Abstract
Background:
Congenital aortic stenosis (AS) is a rare disease. Treatment options for newborns are challenging. Newborns may have higher reintervention rate and mortality.
Objectives:
The study aimed to identify the factors predictive of reintervention following balloon aortic valvuloplasty (BAV) for AS during infancy.
Methods:
Retrospectively, between 2001 and 2016, echocardiography (echo) and cardiac catheterization data for infants with AS were analyzed, including follow-ups and reinterventions. Percentage reduction was defined as the ratio between the drop of aortic valve (AV) peak gradient and the baseline peak gradient.
Results:
Sixty infants were included and 48 were followed up. Sixteen (27%) patients were neonates. Peak-to-peak gradient at AV was 64 ± 27 mmHg, which was reduced to 27 ± 13 mmHg. Percentage reduction was 53% ±24%. Forty-nine (82%) patients had adequate results (residual AV gradient <35 mmHg). There was no significant aortic insufficiency (AI) before procedure, while 6 (10%) patients had increased AI immediately after BAV. Of 48 patients, 14 (29%) required an additional BAV. Of 48 patients, 8 (17%) required surgical interventions following BAV. Reintervention was associated more with small left ventricular outflow tract (LVOT), high residual AV, and low percentage reduction. Mortality was 8.3%.
Conclusions:
BAV in infancy has a reasonable success rate (82%) with high rate of reintervention. Patent ductus arteriosus-dependent neonates carried the highest risk of mortality. Small LVOT, high AV residual gradient, and low percentage reduction resulted in more reinterventions.
Keywords: Aortic valve disease, congenital heart disease, pediatric intervention, pediatrics, percutaneous intervention
INTRODUCTION
Aortic stenosis (AS) is a rare congenital heart disease with an incidence of 4/10,000.[1] Congenital AS has a wide range of clinical presentations. Newborns may present with shock, but some patients remain asymptomatic until adulthood. The treatment of newborns with critical AS remains a challenge in pediatric interventional cardiology although pediatric cardiac surgeons are reporting good outcomes with direct valvotomy.[2] In a multicenter survey of more than 1000 patients with AS, 58% were infants, almost half of them were newborns who had higher mortality and reintervention rates.[3] Aortic valve (AV) insufficiency and residual AS are the primary factors that are reported to necessitate the surgical reintervention.[4]
The purpose of this study was to identify the factors that were predictive of reintervention and to explicate a simple method for calculating the percentage reduction that may provide additional aid in the management of these patients and facilitate parent counseling.
MATERIALS AND METHODS
We retrospectively reviewed our institutional database and records to identify all infants with AS who required balloon aortic valvuloplasty (BAV) during the period from 2001 to 2016. All patients who had BAV within the 1st year of life were included in the study. Patients were excluded if they ultimately underwent single-ventricle palliation or required direct surgical AV intervention. Demographic data, including age at catheterization (cath), gender, weight, and associated cardiac lesions, were recorded. Patent ductus arteriosus (PDA)-dependent patients were defined as patients who required prostaglandin infusion to maintain their cardiac output. Outcomes after BAV were reviewed.
The baseline echocardiography (echo) study was defined as the most recent study before BAV. Data retrieved from the echo reports included aortic annular diameter, AV peak instantaneous and mean Doppler gradients, left ventricular ejection fraction (LVEF), and initial degree of aortic insufficiency (AI) (none, mild, moderate, and severe). AI was categorized according to the echo laboratory standard, which conforms to currently available standards as defined by the American Society of Echocardiography.[5] Significant AI was defined as moderate or severe AI. Postprocedural echo study was defined as the first study after the valvuloplasty. Postprocedural AV peak instantaneous and mean Doppler gradients were obtained.
The final echo study was defined as the last study performed before surgical repair of AS, death, or the end of the study. Data obtained from the final echo study included degree of AI, LVEF, and peak instantaneous and mean AV gradients.
Procedural data were noted and recorded, including pre- and post-BAV catheter-derived peak-to-peak valve gradient, angiography-determined AV annular diameter, and maximum balloon diameter-to-AV annular diameter ratio (BAR).
