Abstract
The rapid changes that have taken place in recent years in relation to techniques used to image the fetal heart have emphasized the need to have a detailed knowledge ofnormal cardiac anatomy. Without such knowledge, it is difficult, if not impossible, to recognize the multiple facets of congenital cardiac disease. From the inception of fetal echocardiographic screening, the importance of basic knowledge of cardiac anatomy has been well recognized. The current machines used for imaging, however, now make it possible potentially to recognize features not appreciated at the start of the specialty. So as to match the advances made in imaging, we have now revisited our understanding of normal cardiac anatomy in the mid-gestational fetus. This was made possible by our dissection of 10 fetal hearts, followed by production of addition histological sections that mimic the standard ultrasound views. The fetuses ranged in gestational age from between 20 and 28 weeks. We then correlated the obtained anatomic images with the corresponding ultrasonic images used in the standard fetal screening scan. We also interrogated the anatomic sections so as to clarify ongoing controversies regarding detailed features of the normal cardiac anatomy. We have been able to show that the views now obtained using current technology reveal many details of anatomy not always appreciated at earlier times. Knowledge of these features should now permit diagnosis of most congenital cardiac malformations. The anatomic-echocardiographic correlations additionally provide a valuable resource for both the understanding and teaching of fetal echocardiography.
Keywords: Arrhythmias, echocardiography, prenatal diagnosis, ultrasound imaging
INTRODUCTION
It is axiomatic that recognition of the abnormal requires a thorough knowledge of the normal. Indeed, one of the first investigations undertaken by those who were developing the field of fetal echocardiography was to make correlations between the anatomy of the normal fetal heart and the images obtained at that time.[1] As in any complex task, it was immediately evident that such knowledge of the normal arrangement was best appreciated if approached in an orderly and systematic fashion. In this regard, the complex temporal and morphological changes that lead to the formation of the heart, with effective completion of septation, are completed by the 8th week of pregnancy. It is not until the 12th week, however, that the heart is located in its normal position in the thorax, and the atrioventricular (AV) and arterial valves are sufficiently developed to produce a miniature version of the postnatal heart. Even in the 12th week of pregnancy, the heart is no larger than 8 mm. At this stage of development, therefore, it remains difficult to visualize with precision the details of cardiac anatomy as seen during fetal echocardiography. From this stage onward, there is a phase of rapid cardiac growth, such that it doubles in size between weeks 12 and 17 and has tripled in size by the end of the 21st week of gestation, thus facilitating the accurate identification of the various cardiac structures.[2] Taking this into account, we have now prepared new dissections of normal hearts obtained from fetuses aged from 22-week gestation to the end of the 28th week. In this way, we have sought to emulate the original study,[1] providing current guides as to what might now realistically be seen during fetal echocardiographic interrogation.
METHODS
We have dissected 10 fetal hearts (6 males and 4 females) belong to the collection of the Pediatrics Service of the Hospital Perpetuo Socorro of Badajoz, with gestational ages ranging from 20 to 28 weeks, obtaining planes that mimic the standard ultrasonic views. This study has been approved by the bioethical committee on human research (University of Extremadura, registration n°25/2016) and with the Helsinki Declaration. Subsequent to the initial dissection, the hearts were cut using a rotation microtome Microm HM 340E. Serial sections were obtained at 10 microns thickness, and every 25th section was stained with Masson trichrome. The sections that correlated best with the standard ultrasound views were chosen for subsequent anatomic-echocardiographic correlations. We also used the sections to clarify features of cardiac anatomy that remain potentially controversial.
RESULTS
Normal fetal cardiac anatomy
So as to assess and diagnose the many and varied congenital cardiac malformations, it has long been recognized that the normal heart can best be assessed in terms of three so-called segments.[3,4] The first segment is the atrial segment the assessment of which requires recognition of the morphologically right and left atriums (LAs), regardless of their location. The second segment is the ventricular mass, and analysis of this component requires differentiation of the morphologically right and left ventricles (LVs). The third segment of the normal heart, the arterial segment, is initially distinguished in terms of the recognition of the pulmonary trunk (PT) and aorta (Ao). In the fetus, nonetheless, it is also necessary to distinguish between the aortic and ductal arches. Although these are the basic features of the segments, it is now also accepted that, while malformations can involve the great veins entering the heart, it must be possible to distinguish between the atrial chambers irrespective of the venoatrial connections. This is because of the importance of the principle known as the morphological method. This states that structures should be identified on the basis of their intrinsic morphology, and not according to other features which are themselves variable.[5] The fact that, in the normal setting, the major axis of the heart is not parallel to the long axis of the body is also important. This feature needs to be taken into account when describing the relationship of the various cardiac components to each other. All of these aspects are important when seeking mentally to integrate the image of the macroscopic anatomical sections with images now routinely obtained using modern-day echocardiographic equipment. In our study, therefore, we have sought to prepare cuts made from the specimens so as to match the standardized fetal echocardiographic views. In this way, we hope to provide the basic knowledge of fetal anatomy that is a prerequisite for correct interpretation of fetal echocardiographic images.
