Abstract
Purpose
Cardiometabolic disease remains a leading cause of morbidity and mortality in developed nations. Consequently, identifying and understanding factors associated with underlying pathophysiological processes leading to chronic cardio metabolic conditions is critical. Metabolic health, arterial elasticity, and insulin sensitivity (SI) may impact disease risk, and may be determined in part by myofiber type. Therefore, the purpose of this study was to test the hypothesis that type I myofiber composition would be associated with high SI, greater arterial elasticity, lower blood pressure, and blood lipids; whereas, type IIx myofibers would be associated with lower SI, lower arterial elasticity, higher blood pressure, blood lipids.
Methods
Muscle biopsies were performed on the vastus lateralis in 16 subjects (BMI = 27.62 ± 4.71 kg/m2, age = 32.24 ± 6.37 years, 43% African American). The distribution of type I, IIa, and IIx myofibers was determined via immunohistochemistry performed on frozen cross-sections. Pearson correlation analyses were performed to assess associations between myofiber composition, SI, arterial elasticity, blood pressure, and blood lipid concentrations.
Results
The percentage of type I myofibers positively correlated with SI and negatively correlated with systolic blood pressure SBP, diastolic blood pressure, and mean arterial pressure (MAP); whereas, the percentage of type IIx myofibers were negatively correlated with SI and large artery elasticity, and positively correlated with LDL cholesterol, SBP, and MAP.
Conclusions
These data demonstrate a potential link between myofiber composition and cardiometabolic health outcomes in a cohort of premenopausal women. Future research is needed to determine the precise mechanisms in which myofiber composition impacts the pathophysiology of impaired glucose and lipid metabolism, as well as vascular dysfunction.
Keywords: Myofiber, Insulin sensitivity, Arterial elasticity, Blood lipids, Cardiometabolic health
Introduction
The prevalence of cardiometabolic diseases continues to rise and remains the leading cause of morbidity and mortality in modern societies. Insulin resistance, obesity, hypertension, and dyslipidemia are known to cluster as risk factors and often coexist in individuals at risk for cardiometabolic diseases. Therefore, it is critical to understand and identify factors that are associated with the underlying pathophysiological processes that lead to chronic cardiometabolic conditions.
It has become increasingly clear that skeletal muscle function is important for many aspects of cardiometabolic health, including insulin-stimulated glucose disposal, vascular function, and lipid metabolism (Stuart et al. 2013; Laughlin et al. 2015; Olver et al. 2015). Skeletal muscle is a heterogeneous tissue consisting of three general phenotypes, which include oxidative type I myofibers (type I), oxidative/glycolytic type IIa myofibers (IIa), and glycolytic type IIx myofibers (IIx), that differ drastically in function, mitochondrial density, and metabolic properties. It is well known that type I myofibers have higher concentrations of mitochondria, greater oxidative phosphorylation capacity, and capillary density compared to type II myofibers that are characterized by low mitochondrial density, low oxidative phosphorylation capacity, and low capillary density (Zierath and Hawley 2004). The role of skeletal muscle fiber type and the etiology of cardiometabolic diseases have yet to be clearly characterized; however, several studies have shown fewer percentage of type I myofibers in obese and type 2 diabetic individuals compared to lean individuals, suggesting a potential link between fiber type and metabolic health. Further support comes from both rodent studies, which have shown an increased insulin-stimulated glucose uptake in type I myofibers compared to type II myofibers (James et al. 1985; Ploug et al. 1987), and human studies performed in vitro (Zierath et al. 1996) and in vivo (Stuart et al. 2013; Oberbach et al. 2006) which have shown positive correlations between type I myofibers and whole-body insulin sensitivity and greater insulin-stimulated glucose uptake in type I myofibers. Furthermore, Albers et al. recently showed a greater capacity for glucose uptake, phosphorylation and oxidation, and glycogen synthesis in human type I myofibers compared to type II myofibers (Albers et al. 2015). It is therefore conceivable that myofiber composition may impact the risk and development of insulin resistance and type 2 diabetes.
