes to the pathogenesis of S. pneumoniae, Group B Streptococcus and Streptococcus sanguinis.12,13,15,29 In this study, we demonstrated that the virulence of S. suis 2 in a murine infection model was completely abolished by deletion of nox. The survival rates were significantly higher in mice infected with the Δnox mutant than in those infected with the WT and CΔnox strains. This observation could be explained by subsequent experiments, which showed that no bacterial cells could be recovered from the blood and organs of Δnox-infected mice, and that a very low production of inflammatory cytokines was detected in Δnox-infected mice. We also found that the ability of the Δnox strain to grow in murine whole blood was significantly reduced. As NADH oxidase is involved in oxidative stress resistance, it is likely that Δnox displays a defect in evasion of killing by phagocytes in the blood. The impaired growth of Δnox in blood might be partly responsible for its attenuated virulence. To further confirm the involvement of NADH oxidase in S. suis virulence, we performed an experimental infection of pigs. Animals inoculated with the WT and CΔnox strains developed typical clinical symptoms and most of them died, while those inoculated with the Δnox mutant displayed no clinical signs and all survived. In the brain, heart and lungs of pigs infected with the WT and CΔnox strains, severe histopathological lesions were found. By contrast, the Δnox-infected pigs exhibited no obvious pathological changes in these tissues. Together, these results indicated clearly that NADH oxidase is required for successful infection of S. suis 2. Despite the fact that NADH oxidase plays an important role in virulence of S. suis 2, it should be noted that some avirulent strains also harbor the nox gene. This observation may not be entirely surprising, as various virulence factors are present in S. suis,41 and the infection process is complicated, depending on the cooperation of multiple factors.
To illustrate the involvement of the NADH oxidase activity in oxidative stress tolerance and virulence of S. suis 2, 2 inactive forms of NADH oxidase with point mutations of the His11 and Cys44 residues were generated. These two residues are the putative active sites of S. suis NADH oxidase, based on the structure of NADH oxidase of S. pneumoniae and Streptococcus pyogenes.12,42 Both inactive forms showed dramatically decreasing activity, suggesting that these 2 residues are the true active sites of S. suis NADH oxidase. Next, we constructed 2 complementation strains of Δnox with the inactive NADH oxidase, and evaluated their abilities to resist to oxidative stress generated by environmental oxygen and hydrogen peroxide. Our results suggested clearly that the NADH oxidase activity has a key role in oxidative stress tolerance. It is also interesting to note that the complementation strains with the inactive NADH oxidase were more defective than the Δnox mutant under oxidative stress conditions. We speculated that the presence of the inactive forms of NADH oxidase influenced the expression of other oxidative stress-related genes, such as sodA and perR.26,28,43 Additionally, the survival rates of mice infected with the complementation strains with the inactive NADH oxidase were lower than those of Δnox-infected mice, and higher than those of CΔnox-infected mice, indicating that the NADH oxidase activity has a secondary role in virulence.
In conclusion, we have performed a study to examine the specific roles of 4 SpxA1-regulated genes in oxidative stress tolerance and virulence. We showed that the Δnox strain is highly sensitive to oxidative stress agents. Using murine and pig infection models, we demonstrated that NADH oxidase is required for S. suis 2 infection. Moreover, the NADH oxidase activity has a key role in oxidative stress tolerance and a secondary role in virulence. Our results reveal that NADH oxidase plays an important part in SpxA1 function and provide a new insight into the pathogenesis of S. suis 2.
Materials and methods
Bacterial strains, plasmids, primers and culture conditions
Bacterial strains and plasmids used in this study are listed in Table S2. Primers are listed in Table S3. S. suis strains were grown in Tryptic Soy Broth (TSB) or on Tryptic Soy Agar (TSA; Difco Laboratories, Detroit, MI, USA) with 10% (vol/vol) newborn bovine serum at 37°C unless otherwise specified. Escherichia coli strains were cultured in Luria-Bertani (LB) broth or on LB agar at 37°C. When required, antibiotics were added at the following concentrations: for E. coli, spectinomycin, 50 μg/mL; ampicillin, 50 μg/mL; kanamycin, 25 μg/mL; and for S. suis, spectinomycin, 100 μg/mL.