Systematic study of normal fetal cardiac anatomy
Arrangement, axis, and position
The first step of the fetal cardiac interrogation is to describe the arrangement and location of the heart in relation to the overall arrangement of the fetus. In this regard, there are four basic patterns, namely, the usual arrangement, often described as “situs solitus,” its mirror image, or “situs inversus,” and the arrangements of right and left isomerism. The latter two arrangements are frequently grouped together as “visceral heterotaxy,” but the major variations in malformations between the two subsets emphasize the need to distinguish between them.[6]
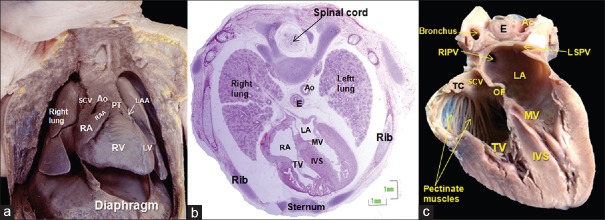
The essence of the usual arrangement is that the majority of the heart is located with the left hemithorax, with its apex also pointing to the left. If the fetal arrangement is also normal, the stomach will similarly be left-sided. The long axis of the heart will be angled at about 45° relative to the axis formed by the vertebral column and the sternum, with approximately 2/3 of the heart to the left of the midline, and 1/3 to the right [Figure 1]. In terms of its size, the normal heart occupies about 1/3 of the fetal thorax, and its transverse diameter of the heart must not exceed half of the transverse diameter of the chest [Figure 1]. Mere presence of the heart in the location as described above, and the finding of normal arrangement of the remaining organs, however, does not guarantee cardiac normality. So as to establish normality, it is essential to assess the features of each individual segment, ensuring that the morphologically right and morphologically left components are appropriately connected across the AV and ventriculoarterial junctions. Normal segmental connections, correctly described as being concordant, exist when the morphologically right atrium (RA) connects to the morphologically right ventricle (RV) through a morphologically tricuspid valve, with the PT arising from the morphologically RV. In such concordant settings, it follows that the morphologically LA will connect to a morphologically LV through a mitral valve, and the LV will give rise to the Ao. It is necessary, therefore, to know the features that provide morphological discrimination between the components of the three segments so as to establish the presence of usual atrial arrangement, along with concordant AV, and ventriculoarterial connections.
Figure 1.
(a) Anterior view of the thorax of a normal 22-week fetus, the heart located in the thoracic mediastinum. (b) Transversal section stained with hematoxylin and eosin from a human fetus at 20 weeks of development. Note the orientation of the heart in relation to the remaining structures. (c) Four chamber view at 25 weeks of gestation. This heart has been opened to show the extent of the pectinate muscles in the morphologically RA. The off-setting of the hinges of the leaflets of the mitral and tricuspid valves is evident. Ao: Aorta, IVS: Interventricular septum, E: Esophagus, LA: Left atrium, LAA: Left atrial appendage, LV: Left ventricle, LSPV: Left superior pulmonary vein, MV: Mitral valve, OF: Oval fossa, PT: Pulmonary trunk, RA: Right atrium, RAA: Right atrial appendage, RIPV: Right inferior pulmonary vein, RV: Right ventricle, SCV: Superior caval vein, TC: Terminal crest, TV: Tricuspid valve
Atrial segment
Morphologically right atrium
Located anteriorly and to the right, the RA comprises five components [Figure 2], namely, the vestibule, the venous component, the septum, the appendage, and a small part of the body of the initial atrial component of the heart tube.[7,8] Of these components, it is the appendage that serves to identify the morphological rightness.[9] The venous component receives the superior and inferior caval veins, as well as the coronary sinus. The major anatomical feature, which as we will see serves to distinguish morphologically rightness from leftness, is the extent of the prominent pectinate muscles, which originate from the terminal crest. In the morphologically RA, these pectinate muscles extend throughout the vestibular component of the right AV junction [Figure 1], reaching to the cardiac crux.[9] The septal surface is constituted by the floor of the oval fossa (OF) and the anteroinferior buttress that contains the triangle of Koch [Figures 1 and 2]. It should be noted that the so-called “septum secundum” is no more than the cranial infolding between the attachments of the right pulmonary veins to the LA and the caval veins to the RA. This feature is already demonstrable in the sections obtained at mid-gestation [Figure 2]. The cranial fold acts as the frame against which the flap valve forming the floor of the OF will close in postnatal life. The left-sided location of the flap valve, when seen, is also an indication of sidedness [Figures 1 and 2]. Sonographically, nonetheless, it may not always be possible to distinguish these various structures. Note is taken, therefore, of the venoatrial connections. The connection of the inferior caval vein is relatively constant, although it can also connect to an atrial chamber in the setting of right isomerism, and even in a minority of fetuses with left isomerism. In the normal fetus, nonetheless, its orifice is shielded by the Eustachian valve [Figure 2], with the orifice of the coronary sinus guarded by the Thebesian valve. The presence of the coronary sinus is more important, since it is universally absent in the setting of right isomerism. Finding the coronary sinus in the left AV groove, with drainage to the right-sided atrium, therefore, is an excellent marker of usual atrial arrangement. It should also be noted that the coronary sinus, as it extends through the left AV groove, possesses its own walls, which are separate from the walls of the LA[10] [Figure 2]. It is a mistake to suppose that there is a common wall between the coronary sinus and the LA.[11] In the normal arrangement, the Eustachian valve embeds itself in the anteroinferior buttress of the atrial septum, continuing as the tendon of Todaro, which then inserts in the AV component of the membranous septum. These features are readily seen in the histological sections [Figure 3]. The tendon, together with the tricuspid valve and the orifice of the coronary sinus, provide the anatomic landmarks of the triangle of Koch, inside which is located the AV node [Figure 3].
Figure 2.
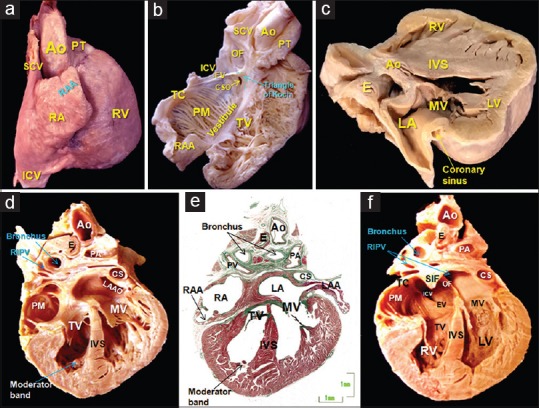
(a) Lateral view of the RA and its appendage, a roughly triangular-shaped offshoot whose apex generally points upward, overlapping the aortic root. (b) The RA and ventricle have been opened like a book to show the different components of the RA, such as the terminal crest and the origin of the pectinate muscles, the vestibule next to the tricuspid valve, the triangle of Koch and the orifice of the coronary sinus. (c) Sagittal section of a 28-week fetal heart showing the coronary sinus which extends through the left atrioventricular groove and is separated from the walls of the LA. (d-f) Frontal sections of a 23-week heart, where image e is a histologic stain using trichromic Masson stain corresponding to image d. Note the different morphology of the right and left atrial appendages and distinguish between the smooth-walled LA and the pectinated RA. Note in image f, the superior interatrial fold between the right pulmonary veins to the LA, and the caval veins to the RA. Ao: Aorta, IVS: Interventricular septum, CS: Coronary sinus, CSO: Coronary sinus orifice, E: Esophagus, EV: Eustachian valve, IVS: Interventricular septum, LA: Left atrium, LAA: Left atrial appendage, LAAO: Left atrial appendage orifice, LV: Left ventricle, LSPV: Left superior pulmonary vein, MV: Mitral valve, OF: Oval fossa, PA: Pulmonary artery, PM: Pectinate muscles, PT: Pulmonary trunk, RA: Right atrium, RAA: Right atrial appendage, RIPV: Right inferior pulmonary vein, RV: Right ventricle, SCV: Superior caval vein, SIF: Superior interatrial fold, TC: Terminal crest, TV: Tricuspid valve
Figure 3.
