Sir,
A 27-day-old male born at term and weighing 2.6 kg presented with heart failure since the 2nd day of life. Clinical examination revealed a tachypneic, emaciated baby with features of hyperdynamic circulation and a systolic precordial murmur, with peripheral oxygen saturation of 95%. On transthoracic echocardiography (TTE) with color Doppler interrogation, both ventricles and pulmonary arteries (PAs) were dilated. A large (5 mm) nonrestrictive aortopulmonary window (APW) was seen well separated from the semilunar valves, left coronary artery (LCA), and PA confluence with predominantly left-to-right shunt [Figure 1]. It was decided to attempt transcatheter closure (TCC) owing to favorable anatomy and high surgical risk in this sick neonate. Under general anesthesia, vascular access was obtained in the right femoral vein (6 French) and left common femoral artery (4 French) with 7.5 cm hydrophilic coated sheaths (Terumo, Japan). Unfractionated heparin at 100 U/kg was administered. The mean aortic (Ao) and PA pressures were 31 and 29 mm Hg, respectively. Ascending aortogram confirmed a 4.8 mm APW located 5 mm away from the left aortic cusp [Figure 2]. The defect was crossed antegradely from the PA using 6 French Judkins Right catheter and 0.035” angled tip hydrophilic-coated glide wire which was parked in the descending thoracic aorta (DTA). The femoral venous sheath was exchanged with a 48 cm Check-Flo Performer Mullins-type guiding sheath with a side flushing port (Cook Medical Inc., USA) and its end parked in the DTA. An Amplatzer Duct Occluder (ADO) (St. Jude Medical, St. Paul, MN, USA) 8–6 device was loaded on a delivery cable and advanced through the sheath [Figure 3]. The aortic disc was partially deployed (mushroomed) in the aortic arch. The assembly was then carefully withdrawn toward the ascending aorta till the disc assumed its natural shape and a gentle tug was felt. This was followed by rapid deployment of the entire device. After TTE and angiographic confirmation of optimum placement [Figure 4] and ruling out any PA obstruction or impingement on LCA, the device was released from the delivery cable. Immediate rise in the aortic pressure and a modest fall in the PA pressure were noted. The procedure time was 70 min with 12 min of fluoroscopy time (radiation dose of 27.8 mGy). Postprocedure recovery was uneventful with preserved lower limb arterial pulses. Aspirin at 3 mg/kg was advised for 3 months, and the baby was discharged on the 5th postprocedure day. TTE before discharge [Figure 5] showed a well-positioned device with no residual shunt or great vessel obstruction findings which persisted at the 2 months’ follow-up visit. This was also accompanied by a gratifying doubling of the body weight to 5.2 kg. Growth trends and TTE imaging [Figure 6] continued to be favorable at the last follow-up at 1 year of age.
Figure 1.

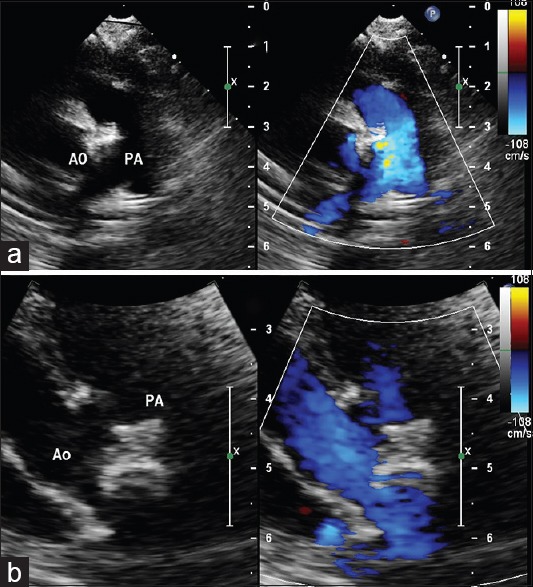
Transthoracic echocardiography with color Doppler demonstrates a large nonrestrictive aortopulmonary window (*) in long (a) and short (b) axes situated well away from the semilunar valves and the pulmonary artery bifurcation
Figure 2.
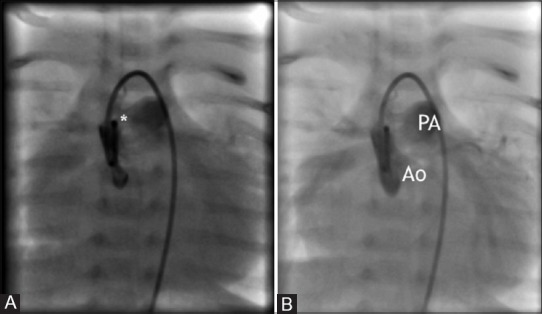
Ascending aortogram with manual injection of contrast medium demonstrates an enlarged cardiac silhouette with a large 4.8 mm aortopulmonary window (*, panel A) with near simultaneous opacification of the pulmonary artery (panel B)
Figure 3.