Construction of mutant strains and functional complementation of the nox deletion
In-frame deletion mutants of the genes 0350, nox, tpx and copA were constructed in the SC19 background using the thermosensitive suicide plasmid pSET4s,44 as previously described.11 The complementation strain CΔnox was generated using E. coli-S. suis shuttle vector pSET2,45 as previously described.46 The mutants (Δ0350, Δnox, Δtpx and ΔcopA) and the complementation strain CΔnox were verified by PCR (Fig. S4A), RT-PCR analysis (Fig. S4B) and direct DNA sequencing (data not shown).
Mutagenesis of NADH oxidase
The conserved residues His11 and Cys44 of NADH oxidase were changed separately to alanine using the Mut Express II Fast Mutagenesis Kit (Vazyme, Nanjing, China), according to the manufacturer's recommendations. A DNA fragment containing the nox gene and its predicted promoter was amplified from the S. suis 2 genome using primer pair Cnox1/Cnox2. The PCR product was purified and cloned into pMD18-T vector, to generate plasmid pMD18T-Cnox. Then, 2 pairs of primers containing the desired substitution amplified separately the entire sequence of pMD18T-Cnox. After digestion with Dpn I and homologous recombination, the products were transformed into E. coli Trans5α competent cells. The resulting plasmids were isolated and termed pMD18T-Cnox(H11A) and pMD18T-Cnox(C44A), respectively. Mutagenesis of the nox gene was verified by DNA sequencing.
Expression and purification of recombinant proteins
The WT and mutant nox genes were amplified from the S. suis 2 genome, plasmids pMD18T-Cnox(H11A) and pMD18T-Cnox(C44A), respectively. The DNA fragments were digested with the BamH I and Hind III enzymes, and then cloned into pET-30a plasmid. After DNA sequencing, the resulting plasmids were transformed into E. coli BL21 (DE3) cells. When the cultures reached the exponential phase (OD600=0.6–0.8), 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce the expression of proteins. Then, the cells were grown for another 4 h at 28°C before harvesting. The expressed proteins were purified by Ni-NTA affinity chromatography (GE Healthcare), according to the manufacturer's recommendations. The quality and concentrations of purified proteins were determined by SDS-PAGE and Qubit 2.0 fluorometer (Invitrogen), respectively. The proteins were stored at -80°C until use.
Measurement of NADH oxidase activity
NADH oxidase activity was measured by monitoring the decrease in NADH absorbance (A340) at 25°C, as described previously.29,47 The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.2 mM EDTA, 0.2 mM NADH, and purified NADH oxidase. One unit of enzyme activity corresponds to the oxidation of 1 μmol of NADH per min at 25°C.
Complementation of the Δnox mutant with the inactive forms of NADH oxidase
Primer pair Cnox1/Cnox2 amplified the DNA fragments containing the mutant nox gene and its predicted promoter from the plasmids pMD18T-Cnox(H11A) and pMD18T-Cnox(C44A), respectively. The DNA fragments were digested with the Pst I and BamH I enzymes, and cloned into pSET2, to generate plasmids pSET2-nox(H11A) and pSET2-nox(C44A). After DNA sequencing, the plasmids were introduced into the Δnox mutant by electroporation. The resulting complementation strains of Δnox with the inactive NADH oxidase were selected with spectinomycin and designated CΔnox(H11A) and CΔnox(C44A), respectively.
Oxidative stress assays
To measure the susceptibility of S. suis strains (the WT, Δ0350, Δnox, Δtpx and ΔcopA) toward oxidative stress, overnight cultures of each strain were diluted in fresh medium (TSB with 10% newborn bovine serum) and cultured at 37°C under various conditions, including static conditions, vigorous shaking, and static conditions with 0.5 mM H2O2. Growth was monitored by measuring the optical density at 600 nm (OD600) every hour.
