Abstract
Spinal synaptic plasticity is believed to drive central sensitization that underlies the persistent nature of neuropathic pain. Our recent data showed that synaptic plasticity in the dorsal horn is cell type-specific: intense afferent stimulation produced long-term potentiation (LTP) in excitatory spinothalamic tract neurons (STTn), whereas it produced long-term depression (LTD) in inhibitory GABAergic interneurons (GABAn). In addition, reactive oxygen species (ROS) were shown to be involved in LTP in STTn (STTn-LTP) as well as in LTD in GABAn (GABAn-LTD). This study examined the roles of two biologically important ROS—superoxide [·O2] and hydroxyl radicals [·OH]—in neuropathic mechanical hyperalgesia and cell type-specific spinal synaptic plasticity. A [·O2] donor induced stronger mechanical hyperalgesia than a [·OH] donor in naïve mice. A [·O2] scavenger showed greater anti-hyperalgesic effect than [·OH] scavengers in the spinal nerve ligation (SNL) mouse model of neuropathic pain. Moreover, a [·O2] donor induced STTn-LTP and GABAn-LTD, but a [·OH] donor induced only GABAn-LTD. In addition, a [·O2] scavenger inhibited STTn-LTP and GABAn-LTD induction (via conditioning stimulus (CS)) in naïve mice and alleviated SNL-induced potentiation and depression, respectively. Also, [·OH] scavenger selectively inhibited GABAn-LTD induction and maintenance as well as SNL-induced depression. These results indicate that mechanical hyperalgesia in SNL mice is the result of the combination of STTn-LTP and GABAn-LTD. Behavioral outcomes compliment electrophysiological results which suggest that [·O2] mediates both STTn-LTP and GABAn-LTD, whereas [·OH] is involved primarily in GABAn-LTD.
Introduction
Central sensitization in the dorsal horn is believed to be the main mechanism behind neuropathic pain25,38. Intense and repetitive nociceptive afferent inputs initiate plastic changes in the synapses relaying nociceptive information to the brain, thus largely encompassing synapses along the spinothalamic tract (STT) pathway15,19,1,36.
Two opposing types of synaptic plasticity develop in the excitatory synapses of STT projection neurons (STTn) and GABAergic inhibitory interneurons (GABAn) in the spinal dorsal horn. These opposing synaptic plastic changes are cell type-specific: long-term potentiation (LTP) of excitatory postsynaptic current (EPSC) develops in STTn as opposed to long-term depression (LTD) in GABAn in response to the same conditioning stimulus (CS)14. It is hypothesized that both LTD in GABAn and LTP in STTn contribute to increased pain transmission and neuropathic pain14,39; however, this hypothesis remains to be addressed.
Evidence has pointed to reactive oxygen species (ROS) being important molecular players in aberrant pain transmission after nerve injury. It is becoming more accepted by pain researchers that increased ROS levels downregulate GABA transmission in the dorsal horn after spinal cord injury8,40. As a result, GABAergic disinhibition then controls the excitatory-inhibitory balance of the dorsal horn and drives enhanced excitatory transmission, leading to neuropathic pain. It also has been shown that dorsal horn neurons produce significant amounts of superoxide radicals in a capsaicin model, and that ROS levels are correlated with the level of secondary hyperalgesia observed33. Additionally, superoxide radical scavengers were shown to reduce dorsal root stimulation-induced LTP of field excitatory post-synaptic potentials (fEPSP) in the superficial medial dorsal horn22. Nevertheless, the causal relationship between ROS and synaptic plasticity in both inhibitory and excitatory circuits of the dorsal horn is yet to be determined.
At a behavioral level, many studies show a clear correlation between increased ROS levels and pathological pain development. For instance, ROS scavengers produce anti-hyperalgesic effects in animal models of neuropathic pain, such as the spinal nerve ligation (SNL) and chronic constriction injury of sciatic nerve models12,16. Conversely, intrathecal administration of an ROS donor—t-butyl-hydroperoxide (tBOOH)—induces pain behaviors in naïve animals39. Furthermore, anti-oxidant superoxide dismutase (SOD2) over-expressing transgenic mice show mitigated capsaicin-induced secondary hyperalgesia, whereas SOD2 knockout mice show significantly higher capsaicin-induced hyperalgesia33.