Loading of the Amplatzer Duct Occluder through the Mullin sheath parked in descending thoracic aorta
Figure 4.

Angiogram confirming optimum device placement across the aortopulmonary window before release
Figure 5.

Transthoracic echocardiography at discharge showing no residual shunt or great vessel obstruction
Figure 6.
Transthoracic echocardiography at 1-year follow-up short axis (a) and long axis (b)
APW is rare, accounting for 0.2%–0.6% of all congenital cardiac defects.[1] It carries high morbidity and mortality if left untreated. Closure is indicated in all cases. Although surgery is the mainstay of treatment, TCC could be considered for isolated defects located away from both semilunar valves and PA bifurcation – the Type IV or “intermediate” type in the classification scheme recommended by the Society of Thoracic Surgeons Congenital Heart Surgery Database Committee. There are a few reports in the literature of TCC using various devices.[2,3,4] However, the above case, to the best of our knowledge, represents the youngest and smallest patient with successful TCC for AP window. The ADO was chosen owing to wide experience with this device and its suitability for anatomy and size of defect. Antegrade crossing from the PA can prove to be difficult and retrograde crossing with formation of an arteriovenous railroad is often necessary. Conceptually, double-disc devices with a “short” waist would be ideal for a “window”-type defect, causing less protrusion in the PA. However, a double-disc device would also be a compromise due to a broader diameter of the PA disk resulting in “more metal” in the PA. For instance, for a 6 mm device size, although the waist/device lengths of the Atrial Septal Occluder and ADO II would be 3 mm and 4 mm (compared to 7 mm for the ADO), the device diameters would have been 14 mm and 12 mm (as against 6 mm for the ADO used in the present case). This case demonstrates the feasibility of nonsurgical management in carefully selected patients in the neonatal age group. A heart team approach and meticulous measures to minimize blood loss, contrast, and radiation dose are mandatory for good outcomes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kutsche LM, Van Mierop LH. Anatomy and pathogenesis of aortopulmonary septal defect. Am J Cardiol. 1987;59:443–7. doi: 10.1016/0002-9149(87)90953-2. [DOI] [PubMed] [Google Scholar]
- 2.Trehan V, Nigam A, Tyagi S. Percutaneous closure of nonrestrictive aortopulmonary window in three infants. Catheter Cardiovasc Interv. 2008;71:405–11. doi: 10.1002/ccd.21366. [DOI] [PubMed] [Google Scholar]
- 3.Stamato T, Benson LN, Smallhorn JF, Freedom RM. Transcatheter closure of an aortopulmonary window with a modified double umbrella occluder system. Cathet Cardiovasc Diagn. 1995;35:165–7. doi: 10.1002/ccd.1810350218. [DOI] [PubMed] [Google Scholar]
- 4.Trehan V, Nigam A. Percutaneous device closure of nonrestrictive aortopulmonary window in a 4 months’ infant. Indian Heart J. 2008;60:254–6. [PubMed] [Google Scholar]