Similar to the relationship between myofiber type distribution and insulin sensitivity, previous investigations have also demonstrated a negative correlation between mean arterial blood pressure and the percentage of type I myofibers (Juhlin-Dannfelt et al. 1979), and a trend toward greater percentage of type II myofibers in individuals with hypertension compared to normotensive controls (Frisk-Holmberg et al. 1983). Thus, there is considerable evidence that skeletal muscle myofiber composition may be linked to blood pressure regulation. Hypertension is considered to be one of the most common modifiable risk factors to reduce mortality; however, the etiology of hypertension is complex and it has been shown that many hypertension-related cardiovascular diseases manifest well before clinically diagnosed hypertension occurs (van Bussel et al. 2011). Endothelial dysfunction and reduced arterial elasticity are both independent predictors of coronary heart disease and stroke, and often present well before clinically diagnosed hypertension (blood pressure ≥140/90 mmHg) (van Bussel et al. 2011). To date, we are aware of no studies conducted in women and only one study in men that has examined associations between muscle myofiber composition and arterial stiffness (Rönnback et al. 2007). This study did not find any associations between myofiber type distribution with arterial stiffness or endothelial function (Groop et al. 1991). However, several studies have shown inverse associations between skeletal muscle mass and arterial stiffness and positive associations between skeletal muscle mass and blood pressure (Lee et al. 2014; Kim et al. 2011; Ochi et al. 2010; Loenneke et al. 2013), suggesting a potential link between skeletal muscle and regulation of vascular hemodynamics.
Skeletal muscle is a critical determinant for overall physical function; however, it is also associated with the risk of many pathological states. Therefore, the purpose of this study was to identify associations between skeletal muscle myofiber distribution with insulin sensitivity, multiple hemodynamic and arterial elasticity measures, and blood lipids in a cohort of healthy premenopausal women. Given that previous research has speculated that higher percentage of type II fibers may be a predisposing factor to cardiometabolic disease, we hypothesized that type I fibers would be associated with greater insulin sensitivity, more favorable hemodynamic and arterial elasticity measures, and lower blood lipids, and that type IIa and IIx myofibers would be associated with lower insulin sensitivity, less favorable hemodynamic and arterial elasticity measures, and higher blood lipids.
Methods
Participants
This cross-sectional study consisted of 16 pre-menopausal women (BMI = 27.62 ± 4.71 kg/m2, age = 32.24 ± 6.37 years). Participants reported normal menstrual cycles and were not taking oral contraceptives or any medications known to influence glucose metabolism, blood pressure, or lipid metabolism. Additional inclusion criteria were normotensive, non-smoker, sedentary as defined by participating in any exercise-related activities less than one time per week, and normoglycemic as evaluated by postprandial glucose response to a 75 g oral glucose tolerance test. All participants provided written informed consent prior to inclusion. All testing was conducted during the follicular phase of the menstrual cycle. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
Body composition measurements
Total and regional body composition were determined by dual-energy X-ray absorptiometry (iDXA, GE-Lunar, Madison, WI, USA). Participants wore light clothing and remained supine in compliance with normal testing procedures. Scans were analyzed with ADULT software, LUNAR-DPX-L version 1,33 (GE Medical Systems Lunar).
Myofiber type distribution
Muscle tissue specimens were collected from the vastus lateralis by percutaneous needle biopsy under local anesthesia (1% lidocaine) with a 5-mm Bergstrom-type biopsy needle using established procedures (Bamman et al. 2004) in the Clinical Research Unit (CRU) of the UAB Center for Clinical and Translational Science. All visible connective and adipose tissues were removed from the biopsy samples with the aid of a dissecting microscope. Portions used for immunohistochemistry were mounted cross-sectionally on cork in optimum cutting temperature mounting medium mixed with tragacanth gum, frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C. The relative distribution of myofiber types I, IIa, and IIx were determined by myosin heavy chain immunohistochemistry using our well-established protocol (Kim et al. 2005).
Arterial elasticity evaluation
Hemodynamic and arterial elasticity variables; systolic (SBP), diastolic (DBP), and mean (MAP) arterial blood pressures, pulse rate, large and small artery elasticity (LAE) (SAE), total vascular impedance (TVI) were measured using non-invasive pulse wave analysis (HDI/Pulse Wave TM CR-2000, Hypertension Diagnostics, Eagan, MN). The arterial pulse wave analysis of the radial artery is based on a modified Windkessel model that allows evaluation of the large conduit arteries and the small microcirculatory arteries (Cohn et al. 1995). Briefly, with participants in the seated position a solid-state pressure transducer array (tonometer) was placed over the radial artery of the dominant arm to record the pulse contour. The waveform was calibrated by the oscillometric method. Once a stable measurement was achieved, a 30-s analog tracing of the radial waveform was digitized at 200 samples per second. Before, during, and after the waveform assessment, an automated oscillatory blood pressure measurement was taken on the contralateral arm. The first maximum waveform observed represents the action of the arteries following cardiac ejection and reflects the large arteries, whereas the second rebound wave reflects compliance of the smaller arteries. TVI was determined from the modified Windkessel model evaluated at the frequency of the measured heart rate (Hales 1964).
