Abstract
Background:
Kawasaki disease (KD) is an acute systemic vasculitis syndrome with a high incidence of coronary aneurysms in untreated children. The majority of aneurysms resulting from KD are known to regress with time.
Aims:
This study aimed to determine the course and outcome of coronary artery dilatation in patients with KD and ascertain whether there are any differences in the outcomes in the different branches.
Setting and Design:
This is a retrospective cohort study of patients diagnosed with KD with midterm follow-up data.
Methods:
Serial echocardiography was performed in all KD patients with coronary dilatation for 1–10½ years. The Kaplan–Meier curve was used for statistical analysis.
Results:
There were 154 patients with coronary dilatation studied. The frequency of coronary dilatation in acute phase was 33.3% and decreased to 7.9% 6–8 weeks later. Each patient could have dilatations at more than one branch, so the total number of dilatations was 245. The median time needed for regression was 2.6 months (mean: 10.5 months) while the median of follow-up duration was 41 months (mean: 23 months). Small- and medium-sized dilatations had more favorable outcomes compared to the giant ones. Location of dilatation did not influence the outcome.
Conclusions:
The majority (77.4%) of small- and medium-sized dilatations regress within 2 years, but giant aneurysms tend to persist. The outcome of coronary dilatation is determined by the diameter and not by the location. Regression rate is faster in smaller dilatations. Left main coronary artery is the most frequent location for dilatation.
Keywords: Coronary dilatation, Kawasaki disease, long-term outcome
INTRODUCTION
Kawasaki disease (KD) is an acute systemic vasculitis syndrome of unknown etiology that mainly affects infants and young children. It was first reported by Tomisaku Kawasaki in 1967 in Japan,[1] but has been found in all races and continents with the highest incidence in Japan. Coronary artery aneurysms develop in 15%–25% of untreated cases and may lead to myocardial infarction, sudden death, or ischemic heart disease.[2] Diagnosis of KD relies on clinical suspicion with no gold standard diagnostic test. Administration of intravenous immunoglobulin (IVIG) has been shown to reduce the incidence of coronary aneurysms significantly to 2%–3% if given within the first 10 days.[3,4] Currently, the treatment of choice is administration of high-dose IVIG combined with aspirin.[5]
Coronary aneurysms or dilatations resulting from KD are known to change over time. These lesions may regress (internal diameter returns to normal), stay unchanged, or progress. Stenotic lesions can develop with or without recanalization or collateralization. Rarely, aneurysms are known to expand and rupture, resulting in significant morbidity.[6] Angiographic resolution within 1–2 years after onset has been observed in approximately 50%–60% of coronary aneurysms, but giant aneurysms tend to persist.[2,7] Stenotic changes may occur after a longer period of time, and there are several hypotheses that many adults with newly diagnosed coronary artery disease may have suffered KD at an earlier age.[2,8] Calcification generally develops 5 or more years after disease onset and becomes more noticeable after a decade.[2,9,10] It is important to predict the clinical course of these aneurysms for optimal long-term management and prevention of further complications. We set out to determine the course and outcome of coronary artery dilatation in patients diagnosed with KD in Jakarta and its suburb in Indonesia and also to ascertain whether there are any differences in the outcomes based on the location of the aneurysms.
METHODS
Design
This study is a retrospective cohort study on the clinical course of patients with coronary artery dilatation since initial diagnosis. The study was approved by the local Ethics and Research Committee. There were 154 patients with coronary dilatation studied. All were treated in five hospitals in Jakarta and its suburb during the acute stage of the illness between January 2003 and July 2013. Echocardiography was done at the time of diagnosis, at 2 weeks after diagnosis, and then 6–8 weeks later or more often in severe cases. Follow-up echocardiography was done regularly every 12 months or more often in severe cases (3–4 months). All patients were invited for an up-to-date echocardiogram assessment except those who had had a recent echocardiogram (in the last 3 months).
Study criteria and measurements
Diagnosis of KD was based on the American Heart Association criteria.[5] All patients received IVIG (2 g/kg) and high-dose aspirin on admission, at the acute stage. For assessment of coronary dilatation, we used a z score based on body surface area for right coronary artery (RCA), left main coronary artery (LMCA), and left anterior descending coronary artery (LAD).[11] KD sequel was defined as coronary lesions persisting beyond 1 month after KD onset.[12] We did not categorize the type of dilatation or aneurysm based on its shape but simply focused on the internal diameter. Coronary artery dilatation was considered as small if the internal diameter was above the normal z score but was <5 mm, medium if 5–8 mm, and giant if >8 mm.[5] Patients were followed up for the duration of minimal 1 year up to maximal 10½ years with a minimum of 2 echocardiographic examinations which were all done by the author (treating physician) and reviewed by two other senior pediatric cardiologists. Coronary artery dilatations were considered to have regressed when the enlarged coronary arteries had normal internal diameters as visualized by echocardiogram.