Despite the studies showing the role of ROS in central sensitization and neuropathic pain, the key evidence linking ROS to cell type-specific synaptic plasticity in the spinal cord (i.e., LTP in STTn and LTD in GABAn) is lacking. Therefore, this study investigates the effects of donors and scavengers of two biologically important ROS—superoxide and hydroxyl radicals—in pain behaviors and synaptic plasticity of STTn and GABAn in normal and neuropathic (SNL) mice. We report here that superoxide radicals mediate LTP in STTn and LTD in GABAn as well as neuropathic pain behaviors. On the other hand, hydroxyl radicals are important only for the LTD in GABAn and contribute to pain primarily through GABAergic disinhibition.
2. Methods
2.1. Animals
Experimental protocols and procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch and are in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals.
2.1.1. Behavior experiments
Ten-week-old C57BL/6J male mice were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were housed on a 12/12 hour light/dark cycle in groups of four in plastic cages with standard bedding and free access to food and water. All behavioral measurements started at 1 pm. All animals were given the chance to acclimate for at least 4 days, including 3 days of handling before any experimental procedures were performed.
2.1.2. Electrophysiology experiments
Three to four-week-old male mice (FVB-Tg(GAD67-GFP) 45704Swn/J, Jackson Laboratory) were used for spinal cord slicing and patch clamp recordings. These mice express GFP exclusively in GABAn, which makes identifying and recording from these cells possible.
2.2. Behavioral testing
Mice were placed on an elevated metal grid and left in plastic chambers to acclimate for 10-15 min. Mechanical sensitivity was assessed by recording the frequency of pain behavior over 10 consecutive von Frey filament (VFF) stimuli, at 20-30 sec intervals. Percent (%) observation frequency of pain-like behaviors was calculated and presented as data in the graphs.
After handling the mice for 3 days, pre-SNL baseline measurements were taken. SNL surgery was then done on Day 0 and the mice were allowed 5 to 7 days to develop mechanical hyperalgesia. On the day of drug administration, pre-drug baseline was measured. Drugs were then administered, and behavioral tests were done 20, 30, 40, 60, and 120 min after the drug administration.
2.3. Spinal nerve ligation (SNL)
Mice were anesthetized with isoflurane (1.5% during induction and 1% during maintenance). A 2 cm incision was made 1 mm to the left of the midline, parallel to the vertebral spines, and the muscles covering the transverse process of the L6 vertebra were removed. A part of the L6 transverse process was removed to expose the L4 and L5 spinal nerves. Without damaging L4 spinal nerve, L5 spinal nerve was ligated using a 7-0 sterile silk thread. The wound was closed by suturing the incision using a 5-0 sterile silk thread and cleaned with alcohol wipes. The mice were then treated once with the analgesic buprenorphine (0.03 mg) immediately after the surgery. Complete recovery was monitored closely before they were transferred to their corresponding cages5.
2.4. Drug treatments
All tested drugs were injected into the intrathecal space by a direct lumbar puncture method using a 10 μL Hamilton syringe28. While mice were under anesthesia (1.5% isoflurane), the needle was introduced dorsally in between the L5 and L6 vertebrae until the tail flick reflex was observed. Five microliters of drug were injected slowly and the needle was held in place for an additional 4-5 sec to prevent drug leakage into the epidural space. The dorsal lower back was disinfected using alcohol wipes before and after injection. Behavior results showed a complete recovery to the pre-drug treatment levels after 4 hr, with each drug treatment.
Behavior experiments were performed to test the following drugs: a superoxide radical donor, potassium superoxide (KO2); a hydroxyl radical donor, tert-Butyl hydroperoxide (tBOOH); a superoxide radical scavenger, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL)23 and the hydroxyl radical scavengers, dimethyl sulfoxide (DMSO) and dimethylthiourea (DMTU)37. To test whether mechanical hyperalgesia is due to ROS-induced GABA dysfunction, the effects of GABAA and GABAB receptor antagonists (Bicuculline and CGP46381)39 in combination with ROS scavengers were also examined. All drugs were diluted in saline just prior to injection.
Electrophysiological experiments were performed to test the following drugs: KO2 (1 mM) and tBOOH (1 mM), TEMPOL (1 mM) and DMTU (20 mM). In both behavioral and electrophysiological experiments, multiple doses were tested for each drug in a preliminary study, and the doses used in the experiments were chosen from among the tested doses.