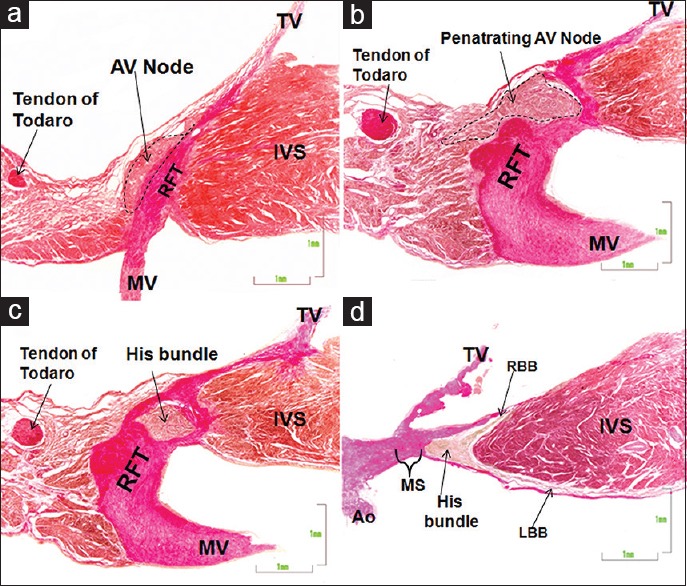
(a-d) This composite figure shows a van Gieson-stained sections of the atrioventricular node (a) plus the penetrating atrioventricular node (b), the His bundle (c), and its right and left bundle branches (d) in a fetal heart of 28 weeks. Ao: Aorta, IVS: Interventricular septum, LBB: Left bundle branch, MS: Membranous septum, MV: Mitral valve, RBB: Right bundle branch, RFT: Right fibrous trigone, TV: Tricuspid valve
Morphologically left atrium
In the normal arrangement, this chamber is located to the left and posterior to the morphologically RA. It possesses the same components as the RA, namely, a venous component, the flap valve forming the septal surface, the vestibule, and the appendage. It also retains the majority of the body of the atrial chamber found in the initial linear heart tube. The ultimate distinguishing feature of its morphologically leftness is the structure of its appendage. Unlike the right appendage, the left appendage is long and narrow [Figure 2], with pectinate muscles virtually confined to its tubular cavity.[9,12] Because the pectinate muscles are confined within the appendage, the majority of the walls of the LA is smooth. In the normal heart, the cranial and posterior component of the atrium receives the four pulmonary veins, usually draining with one at each corner of the roof [Figures 1, 2, 4 and 5]. The feature of the atrial septum in the LA is the presence of the flap valve, which is the embryonic primary septum.[7] In the fetal heart, of course, the upper margin of the flap valve remains separate from the so-called “septum secundum.” As already emphasized, this so-called secondary septum is, in reality, the infolding between the attachments of the pulmonary veins to the LA and the caval veins to the RA [Figures 2 and 4]. It is effaced in the presence of totally anomalous pulmonary venous connection. In the normal arrangement, the blood entering the RA from the inferior caval vein, which is the placental return through the venous duct, is directed through the oval foramen and into the LA by the Eustachian valve. The venous valves, therefore, are a feature of the morphologically RA. The coronary sinus, which drains venous blood from the heart itself, is part of the morphologically left AV groove, even though it drains to the morphologically RA. It is increased in size when associated with persistence of the left superior caval vein. This venous tributary has usually regressed by mid-gestation, persisting as the oblique vein of the LA. Its persistence, however, can be anticipated should the brachiocephalic vein be absent. As already emphasized, the venous channel possesses its own walls, discrete from those of the LA, as it courses through the left AV groove [Figures 2 and 5].
Figure 4.
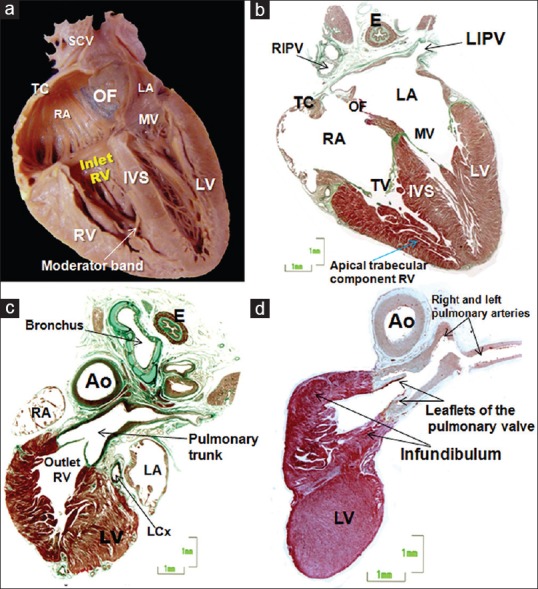
(a and b) Frontal sections of a 26-week heart, where image b is a histologic stain using trichromic Masson stain corresponding to image a. Two of the three components of the RV are revealed: The inlet (tricuspid valve), and the apical trabecular component. Note an important muscular strand, the moderator band, to the anterior papillary muscle and the septal surface reinforced by the septomarginal trabeculation (c and d). Histological sections of the outlet of the RV stained with Masson trichrome of two fetus of 22 (c) and 27 weeks/day) showing the free-standing infundibular sleeve and the attachment of the pulmonary leaflets. Note the anatomic relation of the RV outflow tract with the aortic outflow. Ao: Aorta, E: Esophagus, IVS: Interventricular septum, LA: Left atrium, LCx: Left circumflex artery, LV: Left ventricle, LIPV: Left inferior pulmonary vein, MV: Mitral valve, OF: Oval fossa, RA: Right atrium, RIPV: Right inferior pulmonary vein, RV: Right ventricle, SCV: Superior caval vein, TC: Terminal crest, TV: Tricuspid valve
Figure 5.