Statistical analysis
We used the Kaplan–Meier curve to show the regression of coronary aneurysms over time. An “Event” was defined as regression of the dilatation back to its normal diameter. Furthermore, log-rank tests were performed to prove any difference between the curves, with the point of significance defined by P < 0.05.
RESULTS
Patients with coronary artery dilatations during initial echocardiography on admission were 168 of the total 503 inpatients with KD (33.3%, transient dilatation) and this number decreased to 40 (7.9%, sequelae) in the convalescent period (6–8 weeks after the onset). There were 154 patients with initial dilatation who met the criteria and were eligible for long-term follow-up (14 were lost to follow-up). Age varied between 1 month and 16 years (median: 34 months). The characteristics of patients with coronary artery dilatations are summarized in Table 1.
Table 1.
Characteristics of KD subjects with coronary artery dilatations
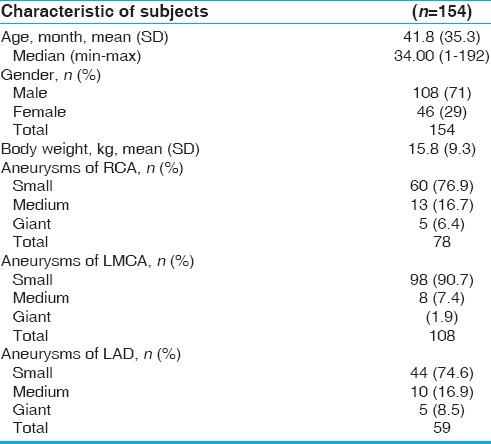
As each patient could have dilatations at more than one location, the number of dilatations found was 245. This consisted of 78 in the RCA, 108 in the LMCA, and 59 in the LAD artery. The LMCA was found to be the most common site for dilatation.
Giant aneurysms only occurred in children with multiple coronary dilatations. None of the children with a single dilatation had a giant aneurysm. At the end of observation (which varied in time), three patients (3.2%) with single-branch dilatation had no regression while patients with multiple-branch dilatation, i.e., 13 (20.6%), still showed dilatation including giant aneurysms.
The regression curve for RCA [Figure 1] showed that small dilatations had the best outcome followed by the medium ones. The giant aneurysms had the worst prognosis, the majority of them showing no improvement until the end of the study. Log-rank test between the three groups showed a statistically significant difference (P < 0.05). The regression curve for the LMCA and the LAD showed the similar results. Approximately 77.4% of small and moderate dilatations regressed within 2-year period.
Figure 1.
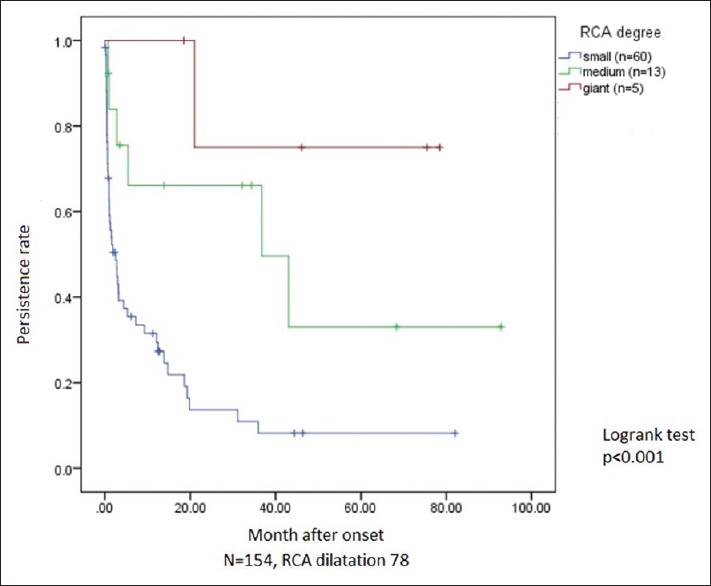
Persistent rate of RCA dilatations showing the difference among small (lower curve), medium (middle curve), and giant (upper curve). RCA: Right coronary artery
To find any difference in regression time curves among the small-sized RCA, LMCA, and LAD dilatations, they were plotted in one field [Figure 2]. No statistically significant difference was seen in terms of regression time between them, and the log-rank test showed P > 0.05. The regression curves for medium-sized RCA, LMCA, and LAD dilatations plotted together revealed similar results.
Figure 2.