2.5. Identification of STTn and GABAn
Three to four-week-old mice were anesthetized (1.5 % isoflurane) and then placed on a rodent stereotactic apparatus supplied with a mouse adapter (Stoelting, Wood Dale, IL). To identify STTn, a retrograde tracer (1% FAST-DiI in 10% ethanol; Invitrogen, Carlsbad, CA) was injected into the ventrobasal thalamus using a 5 μL Hamilton syringe needle. Coordinates of the injection site relative to bregma were: 1.48 mm posteriorly, 1.37 mm laterally, and 3.3 mm deep into the brain using the adjusted ratio of bregma-lambda distance specific for young mice (4.5 mm)14. Dye was allowed 5-7 days post injection to be transported to the lumbar spinal cord, after which the mice were euthanized for spinal cord slicing and patch clamp recording.
2.6. Whole-cell recordings
Whole-cell patch clamp recordings were made from acute spinal cord slices of mice. Spinal cord was removed while mice were under isoflurane anesthesia and transferred into cold high-magnesium artificial cerebrospinal fluid (high Mg CSF) (in mM: 117 NaCl, 3.6 KCl, 2.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 11 Glucose, aerated with 95% O2 and 5% CO2, 0-4 °C). The lumbar part of the spinal cord was cut transversely at 350 μm thickness using a vibratome VT1000S (Leica Biosystems, Buffalo Grove, IL). Cord slices were first incubated in high Mg CSF for 30 min then moved to standard CSF (in mM: 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 11 Glucose; 2 ml/min) for another 30 min at 34 °C. Slices were then kept at room temperature for the rest of the experimental time. The recording chamber was superfused with standard CSF at room temperature. An Olympus fluorescence microscope (BX51W1) was used to visualize STTn and GABAn. For establishing whole-cell patch clamp, we used a glass patch pipette (4-6 MΩ) pulled by Flaming Brown Micropipette Puller, model P-97 (Sutter Instrument, Novato, CA). The pipette was filled with internal solution containing (in mM):120 K-gluconate, 10 KCl, 2 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA, 20 HEPES, and 10 phosphocreatine. All chemicals were from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
Evoked EPSCs were recorded in a voltage-clamp mode using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and pCLAMP 10 data acquisition software (Molecular Devices). EPSCs were evoked by focal electrical stimulation in the vicinity of recorded neurons with a glass micropipette electrode (2-4 MΩ). STTn and GABAn in the laminae I-Il were identified under a fluorescence microscope for recording. A holding potential was -70 mV. Test pulses were given at a 20 sec interval (0.5 ms, 100-170 μA). Recording was made only when the EPSCs were monosynaptic, based on EPSC waveforms with a short latency, single peak, and stable responses to repeated stimuli. All recordings showing polysynaptic response were disregarded. In addition, the glutamatergic nature of EPSCs was verified at the end of recording period by their sensitivities to ionotropic glutamate receptor blockers AP-5 and CNQX.
To induce synaptic plasticity via electrical stimulation, conditioning stimulation (CS) was applied (at 2 Hz for 40 sec = a total of 80 pulses with 0.5 ms pulse width, at the same intensity as the test stimulus [range: 100-170 μA]). Synaptic plasticity was also induced via chemical stimulation; patched neurons were exposed to the chemicals for 10 min right after obtaining baseline recordings (3 min), and then chemicals were washed out with standard CSF for the rest of the recordings. The amplitudes of 3 consecutive EPSCs were averaged and normalized to the baseline recorded before the application of the CS or chemical stimulation for each cell. Plasticity was defined as a greater than 20% change in EPSCs amplitude from baseline over a period of 15 to 25 minutes after CS32.
2.7. Data analyses
Data are expressed as mean ± SEM with N, the number of mice and n, the number of neurons. Data were analyzed by one-way or repeated-measures two-way (time-dependent behavioral/electrophysiological responses) ANOVA with Holm-Sidak multiple comparison tests (pre-drug/baseline responses vs. responses at other time points within a treatment; between treatments at a given time point). P < 0.05 was considered significant. The amplitudes of EPSCs after CS or drug treatments were normalized to the mean amplitude of baseline EPSCs recorded for 3 min. Three EPSCs were obtained per minute and their amplitudes were averaged to represent EPSC amplitudes at 1-min intervals.