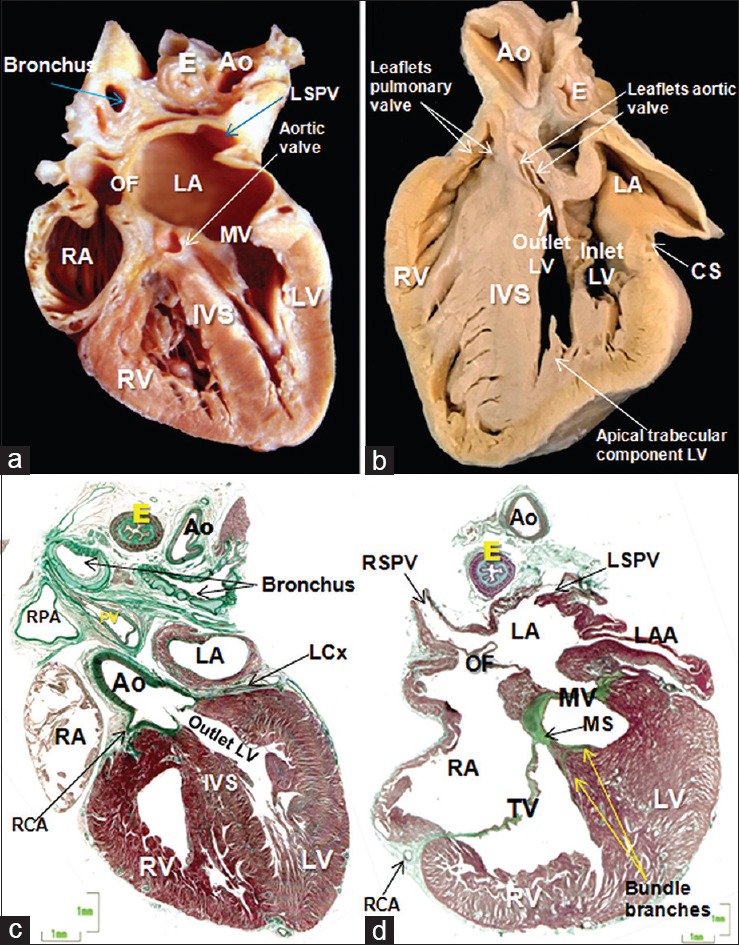
(a) Frontal section of a 25-week heart showing the morphologically LV always develops together with the mitral valve. Note the mitroaortic continuity. (b) Sagittal section of a 28-week fetal heart showing the three components of the LV: the inlet (mitral valve), apical trabecular, and LV outflow tract which is in anatomic relation of the pulmonary valve. (c) Histological section of the outlet of the LV stained with Masson trichrome of 25-week. The two sinuses of the aortic root supporting the coronary arteries are sustained by the left ventricular myocardium. (d) Histological section of 23-week fetal heart. The thin membranous septum separates the cavities of the right and LVs. Ao: Aorta, E: Esophagus, CS: Coronary sinus, IVS: Interventricular septum, LA: Left atrium, LAA: Left atrial appendage, LCx: Left circumflex artery, LV: Left ventricle, LSPV: Left superior pulmonary vein, MV: Mitral valve, MS: Membranous septum, OF: Oval fossa, RA: Right atrium, RCA: Right coronary artery, RSPV: Right superior pulmonary vein, RV: Right ventricle, SCV: Superior caval vein, TC: Terminal crest, TV: Tricuspid valve
Ventricular segment
The morphologically right ventricle
In the setting of concordant AV connections, the RA opens into the RV through the tricuspid valve. The RV itself then extends from the AV to the ventriculoarterial junctions [Figure 4]. It has three components, namely, the inlet, surrounding and supporting the tricuspid valve, the apical trabecular component, and the outlet or infundibulum, which supports the PT [Figure 4]. In the setting of biventricular AV connections, the inlet of the morphologically RV always develops together with the tricuspid valve. In such settings, therefore, the features of the tricuspid valve can be used to define the morphologically RV. Its septal leaflet is inserted at the level of the septum in a slightly apical location relative to the hinge of the mitral valve [Figures 3 and 4]. This is a particularly useful echocardiographic feature. In addition, the tricuspid valve has cordal insertions to the septum. In the final analysis, however, it is the features of the apical trabecular component that determine ventricular rightness. The apical component of the RV is more trabeculated than that of the LV. Its septal surface is reinforced by the septomarginal trabeculation, or septal band, which continues toward the anterior papillary muscle through the moderator band[13] [Figures 2 and 4]. The outlet of the RV is a completely muscular sleeve, which supports the leaflets of the pulmonary valve in uniform fashion [Figure 4]. By mid-gestation, the cushions that initially separated the common outflow tract into the right and left ventricular components have muscularized and become the free-standing infundibular sleeve.[14] This is readily seen in the histological sections, with an extracavitary tissue area interposing between the infundibular sleeve and the aortic root [Figures 4 and 5]. In the normal heart, therefore, it is no longer possible to recognize a discrete outlet or “conal” septum.[15] This feature is now of major importance when describing the different types of ventricular septal defect since the outlet septum only becomes recognizable as a discrete entity when divorced from the crest of the apical muscular septum.[16] It, then, forms the leading edge of the free-standing infundibular sleeve, although it can be virtually absent in the setting of the doubly committed and juxtaarterial defect, remaining in some instances as a fibrous raphe.