To further investigate the role of NADH oxidase in oxidative stress tolerance, the WT, Δnox and CΔnox strains were subjected to a variety of oxidative stress challenges (vigorous shaking, 0.5 mM H2O2, 2 mM SIN-1, and 2 mM paraquat). Overnight cultures were diluted in fresh medium (TSB with 10% newborn bovine serum) adjusted to each specific condition, and growth was evaluated by measuring the OD600 every hour.
To determine whether NADH oxidase activity is involved in oxidative stress tolerance, the susceptibility of S. suis strains (the WT, Δnox, CΔnox, CΔnox(H11A) and CΔnox(C44A)) toward oxidative stress were tested as described above. Overnight cultures of each strain were diluted in fresh medium (TSB with 10% newborn bovine serum) and cultured under various conditions, including static conditions, vigorous shaking, and static conditions with 0.5 mM H2O2. Growth was monitored by measuring the OD600 every hour.
Murine infection model
All animal studies were approved by the Laboratory Animal Monitoring Committee of Huazhong Agricultural University and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals of Hubei Province, China. Five-week-old female BALB/c mice (8 animals per group) were challenged i.p. with S. suis strains (the WT, Δ0350, Δnox, Δtpx and ΔcopA) at a dose of approximately 1 × 108 CFU. The survival of mice was monitored twice daily for the first 2 d and daily for the next 5 d.
To further explore the role of NADH oxidase in virulence, 5-week-old female BALB/c mice (10 animals per group) were inoculated i.p. with ∼1.5 × 108 CFU of the WT, Δnox and CΔnox strains. Mice were monitored for 7 d for clinical signs and survival rates. For estimation of bacterial numbers in blood, brain, liver and spleen, mice were challenged i.p. with ∼2×107 CFU of the WT, Δnox and CΔnox strains. At 6 and 12 h after challenge, 5 mice in each group were sacrificed, and bacterial numbers in blood and in homogenates of brain, liver and spleen were determined by plating.
To examine the involvement of NADH oxidase activity in virulence of S. suis 2, groups of 8 BALB/c mice were inoculated i.p. with ∼1.5 × 108 CFU of the WT, Δnox, CΔnox, CΔnox(H11A) and CΔnox(C44A) strains. The infected mice were monitored for clinical signs and survival time.
Measurement of inflammatory cytokines
To investigate the difference in cytokine release trigged by the WT, Δnox, CΔnox strains and the control P1/7, 5-week-old female BALB/c mice were assigned randomly to 4 groups and each group was challenged i.p. with ∼2 × 107 CFU of one of the indicated strains. At defined time points (3, 6, 9 and 12 h after infection), 6 mice per group were sacrificed and blood samples were collected by cardiac puncture. Serum samples were isolated by centrifugation and preserved at −80°C until analysis. Levels of TNF-α and MCP-1 in serum were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Neobioscience, Beijing, China), according to the manufacturer's recommendations.
Bactericidal assays
The bactericidal assays were performed as described elsewhere,48 with some modifications. Heparinized whole blood was collected from BALB/c mice. The WT, Δnox and CΔnox strains were harvested at early stationary phase, washed twice with PBS, and diluted to 1 × 105 CFU/mL. Subsequently, bacterial suspensions (50 μL) were combined with fresh whole blood (450 μL), and the mixtures were incubated at 37°C for 3 h with rotation. Aliquots were removed from the samples at hourly intervals and the number of viable bacteria was determined by plating. The growth factor was defined as the ratio of CFU in each sample after incubation over the CFU in the corresponding inoculum.
Experimental infection of pigs
A total of 20 high-health-status pigs (ages 4–5 weeks) which tested negative by ELISA for S. suis 2 were used. Pigs were randomly divided into 4 groups (5 pigs per group). Animals in groups 1, 2 and 3 were inoculated by intravenous injection of ∼1.3 × 106 CFU of the WT, Δnox and CΔnox strains, respectively. Group 4 was inoculated with PBS as a control. The infected pigs were monitored for clinical signs and survival time. Surviving animals were euthanized on day 7 post-infection. When the infected pigs died or pigs were humanely sacrificed, samples from the brain, heart and lungs were collected for pathological examination, as reported previously.11
Statistical analysis
Data were analyzed using GraphPad Prism 5 (San Diego, USA). Survival rates were analyzed by the log-rank test. Bacterial counts in the tissues of mice were analyzed by a repeated measures test with a Tukey post test. The Mann–Whitney test was used to analyze the production of cytokines in mice. Bacterial growth in blood was analyzed using the 2-tailed unpaired t test. The two-tailed paired t test was used for NADH oxidase activity analysis. A P value of < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Sekizaki (National Institute of Animal Health, Japan) for supplying plasmid pSET4s and pSET2.