Percentage reduction was defined as the ratio between the drop in AV peak gradient and the baseline peak gradient.
Percentage reduction = (PG1−PG2)/PG1 × 100
where PG1 indicates the baseline peak gradient and PG2 indicates the postprocedural peak gradient. Percentage reduction was calculated from both echo gradients and catheter-derived gradients.
Data are expressed as numbers and percentages for categorical variables and as mean ± SD for continuous variables. Data that did not fit a normal distribution are expressed as medians and ranges. Death or surgical repair for AV or severe left ventricular outflow tract (LVOT) obstruction was considered the endpoint of the study. Continuous variables were compared using paired Student's t-tests, and categorical variables were compared using Chi-square tests or Fisher's exact test appropriately. P < 0.05 was considered statistically significant. The statistical analyses were performed with SPSS for Windows, Version 16.0., SPSS Inc., Chicago, IL, USA.
RESULTS
From 2001 to 2016, sixty infants underwent BAV at our institution. Patients ranged in age at BAV from 1 day to 11.4 months, with a median age of 2 months; 16 (27%) patients underwent BAV as neonates (age <30 days). Median weight was 4.2 kg ranging from 2.2 to 9.6 kg. Eight (13%) patients had surgical intervention before BAV for aortic arch repair (seven cases of coarctation and one case as interrupted aortic arch repair). None had surgical aortic valvotomy before BAV.
Five patients died within 1–58 days of BAV accounting for a mortality of 8.3%. Four (80%) of them were newborns and PDA dependent. Of 12 PDA-dependent patients, there were 4 mortalities (33%) (P = 0.005). Seven patients lost to follow-up. Hence, 48 patients were followed up after BAV for a median time of 3.2 years and a maximum of 10.5 years; 16 (33%) patients had follow-up >5 years.
AV annular diameter was 7 ± 1.6 mm. Forty-six (77%) of AV were bicuspid, 11 (18%) unicuspid, and 3 (2%) tricuspid. Before BAV, the peak and mean AV Doppler gradients were 86 ± 26 and 48 ± 14 mmHg, respectively. The baseline left ventricle ejection fraction (EF) was 70% (14–90). Nineteen (31.7%) patients had EF <55% and 8 (42%) of them were newborns.
At the time of cath, peak-to-peak gradient from the left ventricle to the ascending aorta was 64 ± 27 mmHg, which was reduced by BAV to 27 ± 13 mmHg. The average decrease in AV gradient was 37 ± 23 mmHg. Percentage reduction was 53% ±24%. The BAR was 0.91 ± 0.14. The results of ballooning were considered adequate (residual AV gradient <35 mmHg) in 49 (82%) patients. Eleven (18%) patients had residual AV gradients >35 mmHg. Of those, four required a second BAV, five required surgical repair (three had both BAV and surgery), and five were free from reintervention.
None of the patients had a significant AI before procedure, while 6 (10%) had significant AI immediately after BAV. Two of those patients died during the same admission. One of them had Konno procedure and AV replacement at the age of 8 years. The rest still had significant AI at the last follow-up.
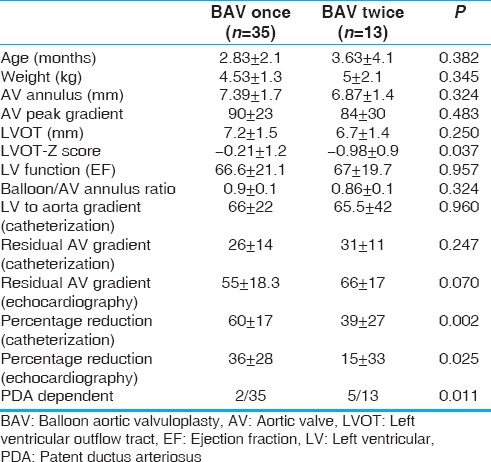
An additional valvuloplasty was performed in 14 (29%) patients 9 months (1–66) after the initial BAV. (One of them died and was not included in follow-up statistics). Freedom from repeat valvuloplasty was 72% and 59% at 2 and 5 years, respectively [Figure 1]. Patients who required an additional BAV had low cath percentage reduction of 39 ± 27 and echo percentage reduction of 15 ± 33 versus those who had BAV once with percentage reduction of 60 ± 17 (cath; P = 0.002) and 36 ± 28 (echo; P = 0.025) during the initial intervention [Table 1].