Morphologically left ventricle
As with its partner, it possesses an inlet, an apical trabecular component, and an outlet [Figure 5]. Moreover, as with the RV, its walls are limited by the AV and ventriculoarterial junctions. The inlet surrounds and supports the mitral valve, which possesses two leaflets, closing along a solitary line of apposition. The leaflets are located murally and anteriorly, with the mural leaflet hinged from the parietal AV junction. The septal hinge is usually toward the atrium when compared to that of the tricuspid valve. The aortic, or anterior, leaflet is almost always in fibrous continuity with the leaflets of the aortic valve [Figure 5], hence its title of aortic leaflet. The key feature of the mitral valve, however, is that its tendinous cords are attached to the paired left ventricular papillary muscles, with no septal attachments. It is also the case that the papillary muscles are located inferoseptally and superolaterally. It is a mistake to describe them as being anteromedial and posterolateral. The apical component of the LV is smoother than that of the RV. It lacks any structure comparable to the septomarginal trabeculation or septal band, and its trabeculations are fine and crisscrossing. Since there is fibrous continuity between the leaflets of the aortic and mitral valves, the outlet of the LV is abbreviated when compared to its right ventricular counterpart [Figures 4 and 5]. As already emphasized, it is not possible, in the normal heart, to identify a discrete “outlet septum.” Instead, there is an extracavitary area of fibroadipose tissue interposing between the aortic root and the free-standing subpulmonary infundibulum. Only the two sinuses of the aortic root supporting the coronary arteries are supported by left ventricular myocardium [Figure 5].
The ventricular septum separates the cavities of the right ventricles and LVss. It is muscular except in its basal portion, which is thin and membranous [Figure 3]. This component forms an integral part of the aortic root but has no relationship to the pulmonary root [Figures 3 and 5]. By mid-gestation, the septal leaflet of the tricuspid valve has usually delaminated to sufficient degree to divide the membranous septum into its AV and interventricular components [Figure 3]. The AV conduction axis penetrates from the apex of the triangle of Koch through the AV component of the membranous septum. The branching component of the axis is then sandwiched between the interventricular part of the membranous septum and the crest of the muscular ventricular septum [Figures 3 and 6]. It is at this location that ventricular septal defects are most frequently encountered, although such defects can also be found within the substance of the muscular septum or between the outflow tracts when there has been failure of muscularization of the subpulmonary infundibulum.[7] The thin fibrous tissue of the membranous septum, however, often underscores a loss of the acoustic signal when insonating the area between the aortic wall and the RV. This can hinder the detection of the typical perimembranous ventricular septal defect.
Figure 6.
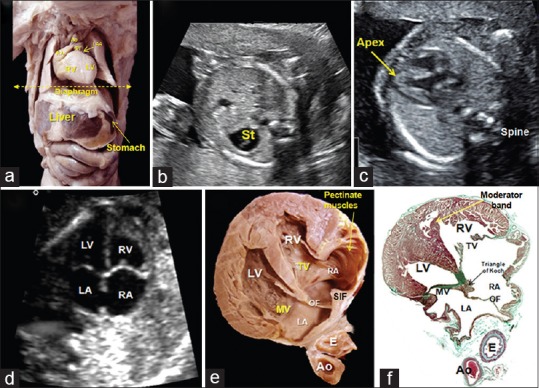
(a) The thorax and abdomen of a 20-week human fetus dissected to show the orientation of the heart in relation to the remaining organs (liver, stomach). The echocardiographic first plane (yellow-dotted line) starting just below the level of the diaphragm. (b) Abdominal echocardiographic view showing the fetal stomach to the left. (c) View of the apex pointing to the left. (d-f) A composite of echocardiogram (d), dissection (e), and histological section (f) illustrating the findings in the four-chamber plane. The LV is dissected anterior to the annulus of the mitral valve. Ao: Aorta, E: Esophagus, LA: Left atrium, LV: Left ventricle, MV: Mitral valve, OF: Oval fossa, PT: Pulmonary trunk, RA: Right atrium, RV: Right ventricle, St: Stomach, SIF: Superior interatrial fold, TV: Tricuspid valve
The arterial segment
Pulmonary trunk
In the normal heart, the PT is supported by the free-standing infundibular sleeve of the RV [Figure 4]. The feature of the pulmonary root is that all three valvular sinuses are uniformly supported by outflow tract myocardium. It branches into the right and left pulmonary arteries [Figures 4 and 7] but continues in fetal life as the ductal arch. It spirals around the aortic root as its branches extend into the mediastinum. The presence of parallel trunks should always alert to the likelihood of abnormal ventriculoarterial connections.
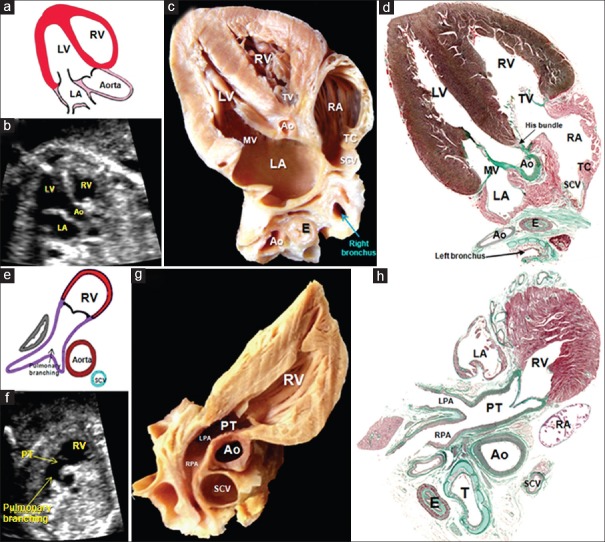
Figure 7.