Hyperinsulinemic euglycemic clamp
The clamp study was performed at the UAB Clinical Research Unit (CRU). Subjects were admitted to the CRU at 8:00 AM after an overnight fast. A catheter was placed in the antecubital space of the right arm for repeated blood draws, The IV line was kept open with infusion of normal saline (0.9% NaCl; pH 7.4). A second catheter with ports for insulin and glucose was inserted into the antecubital space of the left arm. Continuous infusion of human regular insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) was started at a rate of 40 mU m−2 min−1 and continued for 120 min. Plasma glucose was measured with a YSI glucose analyzer at 5-min intervals throughout the clamp. Euglycemia was targeted for 90 mg/dL by variable infusion of 20% D-glucose. Insulin-stimulated glucose disposal rates (M-value) were calculated as the average value during the final 30 min of insulin infusion. M-value is the glucose infusion rate per kg body weight per minute (mg/kg/min). The glucose clamp-derived index of insulin sensitivity (SI) was defined as M/(G × ΔI) corrected for body weight (where M is the steady-state blood glucose concentrations (milligrams per dL), and ΔI is the difference between basal and steady-state plasma insulin concentrations (microunits per mL).
Laboratory analyses
Assays were performed in the DRC human physiology core. Serum glucose, total cholesterol, high density lipoprotein, and triglycerides were measured using an automated glucose analyzer (Sirrus analyzer; Stanbio Laboratory, Boerne, TX, USA), and serum insulin was measured using immunofluorescent methods with an AIA-600 II analyzer (TOSOH Bioscience, South San Francisco, CA, USA) as per manufacturers’ instructions. LDL was calculated using the Friedewald formula.
Statistical analyses
Descriptive characteristics are reported as means and standard deviations. All variables were evaluated for residual normality and logarithmic transformations were performed when appropriate. Simple Pearson correlations were used to examine associations between myofiber types I, IIa, IIx with SI, arterial elasticity measures, and blood lipids. Partial correlations, controlling for the confounding effects of percent body fat, were also performed for each variable. For all analyses, a P value less than 0.05 was deemed statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 23.0, Chicago, IL, USA).