Figure 1.
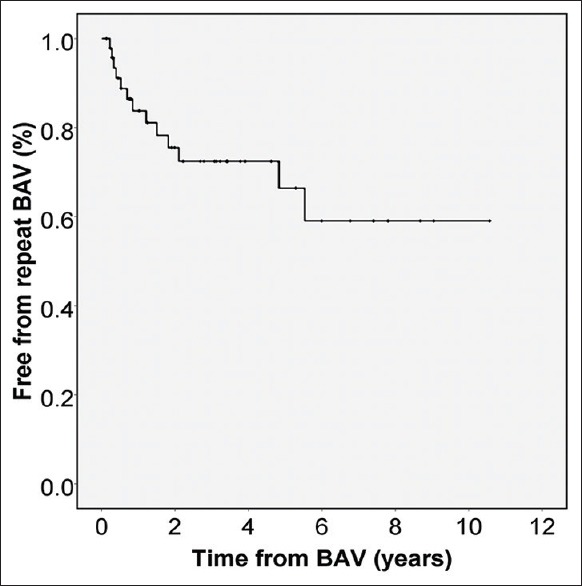
Kaplan–Meier curve displays freedom from repeat valvuloplasty
Table 1.
Comparing patients requiring additional balloon aortic valvuloplasty and those who did not
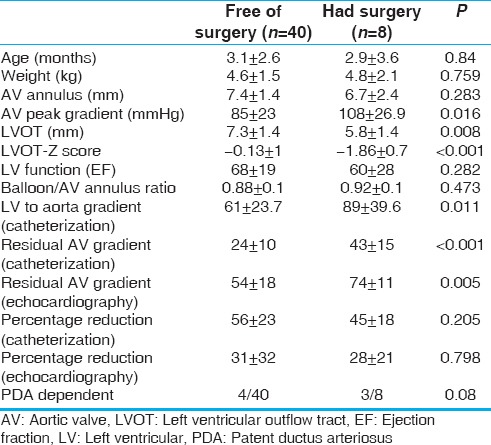
Surgical repair for residual LVOT obstruction was done in 8 (17%) patients at a median of 17 months (0.3–120 months) after the initial BAV. Four of them had twice BAV before surgery. Freedom from surgical repair was 79% and 53% at 5 and 10 years, respectively [Figure 2]. Surgical procedures were Ross–Konno in four patients while aortic valvotomy, Konno and AV replacement, Norwood with Sano shunt, and subaortic membrane resection was performed in one patient each. Those who required surgical repair had smaller LVOT (P = 0.001) and higher residual AV gradients (P < 0.001) [Table 2].
Figure 2.
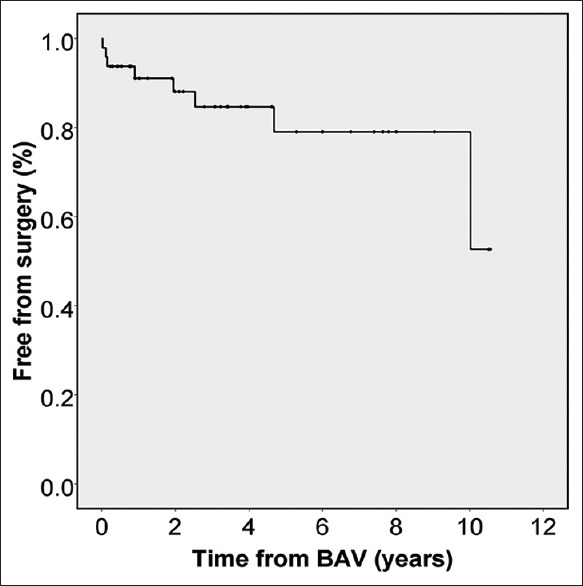
Kaplan–Meier curve displays freedom from surgical procedure after valvuloplasty
Table 2.