3. Results
3.1. Shaking behavior best represents neuropathic mechanical pain status
Despite the common use of withdrawal response to VFF stimuli as a reliable method to detect mechanical allodynia and hyperalgesia in neuropathic pain models, the debate about the validity of the test, in terms of reflecting pain perception, continues to exist13. Researchers have thus developed several methods to improve the quality of the obtained results and reduce inter-experimenter variability4,5,31,35. However, the question remains whether the observed response represents complex pain perception or is just a simple spinal reflex.
Several types of complex behaviors have been reported throughout literature as signs of positive pain responses, such as paw licking, guarding, and shaking29. Any one of these responses is usually considered a positive pain response. In an attempt to refine the VFF method in this study, we categorized the withdrawal response into several different responses: flinch, shake, lick, and hold. Flinch is defined by a quick (< 1 sec) paw withdrawal after which the paw is replaced back on the metal mesh floor in its previous position. Shake is defined by withdrawing the paw, shaking the paw at least twice while holding it up before replacing it back on the mesh floor. Lick is defined as licking the paw at least once after stimulation. Hold is defined by holding the paw towards the abdomen and maintaining the holding position for longer than 4 seconds before replacing it back on the mesh. Common pain behavior quantification methods combine all the explained behaviors under “paw withdrawal”.
The mouse hind paw is illustrated in Figure 1A. The most pain-sensitive spot after SNL surgery is marked under the shaded, which represents the area where VFF stimulations were applied. Any responses due to stimulations outside the marked area were discarded.
Figure 1.
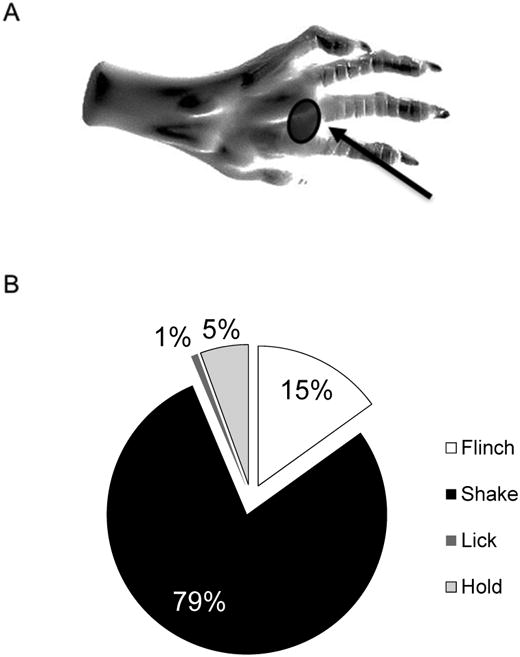
Shake behavior best represents mechanical pain in the mouse. (A) Graphic illustration of a mouse hind paw showing the area stimulated (shaded area) by von Frey filaments (VFF) in pain testing (digital illustration of the rat paw: http://toxic929.deviantart.com/art/Rat-Paw-Sketch-213935890). (B) Pie chart showing relative occurrence (%) of four subcategorized paw withdrawal behaviors (flinch, shake, lick, and hold)in response to VFF stimulation in the spinal nerve ligation (SNL) mouse model of neuropathic pain. Unlike quick reflex-like flinch behavior, shake behavior, the most prevalent withdrawal response type, was often occurred with lick and hold behaviors, and thus was chosen to represent neuropathic mechanical pain in this study. The relative occurrences were calculated from total 74 withdrawal responses in 9 mice.
In response to VFF (#3.00, 0.1 g force) stimulation, mice showed each finch, shake, lick and hold behavior. The relative occurrence of flinch behavior alone, being the closest to a simple reflex, was 15% (out of total 74 withdrawals observed in 9 mice). Shake behavior was the most prevalent (79%) and often occurred with lick and hold; lick alone was only 1% and hold alone, 5% (Fig. 1B). The significant overlap of shake with lick and hold, but not with flinch, suggests that shake is less likely to represent a simple spinal reflex. Therefore, according to the obtained observation frequencies, we chose shake behavior as the pain perception behavior to be used in the rest of our experiments. The number of paw shake occurrences in 10 VFF stimulations was measured and designated as paw shake frequency in all the behavioral experiments' figures to follow.