Funding
This work was supported by grants from the National Basic Research Program (No. 2011CB518805), the National Natural Science Foundation of China (No. 31372466, No. 31502080 and No. 31302123), the Fundamental Research Funds for the Central Universities (No. 2014PY012 and No. 2014PY037), and the PhD Candidate Research Innovation Project of Huazhong Agricultural University (No. 2014bs15).
References
- [1].Baig A, Weinert LA, Peters SE, Howell KJ, Chaudhuri RR, Wang JH, Holden MTG, Parkhill J, Langford PR, Rycroft AN, et al.. Whole genome investigation of a divergent clade of the pathogen Streptococcus suis. Front Microbiol 2015; 6:1191; PMID:26583006; http://dx.doi.org/ 10.3389/fmicb.2015.01191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Segura M, Zheng H, de Greeff A, Gao GF, Grenier D, Jiang YQ, Lu CP, Maskell D, Oishi K, Okura M, et al.. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 1. Future Microbiol 2014; 9:441-4; PMID:24810343; http://dx.doi.org/ 10.2217/fmb.14.14 [DOI] [PubMed] [Google Scholar]
- [3].Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol 2005; 107:63-9; PMID:15795078; http://dx.doi.org/ 10.1016/j.vetmic.2005.01.003 [DOI] [PubMed] [Google Scholar]
- [4].Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis 2007; 7:201-9; PMID:17317601; http://dx.doi.org/ 10.1016/S1473-3099(07)70001-4 [DOI] [PubMed] [Google Scholar]
- [5].Huong VTL, Ha N, Huy NT, Horby P, Nghia HDT, Thiem VD, Zhu XT, Hoa NT, Hien TT, Zamora J, et al.. Epidemiology, Clinical Manifestations, and Outcomes of Streptococcus suis Infection in Humans. Emerg Infect Dis 2014; 20:1105-14; PMID:24959701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feng YJ, Zhang HM, Ma Y, Gao GF. Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent. Trends Microbiol 2010; 18:124-31; PMID:20071175; http://dx.doi.org/ 10.1016/j.tim.2009.12.003 [DOI] [PubMed] [Google Scholar]
- [7].Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infec 2014; 3:e45; http://dx.doi.org/ 10.1038/emi.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, Derr AM, Quivey RG, Lemos JA. Two Spx Proteins Modulate Stress Tolerance, Survival, and Virulence in Streptococcus mutans. J Bacteriol 2010; 192:2546-56; PMID:20233935; http://dx.doi.org/ 10.1128/JB.00028-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen L, Ge XC, Wang XJ, Patel JR, Xu P. SpxA1 Involved in Hydrogen Peroxide Production, Stress Tolerance and Endocarditis Virulence in Streptococcus sanguinis. Plos One 2012; 7:e40034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kajfasz JK, Mendoza JE, Gaca AO, Miller JH, Koselny KA, Giambiagi-deMarval M, Wellington M, Abranches J, Lemos JA. The Spx Regulator Modulates Stress Responses and Virulence in Enterococcus faecalis. Infect Immun 2012; 80:2265-75; PMID:22508863; http://dx.doi.org/ 10.1128/IAI.00026-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zheng CK, Xu JL, Li JQ, Hu LH, Xia JD, Fan JY, Guo WN, Chen HC, Bei WC. Two Spx Regulators Modulate Stress Tolerance and Virulence in Streptococcus suis Serotype 2. Plos One 2014; 9:e108197; http://dx.doi.org/ 10.1371/annotation/c46d8841-f33e-45ea-9c17-b754e1017641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Auzat I, Chapuy-Regaud S, Le Bras G, Dos Santos D, Ogunniyi AD, Le Thomas I, Garel JR, Paton JC, Trombe MC. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol Microbiol 1999; 34:1018-28; PMID:10594826; http://dx.doi.org/ 10.1046/j.1365-2958.1999.01663.x [DOI] [PubMed] [Google Scholar]