(a-d) A composite of drawing (a), echocardiogram (b), dissection (c), and histological section (d) illustrating the findings in the third plane: the aortic outflow tract, also called the echocardiographic cut “five-chamber view” showing the aorta leaving the LV in a central position, the subaortic membranous septum, and the area of mitroaortic continuity. (e-h) A composite of drawing (a), echocardiogram (f), dissection (g), and histological section (h) illustrating the fourth plane: the short axis of the right ventricular outflow tract. In this section, T is possible to discern the features of ventriculoarterial concordance, as well as the support of the pulmonary valve and the branching of the PT into the right and left pulmonary arteries. Ao: Aorta, E: Esophagus, LA: Left atrium, LPA: Left pulmonary artery, LV: Left ventricle, MV: Mitral valve, PT: Pulmonary trunk, RA: Right atrium, RPA: Right pulmonary artery, RV: Right ventricle, SCV: Superior caval vein, TC: Terminal crest, TV: Tricuspid valve
Aorta
The Ao emerges from the LV in a central position within the base of the heart. Having ascended into the mediastinum, where it gives rise to the brachiocephalic arteries, it continues as the isthmus, which in the fetus unites with the arterial duct to become the descending Ao. The aortic root itself possesses three sinuses, with the sinuses adjacent to the pulmonary root giving rise to the right and left coronary arteries. It is this pattern of branching that serves to distinguish the Ao from the PT, although on occasion coronary arteries can arise from the pulmonary root. The more distal branches, nonetheless, will always serve as distinguishing features.
The arterial duct
Patency of the arterial duct, or ductus arteriosus, derived from the left sixth pharyngeal arch artery, is a characteristic feature of the fetal heart. The channel closes postnatally, becoming the arterial ligament. During fetal life, in the absence of gas exchange, the lungs receive only a small amount of blood volume pumped from the RV. Most of the blood reaching the PT is directed to the descending Ao through the arterial duct, which carries 60% of the total blood volume. Thus, it is the morphologically RV which maintains the thoracic and abdominal aortic circulations through the arterial duct, whereas the LV maintains the coronary and cerebral circulations through the ascending Ao. Both ventricles and arterial trunks have comparable systemic pressures. A key feature of the fetal echocardiographic interrogation, therefore, is to confirm the patency of the arterial duct. The pathway from the PT toward the descending Ao through the duct is the so-called “ductal arch,” as opposed to “aortic arch.” This is discrete from the aortic arch, which is formed by the ascending Ao and the isthmus. Both arches are reproducible in the fetal echocardiographic longitudinal planes. These two arches, however, are not observed simultaneously in the same longitudinal section. This is because the aortic arch is oblique, and is located cranial to the ductal arch, which is orientated in a more sagittal plane. The two arches are shown simultaneously by taking transverse sections at the level of the arches. This is described as the “ductal V.”[17] It corresponds to the convergence of the ductal and the aortic arches to form the descending thoracic Ao [Figure 8]. In the normal heart, the aortic arch will be left-sided, having the trachea to its right, and the arterial duct to its left.
Figure 8.
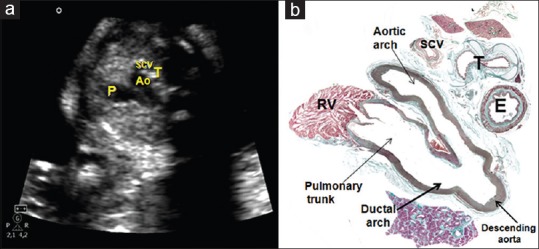
(a and b) A composite of echocardiogram (a) and histological section (b) illustrating a section of the three vessels and trachea (fifth plane). The PT is seen in continuity with the arterial duct. The aorta is in the middle, with the superior vena caval vein to its right side. Behind the caval vein, we see the trachea, and even further posteriorly the esophagus. The arterial duct and the aortic isthmus merge into a “V”. Ao: Aorta, E: Esophagus, PT: Pulmonary trunk, RV: Right ventricle, SCV: Superior caval vein, T: Trachea
Echocardiographic translational cardiac anatomy
Although fetal cardiac anatomy is best approached based on the recognition of the segments, a new echocardiographic approach has been developed. Thus, it is logical to compare the macroscopic and histological views of the heart with the images as routinely produced by those performing ultrasonic interrogation. Use of these views, combined with the knowledge of normal findings, should permit the detection of most cardiac abnormalities. These echocardiographic views, initially described as the “five short axis views,”[18] were subsequently extended to include the arch views.[17] We have simulated these views by making transverse sections starting just below the level of the diaphragm, and moving cranially through the fetal heart [Figure 6].
First plane: The abdominal view
This is the most caudal plane. It is cut transversely just below the diaphragm. It serves to establish the location of the stomach [Figure 6], which usually serves to establish the arrangement of the abdominal organs. When the heart itself is normally located, its apex is on the same side as the stomach. In addition, the abdominal Ao will also be seen to the left, whereas the inferior caval vein is placed normally located anteriorly and to the right of the Ao. In the normal arrangement, the fetal liver will be to the right. Tracing the inferior caval vein will then identify the morphologically RA.
Second plane: The “four-chamber view”
Moving cephalad, this section usually provides a view of the heart from the apex, although there are variations in lateral planes. This section is particularly important. It reveals abnormalities in the location of the ventricular mass and its axis, including the relative size of the contained ventricles [Figure 7]. It also permits the exclusion of any pericardial effusions. More posteriorly, the section shows the relative size of the atrial chambers and should permit identification of at least 2 pulmonary veins [Figure 7]. The sections show the precise structure of the atrial septum, with the flap valve on the left side of the superior interatrial fold. The oval foramen is normally at least 1/3 of the dimensions of the rims of the OF as seen in the RA. In the normal arrangement, it should also be possible to identify the connections of the caval veins to the morphologically RA. Assessment of the ventricular mass will show that, in the normal situation, the right-sided ventricle is more trabeculated than its left-sided partner, with the hinge of the septal leaflet of the tricuspid valve inserted apically when compared to the attachment of the left-sided AV valve [Figure 7]. Analysis of the patterns of ventricular filling reveals the so-called cardiac crux and permit functional assessment of myocardial contractility. Care must be taken, nonetheless, not to confuse the inferior AV groove with the attachments of the AV valvular leaflets.[19] In best circumstances, it should be feasible to confirm the integrity of the ventricular septum, identifying its muscular and membranous components, although we have already emphasized the potential caveat of acoustic “dropout” at the location of the membranous component. In the setting of perimembranous ventricular septal defects, nonetheless, some part of the aortic root, of necessity, will be supported by the RV. This means that careful analysis could show the presence of even minimal overriding of the aortic root. Although the four-chamber view is clearly of great significance, it is only when combined with the more cranial sections that it becomes possible to appreciate the presence of abnormal ventricular-arterial connections, and so-called “conotruncal” malformations involving the ventricular outflow tracts.
