Abstract
Many malignant tumors exploit nitric oxide (NO) for a survival, growth, and migration/invasion advantage, and also to withstand the cytotoxic effects of chemo- and radiotherapies. Endogenous NO has also been shown to antagonize photodynamic therapy (PDT), a unique minimally invasive modality involving a photosensitizing (PS) agent, PS-exciting light in the visible- to near-infrared range, and molecular oxygen. The anti-PDT effects of NO were discovered about 20 years ago, but the underlying mechanisms are still not fully understood. More recent studies in the author’s laboratory using breast, prostate, and brain cancer cell lines have shown that inducible NO synthase (iNOS/NOS2) is dramatically upregulated after a PDT challenge using 5-aminolevulinic acid (ALA-) –induced protoporphyrin IX as the PS. The parallel increase in NO resulted not only in a greater resistance to cell killing but also in a striking increase in the growth and migration/invasion rate of surviving cells. These in vitro findings and their recent recapitulation at the in vivo level are discussed in this article, along with how iNOS/NO’s negative effects on PDT can be attenuated by the use of select iNOS inhibitors as PDT adjuvants.
Keywords: NO, iNOS, iNOS inhibitors, photodynamic therapy, apoptosis resistance, hyperaggressiveness
I. INTRODUCTION
Many malignant tumors overexpress and/or activate pro-survival signaling mediators in an attempt to compensate for an inadequate vascular supply of nutrients and oxygen. Low-level nitric oxide (NO) produced by tumor vascular cells or tumor cells per se is one such mediator that has garnered increasing attention in recent years.1–4 Tumor cell–derived NO is typically generated by inducible nitric oxide synthase (iNOS or NOS2).5–9 It is now clear that NO not only reinforces tumor cell survival under hostile conditions but also imposes a resistance to various therapeutic interventions.10–12 One of these is photodynamic therapy (PDT), a minimally invasive, site-specific modality based on focused light activation of a tumor-localized photosensitizing agent with subsequent generation of singlet oxygen (1O2) and/or other cytotoxic reactive oxygen species (ROSs).13–15 In addition to site specificity, PDT has the advantage of overcoming the resistance of certain tumors to radioor chemotherapy and also enhancing antitumor immunity.14,15 Moderate PDT challenges aimed at killing cells by apoptosis rather than necrosis to minimize inflammatory aftereffects can elicit cell resistance, and some of this has been shown to be NO-mediated. The first PDT studies to demonstrate such a role for endogenous NO were carried out using Photofrin as the photosensitizer and mice bearing various syngeneic tumors, including radiation-induced fibrosarcoma (RIF) and EMT6 breast tumors.16,17 A key finding was that the PDT efficacy could be significantly improved by administering a NOS inhibitor such as L-NAME before or after irradiation. Importantly, the extent of improvement correlated with constitutive NO output of the tumors, those with the highest yield responding the best and those with the lowest output responding the worst.17 Since one of the tumor-disabling effects of PDT is microvasculature constriction and NO opposes this by vasorelaxation, the NOS inhibitor effects were explained on this basis.16,17 More recent work by other investigators,18 using mouse tumors sensitized with 5-aminolevulinic acid (ALA)–induced protoporphyrin (PpIX), confirmed that NO output of a given tumor can predict the extent to which PDT can be improved by NOS inhibition. In this case,18 as in the others,16,17 anti-PDT vascular effects were invoked.
Although the in vivo studies16–18 just discussed were groundbreaking in dealing with the effects of endogenous NO on PDT efficacy, they left certain questions unanswered: (1) whether tumor cells themselves, in addition to vascular endothelial cells, for example, might generate antagonistic NO; (2) if so, whether iNOS is the most significant NO donor; (3) whether resistance is solely due to constitutive NOS/NO or whether upregulation due to PDT stress contributes; and (4) what mechanisms of NOS expression and NO signaling for PDT resistance exist. The following sections describe studies in the author’s laboratory that have addressed some of these questions.
II. I NOS/NO-MEDIATED RESISTANCE TO PDT: IN VITRO STUDIES WITH VARIOUS HUMAN CANCER CELL LINES
In early experiments, we tested NO’s ability to protect cancer cells against PDT-like killing by acting as a chain-breaking antioxidant—that is, a free radical scavenger. Such effects had already been described for relatively simple systems, including unsaturated lipids in micellar or liposomal form undergoing peroxyl radical (LOO•)–mediated chain peroxidation.19,20 We started with a human breast tumor subline, COH-BR1 cells,21 which is relatively sensitive to chain lipid peroxidative damage because it lacks GPx4, a selenoperoxidase that catalyzes reductive detoxification of lipid hydroperoxide (LOOH) intermediates.22,23 The COH-BR1 cells were sensitized with PpIX via forced ALA metabolism, the PpIX accumulating initially in the mitochondria, after which most of it was allowed to diffuse to the plasma membrane.24,25 When these cells were irradiated with broadband visible light in the presence of a lipophilic iron chelate, Fe(HQ)3 (~ 0.5 µM), and an iron reductant, ascorbate (~ 1 mM), a time-dependent increase in plasma membrane lipid peroxidation and loss of viability via necrosis occurred. Light, Fe(HQ)3, or ascorbate alone had little or no effect, indicating that LOOH “priming” via photodynamic action was necessary.26 A striking inhibition of chain peroxidation and necrosis was observed when the chemical NO donor spermine-NONOate (SPNO) at a nontoxic concentration (e.g., 0.2 mM) was included immediately before irradiation.24,25 These effects were attributed to direct interception of chain-carrying lipid-derived radicals by NO at the plasma membrane.