- [13].Yamamoto Y, Pargade V, Lamberet G, Gaudu P, Thomas F, Texereau J, Gruss A, Trieu-Cuot P, Poyart C. The Group B Streptococcus NADH oxidase Nox-2 is involved in fatty acid biosynthesis during aerobic growth and contributes to virulence. Mol Microbiol 2006; 62:772-85; PMID:16999835; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05406.x [DOI] [PubMed] [Google Scholar]
- [14].Derr AM, Faustoferri RC, Betzenhauser MJ, Gonzalez K, Marquis RE, Quivey RG. Mutation of the NADH Oxidase Gene (nox) Reveals an Overlap of the Oxygen- and Acid-Mediated Stress Responses in Streptococcus mutans. Appl Environ Microb 2012; 78:1215-27; http://dx.doi.org/ 10.1128/AEM.06890-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muchnik L, Adawi A, Ohayon A, Dotan S, Malka I, Azriel S, Shagan M, Portnoi M, Kafka D, Nahmani H, et al.. NADH Oxidase Functions as an Adhesin in Streptococcus pneumoniae and Elicits a Protective Immune Response in Mice. Plos One 2013; 8:e61128; PMID:23577197; http://dx.doi.org/ 10.1371/annotation/7fbc524a-4035-401e-8cc7-93393afc35fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park JC, Kim Y, Lee HS. Involvement of the NADH oxidase-encoding noxA gene in oxidative stress responses in Corynebacterium glutamicum. Appl Microbiol Biot 2015; 99:1363-74; http://dx.doi.org/ 10.1007/s00253-014-6327-x [DOI] [PubMed] [Google Scholar]
- [17].Cha MK, Kim WC, Lim CJ, Kim K, Kim IH. Escherichia coli periplasmic thiol peroxidase acts as lipid hydroperoxide peroxidase and the principal antioxidative function during anaerobic growth. J Biol Chem 2004; 279:8769-78; PMID:14676195; http://dx.doi.org/ 10.1074/jbc.M312388200 [DOI] [PubMed] [Google Scholar]
- [18].Missall TA, Pusateri ME, Lodge JK. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol Microbiol 2004; 51:1447-58; PMID:14982637; http://dx.doi.org/ 10.1111/j.1365-2958.2004.03921.x [DOI] [PubMed] [Google Scholar]
- [19].Wang G, Olczak AA, Walton JP, Maier RJ. Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect Immun 2005; 73:378-84; PMID:15618175; http://dx.doi.org/ 10.1128/IAI.73.1.378-384.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].La Carbona S, Sauvageot N, Giard JC, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, Alkyl hydroperoxide reductase and Thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Molecular microbiology 2007; 66:1148-63; PMID:17971082; http://dx.doi.org/ 10.1111/j.1365-2958.2007.05987.x [DOI] [PubMed] [Google Scholar]
- [21].Horst SA, Jaeger T, Denkel LA, Rouf SF, Rhen M, Bange FC. Thiol Peroxidase Protects Salmonella enterica from Hydrogen Peroxide Stress In Vitro and Facilitates Intracellular Growth. J Bacteriol 2010; 192:2929-32; PMID:20304995; http://dx.doi.org/ 10.1128/JB.01652-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Somprasong N, Jittawuttipoka T, Duang-Nkern J, Romsang A, Chaiyen P, Schweizer HP, Vattanaviboon P, Mongkolsuk S. Pseudomonas aeruginosa Thiol Peroxidase Protects against Hydrogen Peroxide Toxicity and Displays Atypical Patterns of Gene Regulation. J Bacteriol 2012; 194:3904-12; PMID:22609922; http://dx.doi.org/ 10.1128/JB.00347-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hajaj B, Yesilkaya H, Benisty R, David M, Andrew PW, Porat N. Thiol Peroxidase Is an Important Component of Streptococcus pneumoniae in Oxygenated Environments. Infect Immun 2012; 80:4333-43; PMID:23027531; http://dx.doi.org/ 10.1128/IAI.00126-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Poyart C, Pellegrini E, Gaillot O, Boumaila C, Baptista M, Trieu-Cuot P. