Abstract
BACKGROUND:
Premenstrual syndrome (PMS) is the emergence of periodic one or more symptoms of symptoms before menstruation and in the first few days of menstruation. Lifestyle is one of the series of factors that affect the health of people. Activity, smoking, and food intake are factors associated with lifestyle, and evidence suggests that women and girls with PMS do not have an adequate life span. The aim of this study was to determine the factors associated with PMS in female high school students.
METHODS:
This cross-sectional correlation study was conducted in 200 female high school students in Sabzevar city using multistage random sampling in the academic year of 2016–2017. Data gathering tools include the temporary diagnostic questionnaire of the PMS, and Beck Depression questionnaire; the nonresonant tape was accurate to 0.1 cm and the digital scale was accurate to 0.1 kg. We analyzed the data using SPSS software and Mann–Whitney U-test.
RESULTS:
The results of this study showed that there is a significant relationship between PMS and fried foods (P = 0.017), sweet drink (P = 0.018), fast food (P = 0.048), fruit (P = 0.012), no habitual exercise (P = 0.006), family history of PMS (P = 0.002), hip circumference (P = 0.04), and body mass index (P = 0.04).
CONCLUSION:
There is a relationship between PMS and some anthropometric indices and nutritional/metabolic factors. Therefore, having a proper lifestyle is effective in reducing PMS.
Keywords: Anthropometric, female, nutrition, premenstrual syndrome
Introduction
Premenstrual syndrome (PMS) is a psychotic neuroendocrine disorder with biological, psychological, and social parameters.[1] Symptoms are divided into two categories: physical and psychological, and PMS can be considered as a periodic recurrence of a combination of disruptive, physical, psychological, and behavioral changes during the luteal phase of the menstrual cycle that interferes with family, social, and occupational activities.[2]
PMS was described by the American Psychiatric Association in 1987 as the Luteal Dysfunctional Disorders, and was classified in 1992 along with other symptoms, such as nervousness, called Dysfunctional Premenstrual Diagnostic and Statistical Manual of Mental Disorders.[3] Physical symptoms include painful tenderness of the breast, flatulence, abdominal pain, weight gain, edema, headache, back pain, nausea, bowel movements, acne, and psychotic symptoms including irritability, anxiety, nervousness, depression, excessive tiredness and weakness, confusion, changes in mood, sleep pattern, and appetite.[4,5,6] Symptoms of PMS may cause many problems, including physical impairment, mental health, and severe functional impairment in women's social and occupational contexts. Symptoms in adolescents may negatively affect their academic performance and their social interactions. Studies have also shown that adolescents with PMS are in poor health.[7] In Iran, the prevalence of PMS was 52.9%, with 34.5% suffering from this syndrome with severe symptoms.[8] Hypotheses about the cause of PMS have been proposed including increased estrogen levels, reduced progesterone levels, changes in estrogen-progesterone ratio, increased aldosterone activity, increased renin-angiotensin activity, impaired secretion of internal opioids, hypoglycemia without causes, opioid deficiencies, Vitamins B6, B1, and A or minerals such as magnesium and calcium, excessive prolactin secretion, and prostaglandin disorders.[4,5] However, in the study by Fathizadeh and Khodakarami, there was a significant relationship between low body mass index (BMI) and menstrual disorders.[9] Bakhshani et al. found a meaningful relationship between menstrual disorders and obesity and hyperandrogenism.[10] Above-mentioned studies have investigated the effect of exercise or hormonal disorders more than anthropometric indices. Furthermore, various studies have shown relationship between PMS and some nutritional disorders in adolescents caused obesity or slimming.[11,12,13] Psychosomatic disorders such as gastrointestinal problems, dizziness, and headache that can be seen in adolescents overlap with symptoms of PMS.[14] Other researches also consider factors such as nutrition, contraceptive pills, social and cultural factors, lack of exercise habits, and the presence of psychological stress, genetics, lifestyle in the development of PMS.[3] Healthy weight and appropriate anthropometric indices, followed by fitness, result in a healthy lifestyle, including physical activity and proper nutrition.[15] In a number of studies, was not clearly determined the effect of weight and BMI on PMS, but has been shown the positive effect of abdominal obesity.[13] One of the possible factors in the prevalence of this syndrome is high BMI. In some studies, BMI is directly or indirectly related to the body's hormonal balance mechanism.[14,26] It is likely that obesity and overweight will play a role in the etiology of some menstrual problems. Masho et al. showed that the prevalence of PMS in obese women is approximately two times that of nonobese women.[12] However, in another study, no relationship was found between PMS and obesity.[16]
Although studies have been done on PMS, the relationship between PMS and anthropometric indicators is ambiguous.