Follow-up experiments revealed that low-level NO from a natural source could also protect against necrosis from chain lipid peroxidation. For example, when COH-BR1 cells with disseminated PpIX were irradiated during coincubation with iNOS-expressing RAW 264.7 macrophages that had been activated with lipopolysaccharide 12 h earlier, a large L-NAME–inhibitable resistance to stress-induced necrosis was observed,25,27 implying that this exogenous NO was acting similarly to SPNO-derived NO. These cytoprotective effects were observed while NO was being actively released/generated by SPNO or activated macrophages; that is, they were “immediate” effects. Interestingly, protection could also be observed when a long delay (e.g., 20 h) was imposed between SPNO or macrophage exposure and irradiation of sensitized COH-BR1 cells. Since NO was no longer being released/generated and early levels had dissipated, these long-term or “delayed” effects were attributed to signaling activity rather than any radical scavenging by NO.25,27 At least two antioxidant effector proteins were found to play a key role in the delayed resistance: heme oxygenase-1 (HO-1) and ferritin, both of which were substantially upregulated 20 h after donor exposure.25 The level of cytosolic free iron was simultaneously diminished,25,27 consistent with the observed reduction of iron-catalyzed lipid peroxidation. These studies provided an early example of the mechanistic diversity of NO as a pro-survival effector for photostressed cancer cells.
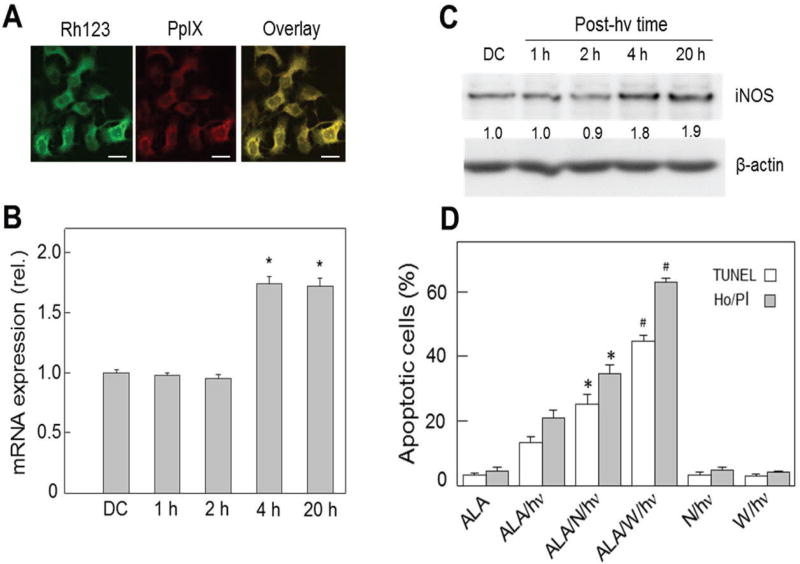
Subsequent in vitro work led to the discovery that low-level NO produced by stressed tumor cells themselves played a major role in photokilling resistance. These experiments were carried out using ALA-induced PpIX that was restricted to mitochondria, where it originates as a heme precursor. Under the sensitization conditions used—for example, for COH-BR1 cells—fluorescence imaging showed that PpIX colocalized with the mitochondrial marker Rhodamine-123 (Fig. 1A). After these cells were exposed to a relatively modest light fluence (~ 1 J/cm2), a significant increase in iNOS transcription and translation was observed.28 The steady-state mRNA level nearly doubled 4 h after irradiation and remained there for at least 16 h longer (Fig. 1B). iNOS protein increased in approximately the same time and to the same extent relative to a dark control (Fig. 1C). These cells expressed a trace of eNOS but no nNOS; unlike iNOS, however, eNOS remained constant after irradiation.28 As shown in Fig. 1D, ~ 25% of the COH-BR1 cells died via Hoechst-assessed apoptosis 20 h after ALA/light treatment, which reflected PpIX-sensitized mitochondrial damage, whereas no significant necrosis was detected.28 When these cells were irradiated in the presence of a nonspecific low-affinity NOS inhibitor (L-NAME) or an iNOS-specific inhibitor (1400W), there was a marked increase in apoptosis. At 100-fold lower concentration than L-NAME, 1400W elevated apoptosis to > 60% (Fig. 1D). Neither inhibitor had any significant effect on control apoptosis, suggesting that hyper-resistance was mainly due to photostress-induced iNOS/NO. The NO scavenger cPTIO also caused a substantial increase in apoptosis of photostressed cells over that seen in ALA-only or light-only controls.28 Confirmation for NO involvement was obtained by observing that the steady-state NO level detected by DAF-2 fluorescence increased nearly 8-fold over background 20 h after irradiation, with 1400W strongly attenuating this.28 These findings, and similar ones for other breast cancer cell types,28–30 appear to be unique for any type anticancer intervention. Although additional resistance mechanisms might have come into play after a photochallenge, it is clear from the large magnitude of iNOS inhibitor and NO scavenger effects that iNOS/NO played a major role in this regard.
FIG. 1.