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect Immun 2001; 69:5098-106; PMID:11447191; http://dx.doi.org/ 10.1128/IAI.69.8.5098-5106.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Esteve-Gassent MD, Elliott NL, Seshu J. sodA is essential for virulence of Borrelia burgdorferi in the murine model of Lyme disease. Molecular microbiology 2009; 71:594-612; PMID:19040638; http://dx.doi.org/ 10.1111/j.1365-2958.2008.06549.x [DOI] [PubMed] [Google Scholar]
- [26].Tang YL, Zhang XY, Wu W, Lu ZY, Fang WH. Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice. Vet Microbiol 2012; 158:360-6; PMID:22424868; http://dx.doi.org/ 10.1016/j.vetmic.2012.02.028 [DOI] [PubMed] [Google Scholar]
- [27].El Shafey HM, Ghanem S. Regulation of expression of sodA and msrA genes of Corynebacterium glutamicum in response to oxidative and radiative stress. Genet Mol Res 2015; 14:2104-17; PMID:25867357; http://dx.doi.org/ 10.4238/2015.March.20.21 [DOI] [PubMed] [Google Scholar]
- [28].Fang LH, Shen HX, Tang YL, Fang WH. Superoxide dismutase of Streptococcus suis serotype 2 plays a role in anti-autophagic response by scavenging reactive oxygen species in infected macrophages. Veterinary Microbiol 2015; 176:328-36; PMID:25726301; http://dx.doi.org/ 10.1016/j.vetmic.2015.02.006 [DOI] [PubMed] [Google Scholar]
- [29].Ge X, Yu Y, Zhang M, Chen L, Chen W, Elrami F, Kong F, Kitten T, Xu P. Involvement of NADH Oxidase in Competition and Endocarditis Virulence in Streptococcus sanguinis. Infect Immun 2016; 84:1470-7; PMID:26930704; http://dx.doi.org/ 10.1128/IAI.01203-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Toes ACM, Daleke MH, Kuenen JG, Muyzer G. Expression of copA and cusA in Shewanella during copper stress. Microbiol-Sgm 2008; 154:2709-18; http://dx.doi.org/ 10.1099/mic.0.2008/016857-0 [DOI] [PubMed] [Google Scholar]
- [31].Djoko KY, Franiek JA, Edwards JL, Falsetta ML, Kidd SP, Potter AJ, Chen NH, Apicella MA, Jennings MP, McEwan AG. Phenotypic Characterization of a copA Mutant of Neisseria gonorrhoeae Identifies a Link between Copper and Nitrosative Stress. Infect Immun 2012; 80:1065-71; PMID:22184419; http://dx.doi.org/ 10.1128/IAI.06163-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marrero K, Sanchez A, Gonzalez LJ, Ledon T, Rodriguez-Ulloa A, Castellanos-Serra L, Perez C, Fando R. Periplasmic proteins encoded by VCA0261-0260 and VC2216 genes together with copA and cueR products are required for copper tolerance but not for virulence in Vibrio cholerae. Microbiol-Sgm 2012; 158:2005-16; http://dx.doi.org/ 10.1099/mic.0.059345-0 [DOI] [PubMed] [Google Scholar]
- [33].Binesse J, Lindgren H, Lindgren L, Conlan W, Sjostedt A. Roles of Reactive Oxygen Species-Degrading Enzymes of Francisella tularensis SCHU S4. Infect Immun 2015; 83:2255-63; PMID:25802058; http://dx.doi.org/ 10.1128/IAI.02488-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol 2007; 179:1842-54; PMID:17641051; http://dx.doi.org/ 10.4049/jimmunol.179.3.1842 [DOI] [PubMed] [Google Scholar]
- [35].Kim JW, Seo SO, Zhang GC, Jin YS, Seo JH. Expression of Lactococcus lactis NADH oxidase increases 2,3-butanediol production in Pdc-deficient Saccharomyces cerevisiae. Bioresource Technol 2015; 191:512-9; PMID:25769689; http://dx.doi.org/ 10.1016/j.biortech.2015.02.077 [DOI] [PubMed] [Google Scholar]