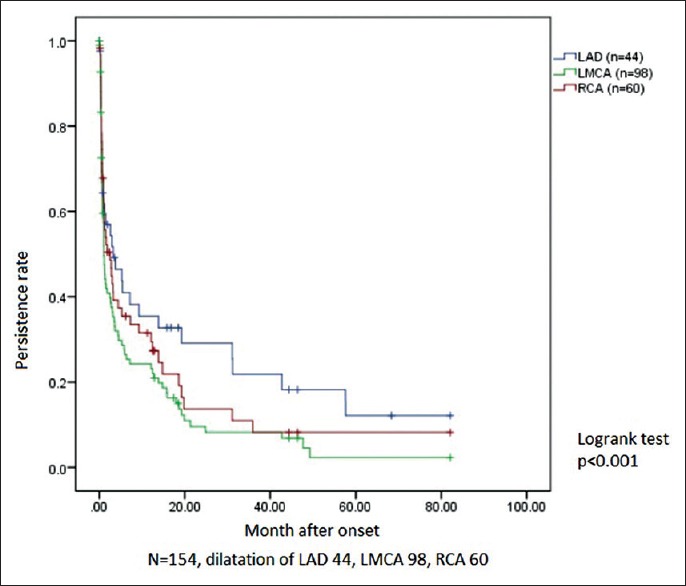
Comparison of persistent rate among small dilatations in RCA (middle curve), LMCA (lower curve), and LAD (upper curve). RCA: Right coronary artery, LMCA: Left main coronary artery, LAD: Left anterior descending
Among the 154 patients, nine (6%) had refractory KD and six responded well with the second dose of IVIG while the remaining three were given the third dose of IVIG, one patient responded well, while the other two needed a combination of infliximab, intravenous methyl prednisolone, and oral cyclosporin despite 3 doses of IVIG. Only one of the refractory KD developed giant aneurysm.
Four patients had recurrent KD at an interval of 6 months to 3 years after onset. They had normal coronary arteries on readmission and on follow-up.
The percentage of giant aneurysms was highest in the LAD which was 5/59 (8.5%), followed by RCA 5/78 (6.5%) and LMCA 2/108 (1.9%). Mortality in this mid- to long-term study was 0.
The median time needed for regression was 2.6 months (mean: 10.5 months) while the median follow-up duration was 41 months (mean: 23 months). We also found that, during observation beyond time-to-event point, there were no changes seen in the diameter of the previously regressed coronary arteries.
DISCUSSION
The frequency of coronary dilatation in KD patients was 33.3% (168 out of the 503 patients) on admission. These initial dilatations were transient ones, but could be regarded as sequelae of KD if they persisted beyond 30 days.[12] Further examination after 6–8 weeks of onset showed that the rate of dilatation decreased to 7.9% (n = 40). The incidence of coronary aneurysms has been reported to be significantly reduced at 2%–3% when immunoglobulin is given within the first 10 days of illness.[2,3,4] Our result (7.9%) is somewhat higher than the published findings, and perhaps this is due to a rather significant proportion (20%, 29 patients) receiving treatment only after day 10 of the onset (late) due to persistence of acute inflammatory signs.
This study has also shown that the majority (77.4%) of small and moderate dilatations regress within 2 years. This is in accordance with other studies which have shown that 50%–60% of coronary aneurysms regress within 1–2 years.[2] The giant aneurysms, however, tended to stay unchanged as previously reported.[2,13]
This study showed that the regression rate was determined by the internal diameter of the initial dilatation rather than the location. No difference in time to regression for similar-sized dilatations was seen between the RCA, LMCA, or LAD. These findings are consistent with results from other studies.[13,14] A previous study showed regression to be positively correlated with the age of onset below 1 year, female sex, fusiform aneurysms rather than saccular, and distal rather than proximal location.[7] In this study, we did not investigate these other factors contributing to regression.
Regressed aneurysms are generally thought to be cured, and follow-up is not universally carried out. However, there are reports that some regressed aneurysms develop into obstructive lesions with morphological and functional changes such as intimal thickening, reduced capacity for dilatation, and abnormal endothelial function.[15,16,17] In this study, although we did not have any histological data, there was no further echocardiographic dilatation in the regressed coronary arteries.
Each patient could have dilatation at more than one location, and the total number of dilatations found was 245 in the 154 patients. This consisted of 78 in the RCA, 108 in the LMCA, and 59 in the LAD. We did not group the cases with single-, double-, or triple-branch coronary lesions as the total number was not so large and our aim was to observe the clinical course of each branch. To the best of our knowledge, there has not been any study showing that the aneurysm at one branch would affect the clinical course of aneurysm at the other. We also did not divide the long-term clinical course of the delayed cases (29 patients) separately due to their small number, but all giant aneurysms belonged to this group. This fact supports the importance of early treatment. Perhaps, if they had been treated earlier, the results would have been much better.