Stress-induced resistance to apoptotic photokilling in ALA/light-challenged breast cancer cells: role of upregulated iNOS. COH-BR1 cells at ~ 60% confluency in serum-free medium were dark-incubated with 1-mM ALA for 45 min, switched to ALA-free medium, then irradiated with broad-band visible light for 15 min (delivered light fluence ~ 1 J/cm2). Where indicated, L-NAME (1 mM) or 1400W (10 µM) was present (added 15 min before ALA). (A) Mitochondrial localization of PpIX in ALA-treated cells, as detected by confocal fluorescence microscopy; Rh123, Rhodamine-123. (B) Quantitative RT-PCR-assessed iNOS mRNA level at various post-hν incubation times; DC, dark control (ALA-only). (C) Western blot of iNOS at indicated post-hν times; numbers below bands are iNOS/β-actin ratios normalized to DC. (D) Effects of L-NAME (N) and 1400W (W) on 20-h post-hν extent of apoptosis determined by TUNEL or Hoechst/propidium iodide (Ho/PI) assay; control (ALA- or light-alone) apoptosis was < 5% (reprinted with permission from John Wiley and Sons, Copyright 2011).28
The signaling events associated with iNOS upregulation and NO-mediated hyper-resistance in photostressed cells have been explored, although much remains to be learned, particularly in the latter category. Our studies with ALA/light-treated breast and prostate cancer cell lines have clearly demonstrated that activation of transcription factor NF-κB downstream of oxidative activation of kinase Akt is responsible for iNOS induction.30,31 Regarding possible mechanisms of NO-imposed resistance, we found that ODQ, an inhibitor of NO-activated soluble guanylyl cyclase, failed to stimulate cell photokilling.30 This ruled out any involvement of cyclic-GMP (cGMP)–activated PKG, a pro-survival kinase,32 in cytoprotection. These results contrasted with those of others using different cancer cells and photosensitization conditions,33 suggesting that cGMP-mediated cytoprotection by NO is specific to cell and photodynamic conditions. Looking at other possibilities, we found that the inhibitor of the apoptosis protein, survivin, was upregulated by photostress, whereas the tumor suppressor p53 was downregulated, both responses being attenuated by 1400W or cPTIO.30 In addition, photostress NO allowed only a transient phosphorylation activation of the pro-apoptotic MAPKs JNK and p38α; inhibition or knockdown of iNOS intensified and prolonged these responses, resulting in greater apoptotic photokilling.30 Consistently, NO also signaled for upregulation of the antiapoptotic Bcl-xL and downregulation of the pro-apoptotic Bax.34 A major unsettled issue is how NO might modify key pro-survival versus antisurvival effector proteins in order to escape lethality, at least when photooxidative pressure is not overwhelming. Modification of select cysteine residues via S-nitrosation35,36 is assumed to occur in the wake of postirradiation iNOS/NO induction, but crucial effector targets remain to be identified.
In considering signaling events, it is important to point out that Rapozzi et al.37–39 have recently shown that endogenous NO can elicit not only cytoprotective responses, as we have reported,28–30,34 but also cytotoxic responses, depending on the level of NO generated by photodynamic action. For example, low-level NO arising from relatively modest photooxidative pressure on pheophorbide-sensitized melanoma (B78-H1) or prostate cancer (PC3) cells resulted in cytoprotection via upregulation of NF-κB, YY1, and SNAIL but downregulation of RKIP.37–39 In addition to its cytoprotective effects, low-level iNOS-derived NO stimulated an epithelial-to-mesenchymal transition (EMT), which is characteristic of metastatic invasion. In striking contrast, high-level NO produced by more intense photooxidative pressure resulted in a reversal of these responses: reduced cytoprotection via downregulation of NF-κB, YY1, and SNAIL; upregulation of RKIP; and diminished EMT.37–39 Although this was not specifically addressed in these studies,37,38 it is likely that the cytoprotective NO derived initially from iNOS transcription by stress-activated NF-κB and, consistent with our recent evidence,40 more NO was generated via its positive feedback on NF-κB, although ultimate NO remained at a relatively low (cytoprotective) steady-state level. In contrast, when NO reached a sufficiently high (cytotoxic) level, it would have exerted negative feedback on NF-κB, resulting in downregulation of survival signaling, as described previously. How NO, depending on its steady-state level, can regulate NF-κB as proposed remains to be elucidated.