- [36].Fang FC. Antimicrobial actions of reactive oxygen species. Mbio 2011; 2:pii: e00141-11; PMID:21896680; http://dx.doi.org/ 10.1128/mBio.00141-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Diezmann S, Dietrich FS. Saccharomyces cerevisiae: population divergence and resistance to oxidative stress in clinical, domesticated and wild isolates. Plos One 2009; 4:e5317; PMID:19390633; http://dx.doi.org/ 10.1371/journal.pone.0005317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nakano S, Kuster-Schock E, Grossman AD, Zuber P. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. P Natl Acad Sci USA 2003; 100:13603-8; http://dx.doi.org/ 10.1073/pnas.2235180100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kajfasz JK, Rivera-Ramos I, Scott-Anne K, Gregoire S, Abranches J, Lemos JA. Transcription of Oxidative Stress Genes Is Directly Activated by SpxA1 and, to a Lesser Extent, by SpxA2 in Streptococcus mutans. J Bacteriol 2015; 197:2160-70; PMID:25897032; http://dx.doi.org/ 10.1128/JB.00118-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Baker JL, Derr AM, Karuppaiah K, MacGilvray ME, Kajfasz JK, Faustoferri RC, Rivera-Ramos I, Bitoun JP, Lemos JA, Wen ZT, et al.. Streptococcus mutans NADH Oxidase Lies at the Intersection of Overlapping Regulons Controlled by Oxygen and NAD(+) Levels. J Bacteriol 2014; 196:2166-77; PMID:24682329; http://dx.doi.org/ 10.1128/JB.01542-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feng YJ, Zhang HM, Wu ZW, Wang SH, Cao M, Hu D, Wang CJ. Streptococcus suis infection An emerging/reemerging challenge of bacterial infectious diseases?. Virulence 2014; 5:477-97; PMID:24667807; http://dx.doi.org/ 10.4161/viru.28595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wallen JR, Mallett TC, Okuno T, Parsonage D, Sakai H, Tsukihara T, Claiborne A. Structural Analysis of Streptococcus pyogenes NADH Oxidase: Conformational Dynamics Involved in Formation of the C(4a)-Peroxyflavin Intermediate. Biochemistry 2015; 54:6815-29; PMID:26506002; http://dx.doi.org/ 10.1021/acs.biochem.5b00676 [DOI] [PubMed] [Google Scholar]
- [43].Zhang TF, Ding Y, Li TT, Wan Y, Li W, Chen HC, Zhou R. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol 2012; 12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 2001; 46:140-8; PMID:11591139; http://dx.doi.org/ 10.1006/plas.2001.1532 [DOI] [PubMed] [Google Scholar]
- [45].Takamatsu D, Osaki M, Sekizaki T. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 2001; 45:101-13; PMID:11322824; http://dx.doi.org/ 10.1006/plas.2000.1510 [DOI] [PubMed] [Google Scholar]
- [46].Zheng CK, Xu JL, Ren SJ, Li JQ, Xia MM, Chen HC, Bei WC. Identification and characterization of the chromosomal yefM-yoeB toxin-antitoxin system of Streptococcus suis. Sci Rep-Uk 2015; 5:13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Higuchi M, Yamamoto Y, Poole LB, Shimada M, Sato Y, Takahashi N, Kamio Y. Functions of two types of NADH oxidases in energy metabolism and oxidative stress of Streptococcus mutans. J Bacteriol 1999; 181:5940-7; PMID:10498705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu P, Pian YY, Li XQ, Liu RF, Xie WL, Zhang CM, Zheng YL, Jiang YQ, Yuan Y. Streptococcus suis Adenosine Synthase Functions as an Effector in Evasion of PMN-mediated Innate Immunity. J Infect Dis 2014; 210:35-45; PMID:24446521; http://dx.doi.org/ 10.1093/infdis/jiu050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.