Results
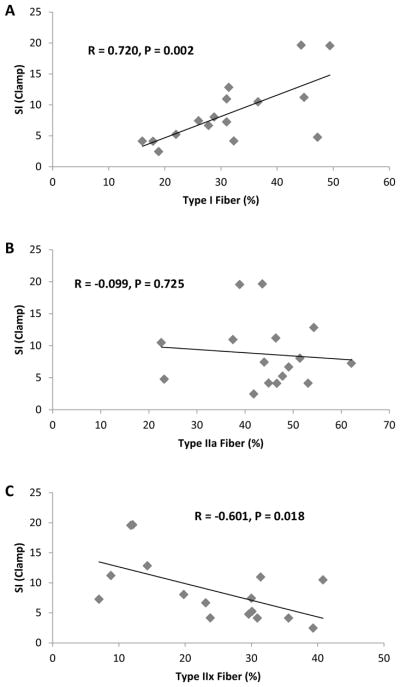
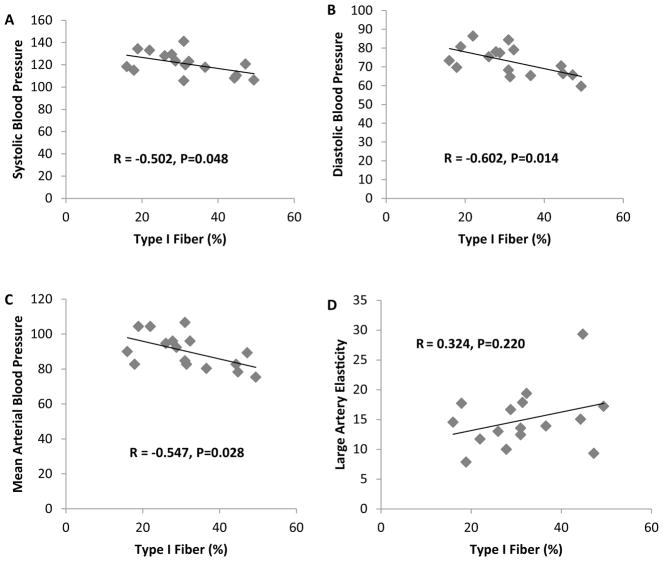
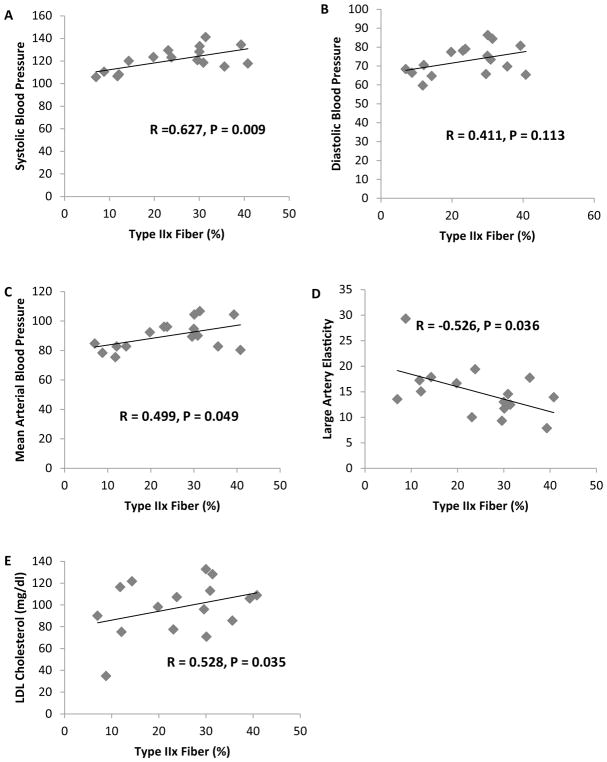
Descriptive statistics are presented in Table 1. Muscle myofiber distribution is presented in Fig. 1. Pearson correlations analyses revealed a significant association between SI and % type I myofibers and a negative correlation between SI and % type IIx myofibers (P < 0.05) (Fig. 2). No significant correlation was observed between SI and % type IIa myofibers. Additionally, partial correlation analyses revealed no differences for any of the results when adjusting for percent body fat (type I myofibers and SI, R = 0.769 and P = 0.001) (type IIx myofibers and SI, R = −0.608 and P = 0.021). A significant negative correlation was observed between type I myofibers and SBP, DBP, and MAP (P < 0.05) (Fig. 3). Additionally, a significant positive association was observed between type IIx myofibers and SBP, MAP, and LDL cholesterol, and a significant negative association was found between type IIx myofibers and LAE (P < 0.05) (Fig. 4). No significant associations were observed between type IIa myofibers and any of the variables measured.
Table 1.
Subject characteristics (mean ± SD)
| Age (years) | 32.2 ± 6.4 |
| Body weight (kg) | 75.2 ± 12.9 |
| BMI (kg/m2) | 27.6 ± 4.7 |
| Body fat (%) | 44.1 ± 6.4 |
| Cholesterol (mg/dL) | 180.6 ± 37.3 |
| Triglycerides (mg/dL) | 100.4 ± 41.6 |
| HDL (mg/dL) | 62.9 ± 18.6 |
| LDL (mg/dL) | 97.6 ± 25 |
| Fasting glucose (mg/dl) | 89.3 ± 9.5 |
| Fasting insulin (μIU/mL) | 9.9 ± 5.9 |
| Insulin sensitivity (10−4 dL kg−1 min−1/(μU/mL)) | 8.9 ± 5.2 |
| Type I myofiber (%) | 31.6 ± 10.5 |
| Type IIa myofiber (%) | 44.2 ± 10.3 |
| Type IIx myofiber (%) | 24.3 ± 10.9 |
| SBP (mmHg) | 120.9 ± 10.5 |
| DBP (mmHg) | 72.7 ± 7.8 |
| MAP (mmHg) | 90 ± 9.7 |
| Pulse rate (BPM) | 76.7 ± 11.1 |
| LAE (mL/mmHg × 10) | 14.9 ± 5.0 |
| SAE (mL/mmHg × 100) | 6.8 ± 1.9 |
| TVI (dyne/s/cm−5) | 119.4 ± 29.8 |
SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, LAE large artery elasticity, SAE small artery elasticity, TVI total vascular impedance
Fig. 1.
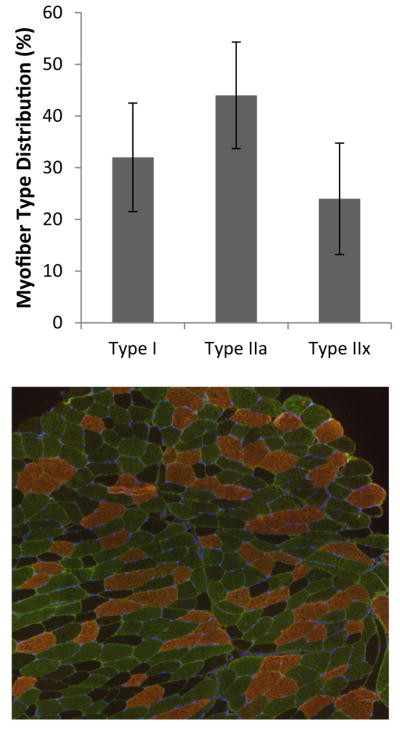
Relative distribution of myofibers by type (I, IIa, and IIx), and representative immunohistological image (type I, copper; type IIa, green; and type IIx, dark/negative) (color figure online)
Fig. 2.