III. ENHANCED GROWTH AND MIGRATORY AGGRESSIVENESS OF TUMOR CELLS SURVIVING A PDT CHALLENGE
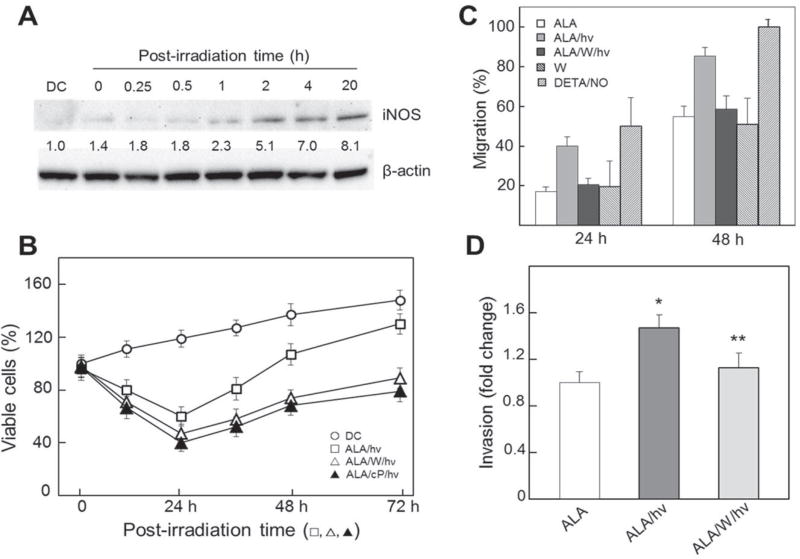
One advantage of clinical PDT over chemo- or radiotherapy is that it can eradicate tumors in a focal manner with minimal damage to surrounding normal tissue. Nevertheless, negative side effects can occur at very high sensitizer/light doses, so moderate doses are usually chosen.13–15 This, combined with the fact that some tumor cells are less sensitized than others (depending on blood supply) and less accessible to irradiation, suggests that many of these cells will survive a PDT challenge. However, their phenotype might not necessarily be the same; for example, growth rate might increase, as observed previously for some in vitro PDT systems but not for possible iNOS/NO involvement.41,42 The author’s group was the first to address this issue, using human prostate carcinoma cells.43,44 Initial experiments revealed that ALA→PpIX-sensitized prostate PC3 cells, like their COH-BR1 counterparts,28–30 exploited iNOS-derived NO to resist apoptotic photokilling. Accordingly, PC3 apoptosis was found to be strongly enhanced by 1400W or cPTIO during postirradiation incubation.43,44 Similarly to COH-BR1 cells, PC3s cells upregulated iNOS after photostress, but did so much more robustly—for example, 10–12-fold over dark controls as soon as ~ 2 h after irradiation and remaining there for at least 18 h longer (Fig. 2A); eNOS remained very low and unchanged, while nNOS was undetectable.43 Thus, apoptotic resistance was predominantly due to stress-induced as opposed to preexisting iNOS. This was considerably more impressive than that observed with COH-BR1 cells (Fig. 1C). More striking yet was the observation the PC3 cells that survived a photochallenge proliferated much more rapidly than dark- or light-only controls over at least a 3-day period, and that inhibiting iNOS or scavenging NO prevented this (Fig. 2B).44 Cell cycle analysis confirmed that growth stimulation was occurring because S-phase occupancy (representing DNA doubling) reached ~3 times the control level after 36 h, and 1400W prevented this increase.43 Other experiments revealed that photostressed PC3 cells also exploited upregulated iNOS/NO to migrate more rapidly, as determined by gap-closure assay. Migration increased in a postirradiation time-dependent fashion up to at least 48 h and was abrogated by 1400W (Fig. 2C). On the other hand, 1400W had no effect on dark (ALA-only) controls, consistent with very low basal levels of iNOS in these cells (Fig. 2A). When the chemical NO donor DETA/NO at a low starting level (10 µM) was used as a positive control, a migration spurt similar to that caused by ALA/light treatment was observed (Fig. 2C). This confirmed that photostress-induced NO could stimulate migratory aggressiveness. Not surprisingly, it was found that this NO also increased the invasiveness of PC3 cells—that is, the ability to traverse a basement membrane simulating a tumor extracellular matrix (Fig. 2D). Accelerated invasion was preceded by modifications of proteins involved in the invasion process, e.g. (i) activation of matrix metalloproteinase-9 (MMP-9), (ii) downregulation of MMP-9 inhibitor TIMP-1, and (iii) upregulation of α6 and β1 integrins. All of these effects were strongly inhibited by 1400W or cPTIO.44 Similar iNOS/NO-enhanced proliferation, migration, and invasion have been observed for prostate cancer DU145 cells that withstand a photochallenge,44 as well as breast cancer MDA-MB-231 and glioblastoma U87 and U251 cells.45,46 Recognition of these striking responses to acute photodynamic stress appears to be unique to these studies31,43–46; they have not been described as yet for other oxidant-based anticancer modalities. Post-PDT switching to a more aggressive growth and migration/invasion phenotype in vivo would raise serious concerns because metastatic progression might result. Unfortunately, this troublesome prospect may be favored by the fact that the intensity of PDT, like that of chemoand radiotherapy, needs to be modulated in order to minimize damage to normal cells (see above), thus allowing a certain fraction of targeted tumor cells to survive.
FIG. 2.