The aim of this study is to determine the role of anthropometric index in the appearance of PMS symptoms by considering nutritional and sports habits and rejection of depression disorders in adolescents; by finding this correlation, we can consider the necessary nutritional education in adolescence.
Methods
This cross-sectional correlation study was conducted to determine the factors associated with PMS in female high school students in Sabzevar city using multistage random sampling in the academic year of 2016–2017.
Sample size was calculated using G*Power software (G*Power is a free-to use software used to calculate statistical power. The program offers the ability to calculate power for a wide variety of statistical tests including t-tests, F-tests, and Chi-square-tests, among others), and according to studies with 95% confidence level and 80% test power, 187 people were calculated. Considering 4% probability of sample loss, 200 people entered the study.[17]
The criteria for entering the study include high school students, fluency in Persian, satisfaction to participate in the study, having regular periodic menstruation courses (21–35 days) and bleeding time 3–10 days, PMS (based on intermittent questionnaire for the diagnosis of PMS), chronic disease (cardiovascular, respiratory, renal, hypertension, asthma, diabetes, epilepsy, migraine, thyroid, anemia, and psychiatric disorders), lack of use of medication continuously during the study (antihypertensive, antidepressant, antihistamine, anticholinergic, and hormonal drugs), no incidence of bad and stressful events during the past 3 months, and lack of severe depression (based on the Beck Depression Inventory, the score was higher than 40). The exclusion criteria were as follows: menstrual cycle is removed from its normal order, a stressful event occurs, the questionnaires will not be completed, and students will be dissatisfied with the continuation of the research during the study period.
Data gathering tool: In the first stage, the questionnaire of personal and midwifery records and assessment of personal lifestyle habits (includes: the demographic characteristics, personal medical history, family history of physical illness, lifestyle habits, and various medical problems of the participants), the temporary diagnostic questionnaire of the PMS (the validity of this questionnaire was confirmed in 2013 by Jafarnejad et al.).[18,19] We measured the reliability of this questionnaire according to the retest method and the Spearman–Brown correlation coefficient as 0.89, Recorded daily symptoms of PMS (The validity of this questionnaire was confirmed in 2013 by Jafarnejad et al.).. The reliability of this questionnaire was done by Cronbach's alpha internal consistency, and the reliability coefficient was calculated as 0.87, and Beck Depression Inventory (This questionnaire with regard to the study by Jafarnejad et al. is a valid and reliable tool). In the second stage, used nonresonant tape was accurate to 0.1 cm and the digital scale was accurate to 0.1 kg.
Initially, students with PMS were invited to participate in the research. After explaining the method of work and the research objectives, students completed the informed consent form of the study, the Temporary Diagnosis of Premenstrual syndrome and Beck Depression (within the first 5 days after menstruation). Then, the daily registration questionnaire for symptoms of PMS was provided to students who had the criteria for entering the study and who were asked to complete them for 2 consecutive months. During this time, the students were encouraged to complete the questionnaire on a regular basis twice per week through phone calls made by the researcher. At the end, questionnaires were collected and people with syndrome were confirmed. In the next stage, anthropometric indices include height, arm circumference, waist circumference, hip and thighs circumference, measured in centimeter with a non elastic strip of meter with a precision of 0.1 cm and weight in kilogram with a precision of 0.1 kg, and BMI with the aid of the formula (weight in kilograms divided by height in meter squared).
The information was coded and entered into SPSS (Version 23)(IBM Company, Armonk, NY) software. P < 0.05 was considered statistically significant.