Correlation analyses between a SI and type I fiber %; b SI and type IIa fiber %; c SI and type IIx fiber %
Fig. 3.
Correlation analyses between a type I fiber % and SBP; b type I fiber % and DBP; c type I fiber % and MABP; d type I fiber % and LAE
Fig. 4.
Correlation analyses between a type IIx fiber % and SBP; b type IIx fiber % and DBP; c type IIx fiber % and MABP; d type IIx fiber % and LAE; e type IIx fiber % and LDL cholesterol
Pearson correlation analyses also revealed several correlations between vascular hemodynamic measures, blood lipids, and SI (Table 2). SI was negatively correlated with MAP and TVI, and positively correlated with SAE (P < 0.05). LAE was negatively correlated LDL cholesterol, SBP, MAP, and TVI (P < 0.05).
Table 2.
Pearson correlation matrix for SI, blood lipids, and vascular hemodynamic measures
| SI | Tchol | Trig | HDL | LDL | SBP | DBP | MAP | LAE | SAE | TVI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | 1 | 0.081 | −0.018 | 0.412 | −0.176 | −0.481 | −0.471 | −0.536 | 0.397 | 0.553 | −0.523 |
| Tchol | 1 | 0.146 | 0.754 | 0.881 | 0.208 | −0.038 | 0.123 | −0.481 | −0.203 | 0.230 | |
| Trig | 1 | −0.135 | −0.015 | −0.348 | −0.222 | −0.132 | −0.234 | 0.051 | 0.148 | ||
| HDL | 1 | 0.427 | 0.108 | 0.004 | 0.038 | −0.186 | 0.077 | −0.063 | |||
| LDL | 1 | 0.345 | 0.014 | 0.198 | −0.500 | −0.376 | 0.247 | ||||
| SBP | 1 | 0.821 | 0.915 | −0.511 | −0.489 | 0.585 | |||||
| DBP | 1 | 0.939 | −0.380 | −0.211 | 0.360 | ||||||
| MAP | 1 | −0.568 | −0.421 | 0.622 | |||||||
| LAE | 1 | 0.413 | −0.757 | ||||||||
| SAE | −0.639 | ||||||||||
| TVI | 1 |
Bold values denote significance (* P < 0.05)
SI insulin sensitivity, Tchol total cholesterol, Trig triglycerides, HDL high density lipoproteins, LDL low density lipoproteins, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, LAE large artery elasticity, SAE small artery elasticity, TVI total vascular impedance
Discussion
The purpose of this study was to identify associations of skeletal muscle myofiber distribution with insulin sensitivity, multiple hemodynamic and arterial elasticity measures, and blood lipids in a cohort of healthy premenopausal women. The main findings were that: (a) type I myofibers were associated with greater SI and lower blood pressure measures; (b) type IIx myofibers were associated with lower SI and LAE, and higher blood pressure and LDL cholesterol measures and (c) SI was associated with healthier measures of vascular hemodynamics, and greater LAE was associated with lower LDL cholesterol and lower MAP, SBP, and TVI. These observations confirm and extend previous investigations that have demonstrated a possible role of myofiber composition and cardiometabolic disease risk factors (James et al. 1985; Ploug et al. 1987; Albers et al. 2015; Juhlin-Dannfelt et al. 1979; Frisk-Holmberg et al. 1983). Our data revealed distinct differences in the potential role of skeletal muscle myofiber type and cardiometabolic health. We showed that type I myofibers, characterized by higher oxidative capacity and capillary density, are associated with a healthier cardiometabolic phenotype, whereas type IIx myofibers were associated with a less healthy cardiometabolic phenotype. To our knowledge, this is also the first study to show an association between type IIx myofibers and lower LAE and higher LDL cholesterol, demonstrating a potential link between myofiber composition with vascular remodeling and LDL concentrations. These observations suggest that myofiber type composition is implicated in numerous health outcomes and may perform a larger role in regulating pathophysiological processes related to health than previously considered.