Elevated growth, migration, and invasion aggressiveness in prostate cancer cells withstanding an ALA/light challenge: iNOS/NO-dependency. Prostate carcinoma PC3 cells in serum-free medium were sensitized with PpIX by incubating with 1-mM ALA for 30 min in the dark. After irradiation (~ 1 J/cm2) in the absence versus the presence of 25 µM 1400W (W) or cPTIO (cP), as indicated, any detached (dead) cells were removed by washing, after which live cells were overlaid with 10% serum-containing medium and incubated for various times prior to different analyses. (A) Western blot of iNOS at different post-hν times out to 20 h; numbers below bands represent iNOS/β-actin ratios normalized to a 24-h dark control (DC). (B) Post-photostress cell viability (0–24 h) and surviving cell proliferation (24–72 h), as determined by MTT assay; means ± SE, n = 3. (C) Surviving cell migration over 24 h and 48 h of post-hν incubation; also represented are nonirradiated cells treated with 10-µM DETA/NO; means ± SD (n = 6). (D) Surviving cell invasiveness over a 48-h post-hν period, corrected for nonviable cells under each condition; means ± SE (n = 3); *P < 0.001 versus ALA; **P < 0.05 versus ALA/hν (reprinted with permission from Elsevier, Copyright 2015).44
IV. BYSTANDER EFFECTS OF NO GENERATED BY PDT-TARGETED CELLS
Because most mature tumors have vascular supply limitations, not all tumor cells are uniformly accessed by preexisting sensitizers or a prosensitizer like ALA. Moreover, during subsequent irradiation, some cells are inevitably less exposed than others because of light field limits, tumor geometry, and other complex factors. This being the case, it is conceivable that cells experiencing the greatest photodynamic stress might respond to it by sending signals to nonstressed or weakly stressed counterparts—bystander cells. Such a phenomenon is well documented for cells exposed to ionizing radiation, and a variety of mobile signaling mediators have been described, including NO.47–50 To determine whether this is applicable to PDT, Bazak et al.51 used a novel approach involving the use of impermeable silicone rings to separate ALA-treated target cells (outside rings) from non-treated bystander cells (inside rings) on a large culture dish. At some point after irradiation, the rings are carefully removed to allow diffusion of small signaling molecules like NO from the targeted cells to the bystanders.51 A particularly striking finding from this seminal study was that after irradiation, iNOS and NO levels increased steadily in the bystander population as well as (expectedly) in the targeted one. This suggested that a “feed-forward” signaling mechanism initiated by target cell iNOS/NO and continuously mediated by NO was in operation. Like surviving targeted cells, bystanders proliferated and migrated at significantly greater rates than controls with nonstressed target cells, and these responses were strongly reduced when an iNOS inhibitor, NO trap, or target cell iNOS knockdown was used.51
In addition to iNOS, several other pro-survival/growth effector proteins were upregulated in bystander populations, including cyclooxygenase-2 (COX-2), Akt, and ERK1/2, each in cPTIO-inhibitable fashion.51 These findings reveal previously unrecognized negative off-target effects of PDT. Because NO from targeted cells can increase bystander growth and migration rates, the troubling prospect might be greater tumor expansion than would occur without PDT. This concern might be allayed through the rational pharmacologic use of iNOS inhibitors, some of which have already been safely used in clinical trials outside the area of cancer or PDT.52,53
V. EFFECTS OF INOS/NO ANTAGONISM TO PDT IN VIVO
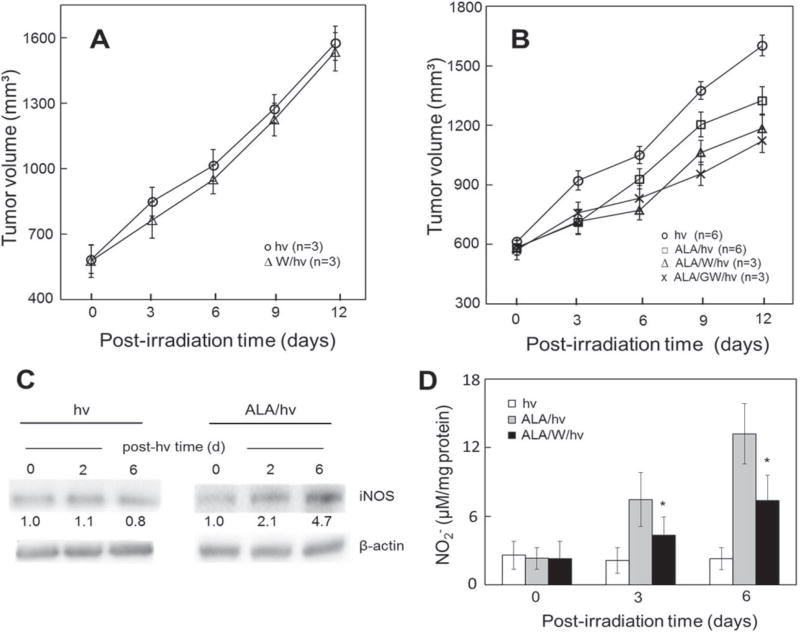
Much of the in vitro evidence for iNOS/NO-based antagonism to PDT (Section II) has recently been validated at the in vivo level in the author’s laboratory. Severe combined immune deficient (SCID) female mice bearing human breast carcinoma MDA-MB-231 tumor xenografts were subjected to ALA-PDT using a 633-nm light source for optimal light penetration into the tumor tissue.46 An iNOS inhibitor (1400W or GW274150) was administered immediately after ALA and then repeatedly after irradiation at one-day intervals. As shown in Fig. 3B, PDT-treated mice exhibited a significant reduction in tumor growth rate compared with light-only controls over a 12-day postirradiation period.46 Importantly, 1400W and GW274150 reduced the growth rate even further and in a highly significant fashion (Fig. 3B). However, these inhibitors caused little, if any, growth reduction in light-only controls (Fig. 3A), implying that preexisting iNOS/NO, in contrast to any stress-upregulated iNOS/NO, had no measurable protumor effects. Immunoblot analysis of post-PDT tumor samples revealed that iNOS had in fact been steadily and substantially upregulated over six days post-PDT relative to light controls (Fig. 3C). Consistent with this were Griess assay data showing that NO-derived nitrite levels in post-PDT tumors were significant higher than those in controls, 1400W strongly blunting the increases (Fig. 3D). Other data dealing with the status of effector proteins revealed that pro-apoptotic Bax was downregulated after PDT, whereas pro-survival/progression survivin, Bcl-xL, and S100A4 were upregulated; once again, 1400W exerted a strong inhibitory effect.46
FIG. 3.