As we measured the internal diameter periodically and for those with persisting aneurysms approximately every 3–4 months, we could not determine the exact time of regression. Hence, there might have been a discrepancy between the time of measurement and time of regression. However, we expect any differences to be negligible.
There were six patients who underwent catheterization; all of them had giant aneurysms. Two patients showed occlusion (total obstruction) of the RCA and LMCA. One patient showed stenotic lesions at the inflow of RCA and inflow of LMCA. Another patient had a coronary artery bypass graft due to severe stenotic lesions at the inflow of RCA and inflow of LAD. This finding is consistent with another study that most stenotic lesions were seen in either inflow or outflow of the aneurysms where the shear stress is high.[10] All patients with dilatation of 8 mm or less were treated with aspirin only, while those with over 8 mm received warfarin as well, of which dose was adjusted to international normalized ratio level of 2–3. One patient with giant aneurysm had a thrombus with partial occlusion at the proximal LAD and resolved with heparin.
There was no mortality noted in our study population. A study published from Japan which looked at the 30 year follow up of patients with Kawasaki Disease found a case fatality rate of 2% in 1970 (before the immunoglobulin era) that decreased to 0.1% (1:1000) in 2002 (after the immunoglobulin era).[18]
This study showed that, even though the LMCA is the most frequent location for dilatation, it has the lowest rate for giant aneurysm compared to RCA and LAD. We do not know the exact reason for this condition but hypothesize that, as the LMCA is a relatively short segment, the development of giant aneurysms is not anatomically feasible.
CONCLUSIONS
We conclude that the frequency of coronary dilatation in the acute phase of KD is 33.3% and reduces to 7.9% in the convalescent phase. The most frequent location for dilatation is the LMCA, followed by the RCA and LAD although the most frequent location for giant aneurysms is the LAD. The majority of dilatations (77.4%) regress within 2 years while giant aneurysms have a tendency to persist. Regression rate is determined by the size of the dilatations and not by their location.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Prof Sukman Tulus Putra MD PhD, Prof. Mulyadi M Djer M.D PhD, and Dr. Lucyana Santoso for their contribution.
REFERENCES
- 1.Kawasaki T. Acute febrile mucocutaneous lymphnode syndrome with accompanying specific peeling of fingers and toes. Jpn J Allergy. 1967;16:178–82. [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 6.Senzaki H. Long-term outcome of Kawasaki disease. Circulation. 2008;118:2763–72. doi: 10.1161/CIRCULATIONAHA.107.749515. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987;75:387–94. doi: 10.1161/01.cir.75.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–7. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki A, Kamiya T, Tsuda E, Shinya T. Natural history of coronary arterial lesions in Kawasaki disease. Prog Pediatr Cardiol. 1997;6:211–8. [Google Scholar]
- 10.Suzuki A, Miyagawa-Tomita S, Nakazawa M, Yutani C. Remodeling of coronary artery lesions due to Kawasaki disease: Comparison of arteriographic and immunohistochemical findings. Jpn Heart J. 2000;41:245–56. doi: 10.1536/jhj.41.245. [DOI] [PubMed] [Google Scholar]
- 11.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW, et al. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–8. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa R, Ogawa S. Long-term prognosis of patients with Kawasaki disease: At risk for future atherosclerosis? J Nippon Med Sch. 2009;76:124–33. doi: 10.1272/jnms.76.124. [DOI] [PubMed] [Google Scholar]
- 13.Akagi T, Rose V, Benson LN, Newman A, Freedom RM. Outcome of coronary artery aneurysms after Kawasaki disease. J Pediatr. 1992;121:689–94. doi: 10.1016/s0022-3476(05)81894-3. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara T, Fujiwara H, Hamashima Y. Size of coronary aneurysm as a determinant factor of the prognosis in Kawasaki disease: Clinicopathologic study of coronary aneurysms. Prog Clin Biol Res. 1987;250:519–20. [PubMed] [Google Scholar]
- 15.Suzuki A, Yamagishi M, Kimura K, Sugiyama H, Arakaki Y, Kamiya T, et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J Am Coll Cardiol. 1996;27:291–6. doi: 10.1016/0735-1097(95)00447-5. [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa R, Ishii M, Sugimura T, Akagi T, Eto G, Iemura M, et al. Coronary endothelial dysfunction after Kawasaki disease: Evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol. 1998;31:1074–80. doi: 10.1016/s0735-1097(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 17.Iemura M, Ishii M, Sugimura T, Akagi T, Kato H. Long term consequences of regressed coronary aneurysms after Kawasaki disease: Vascular wall morphology and function. Heart. 2000;83:307–11. doi: 10.1136/heart.83.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonobe T. A summary of the epidemiologic surveys on Kawasaki disease conducted over 30 years. JMAJ. 2005;48:30–3. [Google Scholar]