Type 2 diabetes and insulin resistance are associated with both micro- and macro-vascular complications that can result in coronary artery diseases, strokes, and peripheral vascular diseases (Laughlin et al. 2015; Olver et al. 2015). Elevated blood pressure is commonly observed in individuals with insulin resistance (Rocchini 1991; Ferrannini et al. 1987; Pollare et al. 1990). Hypertension, insulin resistance, and dyslipidemia are all a part of a constellation of pathophysiological risk factors that are linked to cardiometabolic diseases (Reaven 1988; Ritchie and Connell 2007). The clustering of these risk factors has been well described, such that individuals with one risk factor often present with at least one or more of another risk factor (Ritchie and Connell 2007). Thus, identifying common links between these risk factors is extremely important. Our data support these observations and add to the existing data as we found associations between insulin sensitivity, multiple vascular hemodynamic measures, and LDL cholesterol. In addition to these observations, several early investigations have implicated the possibility that type II fibers may be a predisposing risk factor for cardiovascular diseases (Juhlin-Dannfelt et al. 1979; Henriksen et al. 1990; Bassett 1994). Consistent with these studies, we also found a link between type IIx myofibers and adverse cardiometabolic health outcomes. These findings are important as it has been previously shown in a relatively large sample size (n = 400) that about 25% of North American Caucasian men and women has less than 35% type I myofibers and greater than 65% of type II myofibers (Simoneau and Bouchard 1989), demonstrating the importance of understanding the link between myofiber composition and overall health. Additionally, some studies have shown Black males to have lower type I myofiber distribution compared to Caucasian males (Ama et al. 1986; Nielsen and Christensen 2011), thus it is possible that the implications linking myofiber distribution and cardiometabolic health may be even more important in African Americans and may possibly explain some of the greater risk of developing chronic cardiometabolic diseases in this population. However, it is also important to note that not all studies have demonstrated these associations as Duey et al. (1997) did not find any significant differences between type I myofiber distribution in college-aged Black and White males (Duey et al. 1997). Furthermore, the study conducted by Ama et al. (1986) compared African University students from Africa to Caucasian students in Canada, thus genetic admixture from this study was likely significantly different from African Americans (Ama et al. 1986). Thus, the discrepant findings regarding myofiber distribution between African American’s and Caucasian’s may be explained by differences in gender, genetic admixture, and age differences between these studies.
One possible mechanism that could explain the clustering of these cardiometabolic risk factors with myofiber type is differences in capillary density between type I myofibers and type II myofibers (Lillioja et al. 1987). Our findings are supportive of this as we demonstrate relationships between type IIx myofiber with arterial elasticity, SI, and blood pressure as well as correlations between arterial elasticity with SI and blood pressure. It is possible that higher capillary density in type I myofibers may improve vascular health by reducing vascular resistance and blood pressure. Additionally, greater capillary density and arterial health have also been shown to improve transport of glucose and insulin to target tissues and improve insulin sensitivity (Lillioja et al. 1987). Thus, it is possible that the relationship between insulin sensitivity, arterial elasticity and myofiber type observed in this study is at least in some part due to differences in vascularity observed within type I and IIx myofibers.
Additional support for a role of myofiber distribution and metabolic health risk comes from studies assessing neurological injuries such as spinal cord injury (SCI), in which rapid unloading has been shown to decrease fiber size, increase type IIx myofibers, and lower mitochondrial content in the first 6 months following injury (Grimby et al. 1976; Martin et al. 1992; Castro et al. 1999). This rapid loss of mobility, extreme muscle atrophy, and type IIx fiber shift leads to metabolic abnormalities and increased risk for development of cardiovascular diseases at an earlier age in individuals with SCI compared to able bodied counterparts (Szlachcic et al. 2014). Furthermore, impairments in glucose tolerance and lower GLUT4 protein content have also been shown in individuals with SCI compared with untrained able bodied controls (Yarar-Fisher et al. 2013). Thus, growing evidence demonstrates an important role of myofiber distribution and cardiometabolic health in both healthy individuals, as observed in our study, and individuals that have existing abnormal cardiometabolic health conditions.
Last, this is the first study to our knowledge demonstrating an association between type IIx myofiber content and concentration of LDL cholesterol. An association between elevated LDL cholesterol, blood pressure, and arterial disease was observed over 30 years ago (Martin et al. 1986); however, it is now believed that the primary factor that initiates the development of atherosclerosis is the oxidative modification of LDL cholesterol (Berliner and Heinecke 1996). Furthermore, mitochondrial dysfunction has been shown to be one of the earliest and most prominent features of hypercholesterolemia (Madamanchi and Runge 2007). It well known that type IIx myofibers are characterized by fewer mitochondria, lower oxidative phosphorylation capacity, and lower capillary density (Zierath and Hawley 2004). However, more recently it has also been shown that type II fibers (Quindry et al. 2011) and fast-twitch fiber muscle groups (Chang et al. 2014) may be more closely linked to oxidative stress. Thus, it is possible that the observed associations between myofiber type, insulin sensitivity, blood pressure and arterial elasticity, and LDL cholesterol are mediated by differences between mitochondria content, oxidative capacity, and reactive oxygen species production between type I and IIx myofibers. Future studies should be performed to test these hypotheses.