Antagonistic effects of iNOS/NO on ALA-PDT in a tumor xenograft model. (A) Light-only controls: female SCID mice bearing human breast MDA-MB-231 tumors were injected i.p. with PBS or 1400W (10 mg/kg) in PBS. After 4 h, the mice were anesthetized, placed in opaque restraints with cutouts for tumor exposure, and irradiated, using a 633-nm light source and fluence ~ 95 J/cm2; after irradiation, the mice were reinjected with 1400W once per day until termination; plotted tumor volumes are means ± SEM (n = 3). (B) ALA-PDT: MDA-MB-231 tumor-bearing SCID mice were injected i.p. with ALA (100 mg/kg) or PBS (light-only controls), followed by 1400W (10 mg/kg) or GW274150 (25 mg/kg); after 4 h in the dark, the mice were anesthetized, placed in restraints with cutouts, and irradiated as in (A); after PDT, the mice were kept in subdued light and reinjected with each iNOS inhibitor once daily; measured tumor volumes are means ± SEM (n = 3–6). (C) Western blot showing iNOS levels in tumor samples from light control and ALA-PDT mice; the number below each band indicates iNOS intensity relative to β-actin and normalized to day 0. (D) Nitrite levels in tumor samples after ALA-PDT in the absence versus the presence of 1400W; values determined by Griess assay are means ± SEM (n = 3), (reprinted with permission from Elsevier, Copyright 2017).46
The findings just described were the first to demonstrate that PDT efficacy in an in vivo human tumor model is antagonized by iNOS/NO. It is important to point out that MDA-MB-231 cells surviving an ALA/light challenge in vitro exhibited iNOS/NO-mediated growth, migration/invasion, and effector protein changes virtually identical to those observed in the tumor xenograft model.46 This suggests that the post-PDT stress conditions that developed in vivo closely recapitulated those that developed in isolated cells. Thus, it appears that tumor cell iNOS was primarily responsible for the NO-mediated PDT resistance observed in vivo. Any contribution made by vascular endothelial NOS (eNOS) was probably minimal, since iNOS-specific inhibitors strongly blunted all acquired hyperaggressiveness. It remains to be seen whether this in vivo recapitulation of relatively simple in vitro responses will hold if animals with intact immune systems are used as PDT models. In this case, an additional consideration will be that myeloid-derived cells (MDSCs) may supply NO at relatively high levels that can suppress any PDT-acquired antitumor immunity.14,54–57
VI. SUMMARY AND PERSPECTIVES
The many attractive features of PDT, including tumor site specificity, nontoxicity of individual components (sensitizer, light, O2), and significant efficacy on difficult, tumors such as glioblastomas, continue to make it the therapy of choice for several solid malignancies.14,58,59 It is now well known that many tumors exploit low-level NO to avoid apoptosis; stimulate growth and migration; and resist radiotherapy, chemotherapy, and PDT.1–12,16–18 The NO can derive from tumor cells themselves, but surrounding vascular cells (endothelium, macrophages) can contribute. The in vitro and in vivo PDT studies described in this review are unique in showing that endogenous tumor cell iNOS/NO plays a major role not only in PDT resistance but also in the elevated aggressiveness of residual or bystander cells. Although both constitutive and stress-induced iNOS might be implicated in these responses, the induced enzyme plays an almost exclusive role in several cancer cell types.43,44,46 This evidence is unprecedented because most therapy-based studies up to now have considered only preexisting iNOS/NO and not that possibly altered by the treatment itself. Concerns about a more aggressive, possibly more metastatic phenotype of PDT-surviving cells can be allayed through pharmacologic intervention with iNOS inhibitors, some of which have already seen preclinical testing in non-PDT settings.52,53
Acknowledgments
Studies by the author’s research group were supported by NIH/NCI Grant CA70823, a grant from the Wisconsin Breast Cancer Showhouse for a Cure, and a grant from the Advancing a Healthier Wisconsin Endowment. Johathan Fahey, Reshma Bhowmick, Magda Niziolek-Kierecka, and Jerzy Bazak are thanked for their valuable contributions to much of the research described. The helpful advice and suggestions of Witold Korytowski, Mladen Korbelik, and Neil Hogg are also appreciated.
ABBREVIATIONS
- ALA
5-aminolevulinic acid
- PpIX
protoporphyrin IX
- PDT
photodynamic therapy
- NOS
nitric oxide synthase
- iNOS/NOS2
inducible nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- L-NAME
L-NG-nitroarginine methyl ester
- 1400W
N-[3 (aminomethyl) benzyl]acetamidine
- GW274150
[2-[(1-iminoethyl) amino]ethyl]-L-homocysteine
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide
- NF-κB
nuclear factor-kappa B
- DAF-2DA
4,5-diaminofluorescein diacetate
- SPNO
spermine-NONOate
- ODQ
1H-(1,2,4)oxadiazolo[4,3-a]quinoxalin-1-one
References
- 1.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, Rhodes P, Westmore K, Emson PC, Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995;92:4392–6. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–48. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 3.Fukumura D, Jain RK. Role of nitric oxide in angiogenesis and microcirculation in tumors. Cancer Metastasis Rev. 1998;17:77–89. doi: 10.1023/a:1005908805527. [DOI] [PubMed] [Google Scholar]
- 4.Burke AJ, Sullivan FJ, Giles FJ, Glynn SA. The yin and yang of nitric oxide in cancer progression. Carcinogenesis. 2013;34:503–12. doi: 10.1093/carcin/bgt034. [DOI] [PubMed] [Google Scholar]