3.2. Effects of superoxide and hydroxyl radicals' donors and scavengers on pain behavior
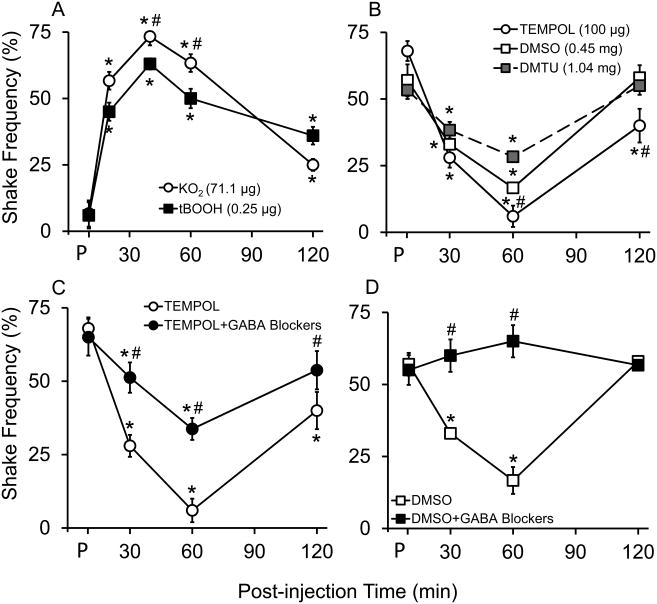
Several doses of each drug were tried in preliminary studies, and the dose with the maximum effect for each drug was chosen for our experiments. The effects of superoxide radical donor (KO2, 71.1 μg) and hydroxyl radical donor (tBOOH, 0.25 μg) on shake pain behavior are shown in Figure 2A. Each ROS donor was injected intrathecally in normal C57BL/6J mice, and percent paw shaking frequency upon VFF (#3.0) stimulation was measured at pre-drug, 20, 40, 60, and 120 min post-drug injection. Both ROS donors increased the shaking responses transiently, peaking at 40 min. The peak response induced by KO2—75 ± 3.3% (N=6) increase in paw shake frequency— was significantly higher than that induced by tBOOH (65 ± 3.6%, N=6, p<0.05 at 40 min by Holm-Sidak test following 2-way ANOVA). As it is understandable that a one-time ROS donors' injection does not represent injury-induced ROS generation, the aim of this experiment was to point out the direct involvement of ROS in pain behavior regardless of the source and mechanism.
Figure 2.
Specific reactive oxygen species (ROS) subtype donors and scavengers affect pain behaviors of naïve and neuropathic mice in different magnitudes. Behavioral responses of 10 weeks old C57BL/6 mice to VFF (# 3.00 = 0.1 g force) stimulations at pre-drug (P) and post-intrathecal drug injections (5 μL). (A) Superoxide radical donor (KO2; 71.1 μg, N=6) induced greater hyperalgesia than hydroxyl radical donor (tBOOH; 0.25 μg, N=6) in naïve mice. (B) Superoxide radical scavenger (TEMPOL; 100 μg, N=8) induced greater anti-hyperalgesia than hydroxyl radical scavengers (DMSO; 0.45 mg and DMTU; 1.04 mg, N=8) in neuropathic mice. (C) Combination of GABAA (Bicuculline, 1 μg) and GABAB (CGP46381, 0.5 μg) receptor antagonists partially blocked TEMPOL's anti-hyperalgesic effect in neuropathic mice (N=8). (D) The GABA receptor antagonists completely blocked DMSO's anti-hyperalgesic effect in neuropathic mice (N=8). Data were analyzed by 2-way repeated measures ANOVA followed by Holm-Sidak multiple comparison test; values at multiple time points within a group were compared with the group's pre-drug value (P). Effects of ROS scavengers on pain behaviors were compared between treatments at each time point. Data were presented as mean ± SEM (*, different from pre-drug within a treatment; #, different between treatments, P<0.05).