Comparing patients who required surgical repair for left ventricular outflow tract obstruction and those who are free of surgery after the first balloon aortic valvuloplasty
Seventeen (35%) patients required reintervention (either BAV or surgery). Risk factors for reintervention were small LVOT (P < 0.001), high residual gradient by cath (P = 0.002) or echo (P = 0.001), low percentage reduction by cath (P < 0.001) or echo (P = 0.01), and those who were PDA dependent [Table 3].
Table 3.
Comparing patients who required reintervention (catheterization or surgery) and those who are free from reintervention
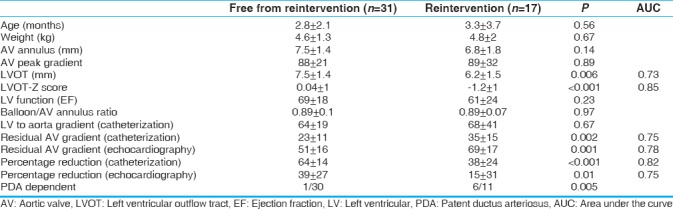
Thirty-one (52%) patients at the end of the study did not require reintervention. They had peak Doppler gradients of 39 ± 17 mmHg and EF of 72% ± 12% at the last echo. Only 6 (19%) of them had significant AI.
DISCUSSION
We reviewed the results of AS in infancy since we thought that this age group has unique risk factors and results. Torres et al.[6] reviewed 373 BAV in a multicenter prospective study for adults and children. 85% of their patients were considered to have adequate results (gradient post-BAV <35 mmHg). Two hundred and twenty-nine were infants, 158 (69%) of them had adequate results, in contrast to our rate of 82%.
In our cohort, we noticed a predictive difference between the actual residual AV gradient and the percentage reduction. High residual AV gradients by echo or cath were associated with the need of surgical repair of AV or LVOT obstruction. However, low percentage reductions by echo or cath were associated with the need of another balloon dilatation. Since there was overlapping between the two groups (cath and surgery; four patients had twice BAV then surgical repair), it was reasonable to study all reinterventions in one group.
An additional BAV was performed in 29% of our patients. This is probably high because of the younger age group in our cohort. In mixed children populations, the rate of reintervention was 16%–23%.[7,8] Ewert et al. reported a reintervention ratio of 16% in neonates and 10% in older patients.[3]
Surgical repair of AV or LVOT obstruction was required in 17% of our patients. The need for surgery is evaluated by multiple factors of LV adequacy like measures of aortic annulus, LVOT, and AV morphology in addition to the gradient across the AV and severity of AI. The nature of varied surgeries reflects the complexity of factors involved in the decision-making. The main surgery performed was the Ross–Konno procedure, while in other series, it was mainly AV repair or AV replacement in older patients.[8,9] Surgical interventions were necessitated by outflow obstructions, rather than aortic insufficiencies.
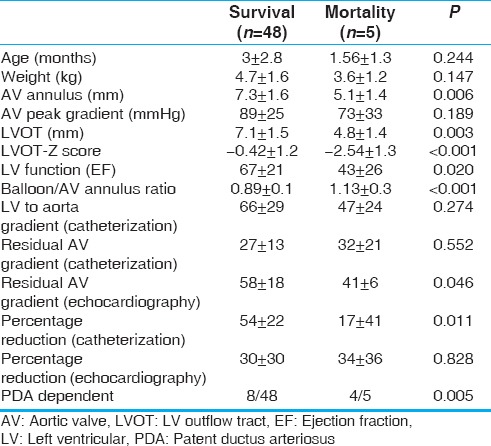
Mortality is high in this group of patients. It was 8.3% in our cohort, which is comparable to previous reports with mortality around 9%–11%.[3,7,9,10] Death was associated with small AV annulus and LVOT-Z score, depressed LV function, high BAR, low percentage reduction by cath, and PDA-dependent systemic circulation [Table 4]. Probably higher BAR is a reflection of low cath percentage reduction and the need for use of larger balloons to try to get the maximum response. Maskatia et al.[9] correlated death to depressed LV function while neonatal BAV and high AV residual gradients were not associated with death in their series. Others described higher mortality, repeat BAV, and higher surgical intervention in newborns when compared to infants and older patients.[7,11] We found that newborns who are PDA dependent are at the highest risk of mortality (33%, P = 0.005).