- 5.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol. 2005;15:277–89. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm EA, Overwijk WW. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010;16:1834–44. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, Rich JN. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostourou V, Cartwright JE, Johnstone AP, Boult JK, Cullis ER, Whitley G, Robinson SP. The role of tumour-derived iNOS in tumour progression and angiogenesis. Br J Cancer. 2011;104:83–90. doi: 10.1038/sj.bjc.6606034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian K, Ghassemi F, Sotolongo A, Siu A, Shauger L, Kots A, Murad F. NOS-2 signaling and cancer therapy. IUBMB Life. 2012;64:676–83. doi: 10.1002/iub.1057. [DOI] [PubMed] [Google Scholar]
- 10.Crowell JA, Steele VE, Sigman CC, Fay JR. Is inducible nitric oxide synthase a target for chemoprevention? Mol Cancer Ther. 2003;2:815–23. [PubMed] [Google Scholar]
- 11.Saleem W, Suzuki Y, Mobaraki A, Yoshida Y, Noda S, Saitoh JI, Nakano T. Reduction of nitric oxide level enhances the radiosensitivity of hypoxic non-small cell lung cancer. Cancer Sci. 2011;102:2150–6. doi: 10.1111/j.1349-7006.2011.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsunaga T, Yamaji Y, Tomokuni T, Morita H, Morikawa Y, Suzuki A, Yonezawa A, Endo S, Ikari A, Iguchi K, El-Kabbani O, Tajima K, Hara A. Nitric oxide confers cisplatin resistance in human lung cancer cells through upregulation of aldo-keto reductase 1B10 and proteasome. Free Radic Res. 2014;48:1371–85. doi: 10.3109/10715762.2014.957694. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benov L. Photodynamic therapy: current status and future directions. Med Princ Pract. 2015;24(Suppl 1):14–28. doi: 10.1159/000362416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson BW, Sitnik-Busch TM, Vaughan LA. Potentiation of photodynamic therapy antitumor activity in mice by nitric oxide synthase inhibition is fluence rate dependent. Photochem Photobiol. 1999;70:64–71. [PubMed] [Google Scholar]
- 17.Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford MR, Chaplin DJ. Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy. Br J Cancer. 2000;82:1835–43. doi: 10.1054/bjoc.2000.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves KJ, Reed MW, Brown NJ. The role of nitric oxide in the treatment of tumours with aminolaevulinic acid-induced photodynamic therapy. J Photochem Photobiol B. 2010;101:224–32. doi: 10.1016/j.jphotobiol.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Korytowski W, Zareba M, Girotti AW. Nitric oxide inhibition of free radical-mediated cholesterol peroxidation in liposomal membranes. Biochemistry. 2000;39:6918–28. doi: 10.1021/bi000393e. [DOI] [PubMed] [Google Scholar]
- 20.Korytowski W, Zareba M, Girotti AW. Inhibition of free radical-mediated cholesterol peroxidation by diazeniumdiolate-derived nitric oxide: effect of release rate on mechanism of action in a membrane system. Chem Res Toxicol. 2000;13:1265–74. doi: 10.1021/tx000160o. [DOI] [PubMed] [Google Scholar]
- 21.Esworthy RS, Baker MA, Chu FF. Expression of selenium-dependent glutathione peroxidase in human breast tumor cell lines. Cancer Res. 1995;55:957–62. [PubMed] [Google Scholar]
- 22.Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation: in situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990;265:454–61. [PubMed] [Google Scholar]
- 23.Hurst R, Korytowski W, Kriska T, Esworthy RS, Chu FF, Girotti AW. Hyperresistance to cholesterol hydroperoxide-induced peroxidative injury and apoptotic death in a tumor cell line that overexpresses glutathione peroxidase isotype-4. Free Radic Biol Med. 2001;31:1051–65. doi: 10.1016/s0891-5849(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 24.Niziolek M, Korytowski W, Girotti AW. Chain-breaking antioxidant and cytoprotective action of nitric oxide on photodynamically stressed tumor cells. Photochem Photobiol. 2003;78:262–70. doi: 10.1562/0031-8655(2003)078<0262:caacao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Niziolek M, Korytowski W, Girotti AW. Nitric oxide inhibition of free radical-mediated lipid peroxidation in photodynamically treated membranes and cells. Free Radic Biol Med. 2003;34:997–1005. doi: 10.1016/s0891-5849(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 26.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B. 2001;63:103–13. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 27.Niziolek-Kierecka M, Korytowski W, Girotti AW. Tumour cell hyperresistance to photodynamic killing arising from nitric oxide preconditioning. In: Kessel D, editor. Proceedings of SPIE: Mechanisms and Techniques in Photodynamic Therapy XVI. Vol. 6427. San Jose, CA: 2007. [Google Scholar]
- 28.Bhowmick R, Girotti AW. Rapid upregulation of cytoprotective nitric oxide in breast tumor cells subjected to a photodynamic therapy-like oxidative challenge. Photochem Photobiol. 2011;87:378–86. doi: 10.1111/j.1751-1097.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick R, Girotti AW. Cytoprotective induction of nitric oxide synthase in a cellular model of 5-aminolevulinic acid-based photodynamic therapy. Free Radic Biol Med. 2010;48:1296–301. doi: 10.1016/j.freeradbiomed.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhowmick R, Girotti AW. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic Biol Med. 2013;57:39–48. doi: 10.1016/j.freeradbiomed.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girotti AW, Fahey JM, Korytowski W. Multiple means by which nitric oxide can antagonize photodynamic therapy. Curr Med Chem. 2016;23:2754–69. doi: 10.2174/0929867323666160812145641. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86:1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 33.Gomes ER, Almeida RD, Carvalho AP, Duarte CB. Nitric oxide modulates tumor cell death induced by photodynamic therapy through a cGMP-dependent mechanism. Photochem Photobiol. 