Results
Among 200 participants in the study, 150 had PMS and 50 had no PMS. The demographic characteristics of the research units showed that most students (75%) were in the range of 15–17 years old and had an average age of 16 ± 2.3 years. The fathers of most students completed diploma education (57.5%), were employees (38.5%), and had income of 15 million rials and lower (62.3%). Most of the student mothers' education was secondary school (70%) and they were homemakers (75%).
Pearson's correlation coefficient shows that there is a meaningful relationship between PMS and fried foods (P = 0.017), sweet drink (P = 0.018), fast food (P = 0.048), fruit (P = 0.012), no habitual exercise (P = 0.006), family history of PMS (P = 0.002), hip circumference (P = 0.04), and BMI (P = 0.04) [Table 1].
Table 1.
Comparison relationship between premenstrual syndrome and some anthropometric indices and nutritional/metabolic factors
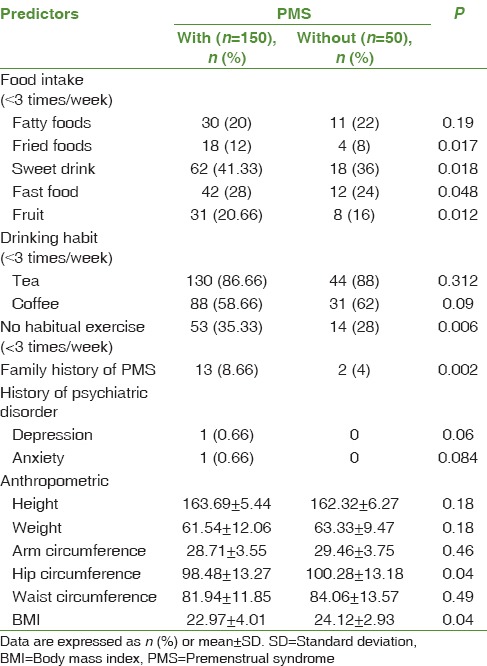
In addition, based on the general linear regression test, it was shown that there was no significant relationship between the demographic variables of high school female students with premenstrual syndrome including age (P = 0.12), father education (P = 0.239), mother education (P = 0.089), and household income (P = 0.112).
Discussion
The aim of this study was to investigate the factors associated with PMS in female high school students of Sabzevar city. In this study, we examined the association of some anthropometric indices, food intake, and lifestyle with PMS. The relationship between anthropometric indices measured with PMS showed that hip circumference and BMI were associated with PMS, and the remaining indices had no relation with syndrome. However, lifestyle, food intake such as fried foods, sweet drink, fast food, and fruit, not having sports habits, and family history of PMS were associated with PMS. Similarly, the study by Cheng et al. showed a positive and significant relationship with food intake such as fried foods, sweet drink, fast food, and fruit, not having sports habits, and PMS.[11]
This study showed that weight has no significant correlation with PMS which is opposed to Masho et al. that in their study reported that the risk of PMS in obese women was 8.2% higher than that of underweight women.[12] Perhaps the reason for this difference is in the age of research units that Masho et al.'s study was on adult women and this study was on adolescents. In adolescents, hormonal disorders are more common. In addition, the beliefs of adult women about the symptoms of PMS may be different of adolescents.[10] A study by Bertone-Johnson et al. also found that the risk of developing PMS increases with weight gain.[3] Studies have shown that there is a relationship between sexual hormonal disorders and central fat mass in women; fatty acids also play a role in the metabolism of sex hormones and are involved the pattern of lipid distribution in the regulation of sex hormones. The increase in fat mass, especially the abdominal fat mass, is related to hyperandrogenism and hyperinsulinism, and sexual hormonal disorders are a possible cause of PMS.[20,21]
In agrees with the results of this study, Tolossa and Bekele showed that there was no significant relationship between PMS and BMI, waist-to-hip ratio (WHR), and waist-to-height ratio (WHTR).[22]
The present study showed a significant relationship between hip circumference and BMI with PMS. Align with this result, a study by Mohammadi et al. showed that there was a significant direct correlation between waist circumference, WHR, and WHTR with PMS, but no significant relationship was found between the other indices and PMS.[13] Weight gain and especially the increase in adipose tissue in the central areas of the body disturb the balance of steroid hormones, including the androgens, estrogen, and sex hormone binding goblins (SHBG). Changes in SHBG lead to changes in the release of androgens and estrogens in the target tissue. Obesity increases production estrogen is associated with body weight and fat percentage.[20,23]