Table 4.
Comparing mortality and survival
CONCLUSIONS
BAV in infancy has a reasonable success rate (82%) with high rate of reintervention. PDA-dependent neonates carried the highest risk of mortality. Small LVOT, high AV residual gradient, and low percentage reduction resulted in more reinterventions.
Limitations
This was a retrospective descriptive study with a relative small number of patients who precluded the establishment of regression modeling. The study population was exclusively composed of infants. A multicenter study with a greater number of patients would probably enable stronger conclusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This paper is dedicated to our beloved friend, colleague, and collaborator Dr. Mahmoud Elbarbary (Consultant Pediatric Cardiac Intensivist; Associate Professor, Clinical Epidemiology and Biostatistics, McMaster University, Canada, and Associate Clinical Professor, University of South Carolina, USA). Sadly, he passed away before publication of this study.
We would like to specially thank Professor Talat Mesud Yelbuz for critical reading and revision of this manuscript.
REFERENCES
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Donald JS, Konstantinov IE. Surgical aortic valvuloplasty versus balloon aortic valve dilatation in children. World J Pediatr Congenit Heart Surg. 2016;7:583–91. doi: 10.1177/2150135116651091. [DOI] [PubMed] [Google Scholar]
- 3.Ewert P, Bertram H, Breuer J, Dähnert I, Dittrich S, Eicken A, et al. Balloon valvuloplasty in the treatment of congenital aortic valve stenosis – A retrospective multicenter survey of more than 1000 patients. Int J Cardiol. 2011;149:182–5. doi: 10.1016/j.ijcard.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Knirsch W, Berger F, Harpes P, Kretschmar O. Balloon valvuloplasty of aortic valve stenosis in childhood: Early and medium term results. Clin Res Cardiol. 2008;97:587–93. doi: 10.1007/s00392-008-0655-8. [DOI] [PubMed] [Google Scholar]
- 5.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–71. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Torres A, Vincent JA, Everett A, Lim S, Foerster SR, Marshall AC, et al. Balloon valvuloplasty for congenital aortic stenosis: Multi-center safety and efficacy outcome assessment. Catheter Cardiovasc Interv. 2015;86:808–20. doi: 10.1002/ccd.25969. [DOI] [PubMed] [Google Scholar]
- 7.Reich O, Tax P, Marek J, Rázek V, Gilík J, Tomek V, et al. Long term results of percutaneous balloon valvoplasty of congenital aortic stenosis: Independent predictors of outcome. Heart. 2004;90:70–6. doi: 10.1136/heart.90.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown DW, Dipilato AE, Chong EC, Lock JE, McElhinney DB. Aortic valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis intermediate and late follow-up. J Am Coll Cardiol. 2010;56:1740–9. doi: 10.1016/j.jacc.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 9.Maskatia SA, Ing FF, Justino H, Crystal MA, Mullins CE, Mattamal RJ, et al. Twenty-five year experience with balloon aortic valvuloplasty for congenital aortic stenosis. Am J Cardiol. 2011;108:1024–8. doi: 10.1016/j.amjcard.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton GE. Transcatheter management of neonatal aortic stenosis. Cardiol Young. 2014;24:1117–20. doi: 10.1017/S1047951114002030. [DOI] [PubMed] [Google Scholar]
- 11.Fratz S, Gildein HP, Balling G, Sebening W, Genz T, Eicken A, et al. Aortic valvuloplasty in pediatric patients substantially postpones the need for aortic valve surgery: A single-center experience of 188 patients after up to 17.5 years of follow-up. Circulation. 2008;117:1201–6. doi: 10.1161/CIRCULATIONAHA.107.687764. [DOI] [PubMed] [Google Scholar]