2002;76:423–30. doi: 10.1562/0031-8655(2002)076<0423:nomtcd>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Bhowmick R, Girotti AW. Signaling events in apoptotic photokilling of 5-aminolevulinic acid-treated tumor cells: inhibitory effects of nitric oxide. Free Radic Biol Med. 2009;47:731–40. doi: 10.1016/j.freeradbiomed.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DD, Jourd’heuil D. S-nitrosation: current concepts and new developments. Antioxid Redox Signal. 2012;17:934–6. doi: 10.1089/ars.2012.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012;320:123–9. doi: 10.1016/j.canlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Rapozzi V, Della Pietra E, Zorzet S, Zacchigna M, Bonavida B, Xodo LE. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide. 2013;30:26–35. doi: 10.1016/j.niox.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Della Pietra E, Simonella F, Bonavida B, Xodo LE, Rapozzi V. Repeated sub-optimal photodynamic treatments with pheophorbide a induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide. 2015;45:43–53. doi: 10.1016/j.niox.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Rapozzi V, Della Pietra E, Bonavida B. Dual roles of nitric oxide in the regulation of tumor cell response and resistance to photodynamic therapy. Redox Biol. 2015;6:311–7. doi: 10.1016/j.redox.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazak J, Fahey JM, Wawak K, Korytowski W, Girotti AW. Enhanced aggressiveness of bystander cells in an anti-tumor photodynamic therapy model: role of nitric oxide produced by targeted cells. Free Radic Biol Med. 2017;102:111–21. doi: 10.1016/j.freeradbiomed.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 41.Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim Biophys Acta. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Edmonds C, Hagan S, Gallagher-Colombo SM, Busch TM, Cengel KA. Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3: targeting survival pathways to increase PDT efficacy in ovarian and lung cancer. Cancer Biol Ther. 2012;13:1463–70. doi: 10.4161/cbt.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhowmick R, Girotti AW. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer Lett. 2014;343:115–22. doi: 10.1016/j.canlet.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fahey JM, Girotti AW. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: role of nitric oxide. Nitric Oxide. 2015;49:47–55. doi: 10.1016/j.niox.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fahey JM, Emmer JV, Korytowski W, Hogg N, Girotti AW. Antagonistic effects of endogenous nitric oxide in a glioblastoma photodynamic therapy model. Photochem Photobiol. 2016;92:842–53. doi: 10.1111/php.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fahey JM, Girotti AW. Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: improved outcome with NOS2 inhibitors. Nitric Oxide. 2017;62:52–61. doi: 10.1016/j.niox.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, Kitai R, Ohnishi T, Kano E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat Res. 2001;155:387–96. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Azzam EI, de Toledo SM, Little JB. Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets. 2006;4:53–64. doi: 10.2174/1568009043481641. [DOI] [PubMed] [Google Scholar]
- 49.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation-induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakovlev VA. Role of nitric oxide in the radiation-induced bystander effect. Redox Biol. 2015;6:396–400. doi: 10.1016/j.redox.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bazak J, Fahey JM, Wawak K, Korytowski W, Girotti AW. Enhanced aggressiveness of bystander cells in an anti-tumor photodynamic therapy model: role of nitric oxide produced by targeted cells. Free Radic Biol Med. 2017;102:111–21. doi: 10.1016/j.freeradbiomed.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 52.Hansel TT, Kharitonov SA, Donnelly LE, Erin EM, Currie MG, Moore WM, Manning PT, Recker DP, Barnes PJ. A selective inhibitor of inducible nitric oxide synthase inhibits exhaled breath nitric oxide in healthy volunteers and asthmatics. FASEB J. 2003;17:1298–317. doi: 10.1096/fj.02-0633fje. [DOI] [PubMed] [Google Scholar]
- 53.Singh D, Richards D, Knowles RG, Schwartz S, Woodcock A, Langley S, O’Connor BJ. Selective inducible nitric oxide synthase inhibition has no effect on allergen challenge in asthma. Am J Respir Crit Care Med. 2007;176:988–93. doi: 10.1164/rccm.200704-588OC. [DOI] [PubMed] [Google Scholar]
- 54.Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7:75–91. doi: 10.1586/eci.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89:873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirano K, Hosoi A, Matsushita H, Iino T, Ueha S, Matsushima K, Seto Y, Kakimi K. The nitric oxide radical scavenger carboxy-PTIO reduces the immunosuppressive activity of myeloid-derived suppressor cells and potentiates the antitumor activity of adoptive cytotoxic T lymphocyte immunotherapy. Oncoimmunology. 2015 Aug;4(8):e1019195. doi: 10.1080/2162402X.2015.1019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fionda C, Abruzzese MP, Santoni A, Cippitelli M. Immunoregulatory and effector activities of nitric oxide and reactive nitrogen species in cancer. Curr Med Chem. 2016;23:2618–36. doi: 10.2174/0929867323666160727105101. [DOI] [PubMed] [Google Scholar]
- 58.Quirk BJ, Brandal G, Donlon S, Vera JC, Mang TS, Foy AB, Lew SM, Girotti AW, Jogal S, LaViolette PS, Connelly JM, Whelan HT. Photodynamic therapy (PDT) for malignant brain tumors—where do we stand? Photodiagnosis Photodyn Ther. 2015;12:530–44. doi: 10.1016/j.pdpdt.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Banerjee SM, MacRobert AJ, Mosse CA, Periera B, Bown SG, Keshtgar MRS. Photodynamic therapy: inception to application in breast cancer. The Breast. 2017;31:105–13. doi: 10.1016/j.breast.2016.09.016. [DOI] [PubMed] [Google Scholar]