A study by Cross et al. indicated that women with PMS compared to women who did not have PMS have a significant increase in the intake of lipids, carbohydrates, and sugars and a specific decrease in protein intake during premenstrual period.[24] According to other studies, the reason for increased intake of food in the luteal phase is the increase in progesterone levels and the decrease in appetite levels during ovulation to the suppressive effects of estrogen appetite, and in the luteal phase, progesterone inhibitory activity increases on estrogen activity.[15] The same hormonal fluctuations in the luteal phase may occur more often in obese people, and the symptoms of PMS are more common in obese people.[15]
Studies have shown that obese women are usually at risk for stress, depression, and sleep deprivation and are usually less likely to exercise.[25] Perhaps one of the reasons why BMI is higher in people with PMS is that people with PMS usually have symptoms of stress, anxiety, depression and more boredom, and have less joy.[25] In the other word, PMS is one the reasons of sedentary life style, and isolation which lead to increasing obesity.[27]
The first limitation of this study was that the severity of PMS with anthropometric risk factors was not monitored. The study was done by cross-sectional method and the limited number of female students could not be a valid causal relationship between anthropometric and PMS. Furthermore, the feed frequency questionnaire has not been used to assess the nutritional status of the students. The strength of this study was that students were followed up for 2 months and then entered the study for the diagnosis of PMS and the PMS diagnostic questionnaire was not used.
Conclusion
The results of this study showed that there is a significant relationship between PMS and some anthropometric indices (hip circumference and BMI), lifestyle, and food intake. By having the right way of life and proper diet, we can reduce the syndrome by creating weight and ideal indicators. Considering this study which was one of the first studies done on female high school students with PMS, it is recommended that studies should be conducted with more sample size and dietary control during premenstrual periods.
Financial support and sponsorship
This study was funded by Sabzevar University of Medical Science.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study is a research project with the code 95085 approved by Sabzevar University of Medical Sciences. For this purpose, we thank the research Vice President of Sabzevar University of Medical Sciences for the cost of this study and all the participants in the study.
References
- 1.Burkman RT. Berek & novak's gynecology. JAMA. 2012;308:516–7. [Google Scholar]
- 2.Moghadasi A, Abbasi M, Yousefi M, Kargarfard M. A comparison of prevalence of premenstrual syndrome symptoms between athlete and non-athlete female students. J Sports Physiol Act. 2009;3:199–208. [Google Scholar]
- 3.Bertone-Johnson ER, Hankinson SE, Willett WC, Johnson SR, Manson JE. Adiposity and the development of premenstrual syndrome. J Womens Health (Larchmt) 2010;19:1955–62. doi: 10.1089/jwh.2010.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohebbi Dehnavi Z, Jafarnejad F, Mojahedy M, Shakeri M, Sardar M. The relationship between temperament warm and cold with symptoms of premenstrual syndrome. IJOGI. 2016;18:17–24. [Google Scholar]
- 5.Mohebbi Dehnavi Z, Torkmannejad Sabzevari M, Rastaghi S, Rad M. A survey on the association of premenstrual syndrome with type of temperament in high school students. IJOGI. 2017;20:15–23. [Google Scholar]
- 6.Jafarirad S, Rasaie N, Darabi F. Comparison of anthropometric indices and lifestyle factors between healthy university students and affected by premenstrual syndrome. Jundishapur Sci Med J. 2016;15:217–27. [Google Scholar]