Strengths of this study included recruitment of a homogenous cohort of healthy premenopausal women with no known cardiovascular or metabolic complications. Further strengths included robust measures of hemodynamic variables in conjunction with the hyperinsulinemic euglycemic clamp technique to assess insulin sensitivity, and muscle biopsies with immunohistochemical analysis to determine myofiber type. Limitations in this study include the cross-sectional study design and the fact that it only included a small sample of women. Thus, these results represent a relatively homogenous sample population and may not be generalized to patients with vascular disease.
Future research is needed to determine the precise mechanisms in which myofiber composition impacts the pathophysiology of impaired glucose and lipid metabolism, as well as vascular dysfunction. Furthermore, studies utilizing interventions known to influence myofiber composition, such as exercise training, should be conducted to determine if myofiber shifts from IIx to IIa or increases in type I myofibers can protect against the development of adverse cardiometabolic health conditions.
Acknowledgments
We would like to recognize Brandon Kane for his assistance in coordinating this study. We would also like to thank the UAB Center for Clinical and Translational Science and the Clinical Research Unit for their assistance with the clamp procedure and muscle biopsies. This work was supported by the NIH Grants P30DK56336, P60DK079626, and 2R01DK049779-11A1.
Abbreviations
- DBP
Diastolic blood pressure
- LAE
Large artery elasticity
- LDL
Low density lipoprotein
- MAP
Mean arterial blood pressure
- SAE
Small artery elasticity
- SBP
Systolic blood pressure
- SI
Insulin sensitivity
- TVI
Total vascular impedance
Footnotes
Author contribution GF wrote the manuscript, assisted with data collection, performed data and statistical analysis, and reviewed and edited the manuscript. GH designed the study, assisted with data collection, assisted with data and statistical analysis, and reviewed and edited the manuscript. BG designed the study, assisted with data collection and analysis, and reviewed and edited the manuscript. SW conducted the muscle biopsies and assisted with study design. PG conducted fiber type experiments and assisted with data collection. JW assisted with fiber type experiments and assisted with data collection.
Compliance with ethical standards
Conflict of interest The author’s declare no conflicts of interest.
Communicated by Carsten Lundby.
References
- Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nøhr J, Højlund K, Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64(2):485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- Ama PF, Simoneau JA, Boulay MR, Serresse O, Thériault G, Bouchard G. Skeletal muscle characteristics in sedentary black and Caucasian males. J Appl Physiol (1985) 1986;61(5):1758–1761. doi: 10.1152/jappl.1986.61.5.1758. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-year-old men and women. J Appl Physiol (1985) 2004;97(4):1329–1337. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- Bassett DR. Skeletal muscle characteristics: relationships to cardiovascular risk factors. Med Sci Sports Exerc. 1994;26(8):957–966. [PubMed] [Google Scholar]
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20(5):707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol (1985) 1999;86(1):350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Tung HC, Chang CY, Tsai YL, Huang JP, Yen TH, Hung LM. Resveratrol exhibits differential protective effects on fast- and slow-twitch muscles in streptozotocin-induced diabetic rats. J Diabetes. 2014;6(1):60–67. doi: 10.1111/1753-0407.12072. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26(3):503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- Duey WJ, Bassett DR, Torok DJ, Howley ET, Bond V, Mancuso P, Trudell R. Skeletal muscle fibre type and capillary density in college-aged blacks and whites. Ann Hum Biol. 1997;24(4):323–331. doi: 10.1080/03014469700005072. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317(6):350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- Frisk-Holmberg M, Essén B, Fredrikson M, Ström G, Wibell L. Muscle fibre composition in relation to blood pressure response to isometric exercise in normotensive and hypertensive subjects. Acta Med Scand. 1983;213(1):21–26. doi: 10.1111/j.0954-6820.1983.tb03683.x. [DOI] [PubMed] [Google Scholar]
- Grimby G, Broberg C, Krotkiewska I, Krotkiewski M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8(1):37–42. [PubMed] [Google Scholar]
- Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and non-insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72(1):96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- Hales S. Haemostaticks II. Hafner Publishing; New York: 1964. Statistical essays. [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259(4 Pt 1):E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- James DE, Jenkins AB, Kraegen EW. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- Juhlin-Dannfelt A, Frisk-Holmberg M, Karlsson J, Tesch P. Central and peripheral circulation in relation to muscle-fibre composition in normo- and hypertensive man. Clin Sci (Lond) 1979;56(4):335–340. doi: 10.1042/cs0560335. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol (1985) 2005;99(6):2149–2158. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- Kim TN, Park MS, Lim KI, Yang SJ, Yoo HJ, Kang HJ, Song W, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Res Clin Pract. 2011;93(2):285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Akter S, Davis JW. Exercise-induced differential changes in gene expression among arterioles of skeletal muscles of obese rats. J Appl Physiol (1985) 2015;119(6):583–603. doi: 10.1152/japplphysiol.00316.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Youm Y, Kim CO, Lee WJ, Choi W, Chu SH, Park YR, Kim HC. Association between skeletal muscle mass and radial augmentation index in an elderly Korean population. Arch Gerontol Geriatr. 2014;59(1):49–55. doi: 10.1016/j.archger.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80(2):415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenneke JP, Fahs CA, Heffernan KS, Rossow LM, Thiebaud RS, Bemben MG. Relationship between thigh muscle mass and augmented pressure from wave reflections in healthy adults. Eur J Appl Physiol. 2013;113(2):395–401. doi: 10.1007/s00421-012-2449-y. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100(4):460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Hulley SB, Browner WS, Kuller LH, Wentworth D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet. 1986;2(8513):933–936. doi: 10.1016/s0140-6736(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol (1985) 1992;72(4):1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Christensen DL. Glucose intolerance in the West African Diaspora: a skeletal muscle fibre type distribution hypothesis. Acta Physiol (Oxf) 2011;202(4):605–616. doi: 10.1111/j.1748-1716.2011.02272.x. [DOI] [PubMed] [Google Scholar]
- Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, Schön MR, Blüher M, Punkt K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006;29(4):895–900. doi: 10.2337/diacare.29.04.06.dc05-1854. [DOI] [PubMed] [Google Scholar]
- Ochi M, Kohara K, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, Miki T. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212(1):327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Olver TD, Ferguson BS, Laughlin MH. Molecular mechanisms for exercise training-induced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci. 2015;135:227–257. doi: 10.1016/bs.pmbts.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Ploug T, Galbo H, Vinten J, Jørgensen M, Richter EA. Kinetics of glucose transport in rat muscle: effects of insulin and contractions. Am J Physiol. 1987;253(1 Pt 1):E12–E20. doi: 10.1152/ajpendo.1987.253.1.E12. [DOI] [PubMed] [Google Scholar]
- Pollare T, Lithell H, Berne C. Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism. 1990;39(2):167–174. doi: 10.1016/0026-0495(90)90071-j. [DOI] [PubMed] [Google Scholar]
- Quindry J, Miller L, McGinnis G, Irwin M, Dumke C, Magal M, Triplett NT, McBride J, Urbiztondo Z. Muscle-fiber type and blood oxidative stress after eccentric exercise. Int J Sport Nutr Exerc Metab. 2011;21(6):462–470. doi: 10.1123/ijsnem.21.6.462. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Rocchini AP. Insulin resistance and blood pressure regulation in obese and nonobese subjects. Special lecture. Hypertension. 1991;17(6 Pt 2):837–842. doi: 10.1161/01.hyp.17.6.837. [DOI] [PubMed] [Google Scholar]
- Rönnback M, Hernelahti M, Hämäläinen E, Groop PH, Tikkanen H. Effect of physical activity and muscle morphology on endothelial function and arterial stiffness. Scand J Med Sci Sports. 2007;17(5):573–579. doi: 10.1111/j.1600-0838.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257(4 Pt 1):E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, Ramsey MW, Stone MH. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98(5):2027–2036. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlachcic Y, Adkins RH, Govindarajan S, Cao Y, Krause JS. Cardiometabolic changes and disparities among persons with spinal cord injury: a 17-year cohort study. Top Spinal Cord Inj Rehabil. 2014;20(2):96–104. doi: 10.1310/sci2002-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58(4):588–595. doi: 10.1161/HYPERTENSIONAHA.111.174557. [DOI] [PubMed] [Google Scholar]
- Yarar-Fisher C, Bickel CS, Windham ST, McLain MM, Bamman AB. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol (1985) 2013;115(5):756–764. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004;2(10):e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath JR, He L, Gumà A, Odegoard Wahlström E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39(10):1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]