Third plane: The aortic outflow tract, also called the “five-chamber view”
This plane, taken slightly cranial to the four-chamber plane, shows the outlet of the LV. The aortic root, in the normal setting, is deeply wedged between the mitral valve and the muscular ventricular septum [Figure 7]. Careful interrogation will show the continuity between the right wall of the aortic root and the membranous septum. As discussed, absence of any aortic override will serve to confirm the integrity of the ventricular septum [Figure 7]. The section should also reveal the presence of fibrous continuity between the leaflets of the aortic and mitral valves in the inner heart curvature.
Fourth plane: The short axis of the right ventricular outflow tract
This section will show the origin of the PT from the free-standing muscular subpulmonary infundibulum. In the setting of concordant ventricular-arterial connections, the right ventricular outflow tract will cross the aortic root at an angle of about 45°. The PT can be traced into its bifurcation into the right and left pulmonary artery, but the major pathway will be seen to continue as the arterial duct. It is insonation at this level that will show the features of the pulmonary root [Figure 7].
Fifth plane: The three vessel and tracheal view
This is the most cephalad of the transverse views. Running from left to right, and from anterior to posterior, the PT will be seen as it extends into the mediastinum, giving rise to the pulmonary arteries, and continuing as the arterial duct. The other vessels in this tripartite view are the Ao and the superior caval vein [Figure 8]. In the normal situation, the trachea will be seen, lying posterior to the caval vein but anterior to the spine. It is in this place that the arterial duct and the isthmus is seen converging to form a “V” [Figure 8]. If the situation is normal, then the PT will be the largest vessel, with the Ao being second largest and the caval vein the smallest. As yet, it has not proved possible to image the cardiac conduction system echocardiographically, although some of its components, such as the penetrating AV bundle, are readily recognized in the histological sections of the heart,[20,21] indirect echocardiographic data, nonetheless, are available to permit inferences to made regarding its normal morphology and function, such as heart rate and AV synchrony.
DISCUSSION
Malformations of the heart are one of the most frequent congenital malformations. Reports of their prevalence around the world range from 2.1 to 12.3 for every 1000 newborns. It has now been recognized for several decades that a standard fetal ultrasonic scan can detect the most severe forms of congenital heart disease, and thus help in planning the delivery of the neonates in specialized centers. This has been shown to improve the survival of these babies.
Our study adds clear and detailed images of normal fetal hearts that have been dissected according to the standard fetal echocardiographic examination, extending the anatomic-echocardiographic correlations that underscored the development of fetal cardiology as a specialty. This normality needs to be appreciated by all those conducting prenatal scans. Any deviation of these findings as seen in the standardized views should alert toward the possibility of an underlying congenital cardiac malformation. Our analysis of the new findings also serves to resolve ongoing controversies regarding normal cardiac anatomy. Taken together, our findings should help in decreasing the morbidity and mortality due to congenital cardiac diseases, which continue to rank as one of the major causes of mortality in childhood throughout the World.
There was considerable skepticism when it was first suggested that it might prove possible echocardiographically to interrogate the heart during fetal life, and to recognize the presence of cardiac malformations. One of the first steps of the initial innovators, therefore, was to carry out echocardiographic-anatomic correlations so as to prove that the various cardiac components could, indeed, be recognized in the fetal scans.[1] Not only has the quality of the images produced during fetal echocardiographic scanning improved remarkably since those early investigations but also has the ability to make anatomic correlations. As we have shown in our current investigation, not only do the histological sections emulate the initial cuts made using gross sectioning, but they also demonstrate with exquisite accuracy such features as the nature of the so-called “septum secundum,” the unequivocal presence of the separate walls of the coronary sinus, and the extent ofthe free-standing muscular subpulmonary infundibular sleeve. Over and above that, assessment of the specimens reveals that each cardiac chamber has its own intrinsic features, now permitting atriums and ventricles to be distinguished one from the other without taking recourse to additional features that themselves might be variable. Thus, at least when assessing the specimens, the so-called morphological method is seen to be just as valuable in the analysis of the fetal heart as in the postnatal situation. It remains to be seen whether, with ongoing improvements in technology, features such as the extent of the atrial pectinate muscles will be visible in the echocardiographic scans. Other features, such as the presence of the coronary sinus in the AV groove, nonetheless, should prove just as useful in determining atrial arrangement as ascertaining the presence of normal venoatrial connections. It remains an obvious fact, however, that such features as venoatrial connections and the morphology of the ventricular inlets should be used as part of the determinant of normality when they are present. In the final analysis, it is the intrinsic features that remain paramount. It is, then, also axiomatic that these features will not reveal themselves unless actively sought. With regard to the arterial segment, it remains the branching pattern of the arterial trunks that serves to distinguish an Ao from a PT from a common arterial trunk. Fortunately, these patterns of branching are particularly well seen during fetal interrogation. It should now be entirely feasible, therefore, to identify those problems afflicting the arterial roots, the ventriculoarterial junctions, and the ventricular outflow tracts. Taken together, it is our belief that our correlations will serve as the guide to diagnosis, in the future, and even during fetal life, of the majority of congenital cardiac malformations. It is also our hope that since the characteristics of the normal heart is the gold standard for recognition of abnormalities, our correlations will function as a teaching tool for not only pediatric cardiologists, but also obstetricians and pathologists.