- 7.Vichnin M, Freeman EW, Lin H, Hillman J, Bui S. Premenstrual syndrome (PMS) in adolescents: Severity and impairment. J Pediatr Adolesc Gynecol. 2006;19:397–402. doi: 10.1016/j.jpag.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Samadi Z, Taghian F, Valiani M. The effects of 8 weeks of regular aerobic exercise on the symptoms of premenstrual syndrome in non-athlete girls. Iran J Nurs Midwifery Res. 2013;18:14–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Fathizadeh N, Khodakarami N. Menstrual disorders in early puberty in girls of 14-17 years old. J Shahrekord Univ Med Sci. 2001;3:41–6. [Google Scholar]
- 10.Bakhshani N, Hasanzadeh Z, Raghibi M. Prevalence of premenstrual symptoms and premenstrual dysphoric disorder among adolescents students of Zahedan. Zahedan J Res Med Sci. 2012;13:29–34. [Google Scholar]
- 11.Cheng SH, Shih CC, Yang YK, Chen KT, Chang YH, Yang YC, et al. Factors associated with premenstrual syndrome-A survey of new female university students. Kaohsiung J Med Sci. 2013;29:100–5. doi: 10.1016/j.kjms.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masho SW, Adera T, South-Paul J. Obesity as a risk factor for premenstrual syndrome. J Psychosom Obstet Gynaecol. 2005;26:33–9. doi: 10.1080/01443610400023049. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi V, Shidfar F, Keshtkar Aghababaee S, Mokhtari P, Mohammadi R, Gohari MR. The relationship of anthropometric indices with PMS and it's severity in female students of Tehran university of medical sciences. Razi J Med Sci. 2013;20:87–94. [Google Scholar]
- 14.GAfari F, Porgaznien T. The relationship of severity premenstrual syndrome with anger in adolescent girls. IJOGI. 2006;9:52–60. [Google Scholar]
- 15.Amiri Farahani L, Heidari T, Narenji F, Asghari Jafarabadi M, Shirazi V. Relationship between pre menstrual syndrome with body mass index among university students. J hayat. 2012;17:85–95. [Google Scholar]
- 16.Kritz-Silverstein D, Wingard DL, Garland FC. The association of behavior and lifestyle factors with menstrual symptoms. J Womens Health Gend Based Med. 1999;8:1185–93. doi: 10.1089/jwh.1.1999.8.1185. [DOI] [PubMed] [Google Scholar]
- 17.Bakhshani NM, Mousavi MN, Khodabandeh G. Prevalence and severity of premenstrual symptoms among Iranian female university students. J Pak Med Assoc. 2009;59:205–8. [PubMed] [Google Scholar]
- 18.Kiani Asiabar A, Heidari M, Mohammadi Tabar S, Kiani Asiabar M. The prevalence of and factors associated with headaches, menstrual characteristics in students. Res Med. 2011;35:63–7. [Google Scholar]
- 19.Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA. Washington (DC): World Bank; 2006. Prevention of Chronic Disease By Means of Diet and Lifestyle Changes; pp. 833–50. [PubMed] [Google Scholar]
- 20.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9:359–72. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- 21.Indusekhar R, Usman SB, O'Brien S. Psychological aspects of premenstrual syndrome. Best Pract Res Clin Obstet Gynecol. 2007;21:207–20. doi: 10.1016/j.bpobgyn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Tolossa FW, Bekele ML. Prevalence, impacts and medical managements of premenstrual syndrome among female students: Cross-sectional study in college of health sciences, Mekelle University, Mekelle, Northern Ethiopia. BMC Womens Health. 2014;14:52. doi: 10.1186/1472-6874-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz M, Speroff L. Philadelphia, PA: Williams and Wilkins; 2005. Clinical Endocrinology and Infertility; pp. 120–30. [Google Scholar]
- 24.Cross GB, Marley J, Miles H, Willson K. Changes in nutrient intake during the menstrual cycle of overweight women with premenstrual syndrome. Br J Nutr. 2001;85:475–82. doi: 10.1079/bjn2000283. [DOI] [PubMed] [Google Scholar]
- 25.Silva MS, Matsuoka J, Faintuch B, Zilberstein B, Gama-Rodrigues J. Psychological fragility of obese women. Clin Nutr. 2003;22:21. [Google Scholar]
- 26.Rad M, Sabzevari MT, Rastaghi S, Dehnavi ZM. The relationship between anthropometric index and primary dysmenorehea in female high school students. J Edu Health Promot. 2018;7:34. doi: 10.4103/jehp.jehp_117_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbasi S, Tufail A, Kalyar J, Ahsan NA. Pre menstrual syndrome in undergraduate medical students: Hostellers versus day-scholars. J Surg Pak Int. 2015;20:3. [Google Scholar]