CONCLUSIONS
Anatomy remains a core discipline in the understanding of echocardiography. In this investigation, we have translated the planes used during routine fetal scanning to the anatomic sections obtained from mid-gestational hearts. Experience has shown that it was the standardization of routine planes of interrogation that established echocardiography as a screening tool for the diagnosis during fetal life of congenital cardiac disease. It is axiomatic; therefore, that knowledge of the underlying anatomy of the heart is just as fundamental as the ongoing development of the techniques used for imaging. It could well be that, in the future, ongoing improvements in imaging will not only permit recognition of all the nuances demonstrated in our histological sections but also the microscopic anatomy of the conduction system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Allan LD, Tynan MJ, Campbell S, Wilkinson JL, Anderson RH. Echocardiographic and anatomical correlates in the fetus. Br Heart J. 1980;44:444–51. doi: 10.1136/hrt.44.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadler TW. In: Langman Medical Embryology. 12th ed. New York: Lippincott Williams & Wilkins, Wolters Kluwer Health, SA.; 2012. Cardiovascular system; pp. 162–200. [Google Scholar]
- 3.Van Praagh R. The segmental approach clarified. Cardiovasc Intervent Radiol. 1984;7:320–5. doi: 10.1007/BF02625121. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RH, Ho SY. Continuing Medical Education. Sequential segmental analysis – Description and categorization for the millenium. Cardiol Young. 1997;7:98–116. [Google Scholar]
- 5.van Praagh R, David I, Wright GB, van Praagh S. Large RV plus small LV is not single RV. Circulation. 1980;61:1057–9. [PubMed] [Google Scholar]
- 6.Sharland G. Abnormalities of cardiac size, position and situs. In: Gurleen Sharland G, editor. Fetal Cardiology Simplified – A Practical Manual. Shrewsbury SY: TFM Publishing Limited; 2013. pp. 55–66. [Google Scholar]
- 7.Anderson RH, Spicer DE, Brown NA, Mohun TJ. The development of septation in the four-chambered heart. Anat Rec (Hoboken) 2014;297:1414–29. doi: 10.1002/ar.22949. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera JA, Sánchez-Quintana D. Cardiac anatomy: What the electrophysiologist needs to know. Heart. 2013;99:417–31. doi: 10.1136/heartjnl-2011-301154. [DOI] [PubMed] [Google Scholar]
- 9.Loomba RS, Hlavacek AM, Spicer DE, Anderson RH. Isomerism or heterotaxy: Which term leads to better understanding? Cardiol Young. 2015;25:1037–43. doi: 10.1017/S1047951115001122. [DOI] [PubMed] [Google Scholar]
- 10.Knauth A, McCarthy KP, Webb S, Ho SY, Allwork SP, Cook AC, et al. Interatrial communication through the mouth of the coronary sinus. Cardiol Young. 2002;12:364–72. doi: 10.1017/s104795110001297x. [DOI] [PubMed] [Google Scholar]
- 11.Van Praagh S, Carrera ME, Sanders SP, Mayer JE, Van Praagh R. Sinus venosus defects: Unroofing of the right pulmonary veins – Anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128:365–79. doi: 10.1016/0002-8703(94)90491-x. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Quintana D, López-Mínguez JR, Macías Y, Cabrera JA, Saremi F. Left atrial anatomy relevant to catheter ablation. Cardiol Res Pract. 2014;2014:289720. doi: 10.1155/2014/289720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso-González R, Dimopoulos K, Ho S, Oliver JM, Gatzoulis MA. The right heart and pulmonary circulation (IX). The right heart in adults with congenital heart disease. Rev Esp Cardiol. 2010;63:1070–86. doi: 10.1016/s1885-5857(10)70211-5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RH, Mohun TJ, Spicer DE, Bamforth SD, Brown NA, Chaudhry B, et al. Myths and realities relating to development of the arterial valves. J Cardiovasc Dev Dis. 2014;1:177–200. [Google Scholar]
- 15.Saremi F, Gera A, Ho SY, Hijazi ZM, Sánchez-Quintana D. CT and MR imaging of the pulmonary valve. Radiographics. 2014;34:51–71. doi: 10.1148/rg.341135026. [DOI] [PubMed] [Google Scholar]
- 16.Mostefa-Kara M, Bonnet D, Belli E, Fadel E, Houyel L. Anatomy of the ventricular septal defect in outflow tract defects: Similarities and differences. J Thorac Cardiovasc Surg. 2015;149:682–80. doi: 10.1016/j.jtcvs.2014.11.087. [DOI] [PubMed] [Google Scholar]
- 17.Yagel S, Arbel R, Anteby EY, Raveh D, Achiron R. The three vessels and trachea view (3VT) in fetal cardiac scanning. Ultrasound Obstet Gynecol. 2002;20:340–5. doi: 10.1046/j.1469-0705.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Yagel S, Cohen SM, Achiron R. Examination of the fetal heart by five short-axis views: A proposed screening method for comprehensive cardiac evaluation. Ultrasound Obstet Gynecol. 2001;17:367–9. doi: 10.1046/j.1469-0705.2001.00414.x. [DOI] [PubMed] [Google Scholar]
- 19.Nadal A, Martínez JM. Embriogénesis y anatomía normal del corazón. In: Galindo J, Gratacós E, Martínez JM, editors. Cardiologia Fetal. Madrid: Editorial Marban; 2014. pp. 7–15. [Google Scholar]
- 20.Sánchez-Quintana D, Yen Ho S. Anatomy of cardiac nodes and atrioventricular specialized conduction system. Rev Esp Cardiol. 2003;56:1085–92. doi: 10.1016/s0300-8932(03)77019-5. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Quintana D, Picazo-Angelín B, Cabrera A, Murillo M, Cabrera JA. Koch's triangle and the atrioventricular node in Ebstein's anomaly: Implications for catheter ablation. Rev Esp Cardiol. 2010;63:660–7. doi: 10.1016/s1885-5857(10)70140-7. [DOI] [PubMed] [Google Scholar]