To better investigate the effect of nerve injury-induced ROS generation on pain behavior, the effects of specific ROS scavengers—TEMPOL, DMTU and DMSO—were then tested for anti-hyperalgesic effect in SNL mice. Baseline pain behavior measurements were taken just before every drug injection, 7 days after SNL. As shown in Figure 2B, all SNL mice developed mechanical hyperalgesia, showing on average 70% shaking frequency 7 days after the surgery. TEMPOL (100 μg, N=8) caused a 93 ±4.0% reduction in paw shaking frequency at 1 hour after injection. DMTU (1.04 mg, N=8) and DMSO (0.45 mg, N=8), however, caused a 32 ± 4.0% and 70± 1.8% reduction in paw shaking behavior, respectively. Two hours after TEMPOL, DMTU, or DMSO injection, the levels of hyperalgesia were returned to the pre-drug treatment levels. The level of anti-hyperalgesia produced by TEMPOL was statistically higher than that by DMSO or DMTU (p<0.05 vs. DMSO and p<0.05 vs. DMTU at 60 min by Holm-Sidak test following 2-way ANOVA).
To isolate GABAergic contribution to the observed anti-hyperalgesic effects of ROS scavengers, the effect of GABA receptor (GABA-R)antagonists was tested in combination with each specific ROS scavenger using the following treatments: TEMPOL (100 μg, N=8), TEMPOL + GABA-R antagonists (Bicuculline, 1 μg and CGP46381, 0.5 μg, N=8), DMSO (0.45 mg, N=8), and DMSO + GABA-R antagonists (N=8). The effects of either TEMPOL or DMSO alone (shown in Fig. 2B) are in comparison to the combined treatments. GABA-R antagonists only partially reversed TEMPOL-induced analgesia (Fig. 2C) but completely negated DMSO-induced analgesia (Fig. 2D).
3.3. LTP develops in STTn via both electrical stimulation (CS) and nerve injury (SNL)
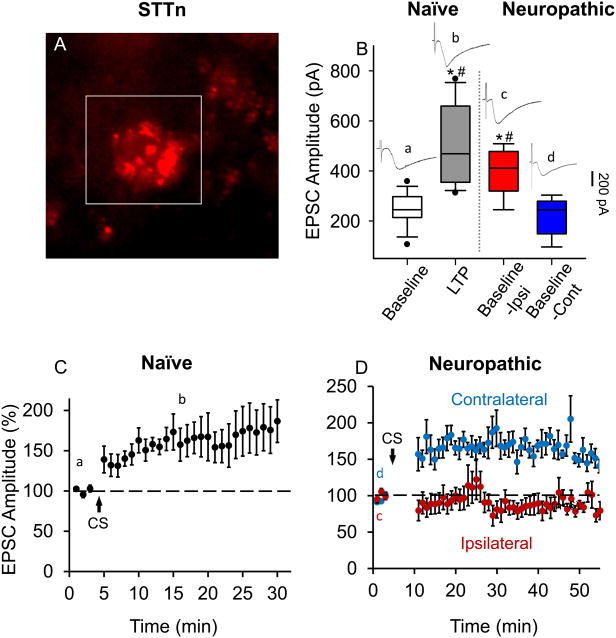
Neuropathic pain-related synaptic plasticity is rarely investigated in neuropathic pain animal models. Rather, it is often investigated in naïve mice and artificially induced via a conditioning stimulus (CS). As reported in our previous study, CS-induced synaptic plasticity in STTn and GABAn of the dorsal horn is cell-type specific14. In this study, we ought to seek evidence for STTn-LTP establishment in spinal nerve-ligated mice (SNL mice) to better correlate our experimental conditions to pathophysiological conditions. Figure 3 explores long-term potentiation in STTn after SNL. STTn were identified using the retrograde tracer DiI (Fig. 3A). After obtaining a whole cell patch condition, we collected and compared raw baseline EPSC values at four states (lowercase letters correspond to regions where representative EPSC traces were collected, as shown in the figures): (a) Baseline (pre-CS, n=9, N=9) in naïve mice, (b) LTP state (post-CS, n=9, N=9) in naïve mice, (c) Baseline in the ipsilateral side of SNL mice (n=6, N=6), and (d) Baseline in the contralateral side of SNL mice (n=6, N=5). Figure 3B shows that the averages of raw EPCS amplitudes taken from states (b) and (c) are significantly higher than the averages of those taken from (a) and (d). This indicates that baseline EPSC levels in STTn in the ipsilateral side of SNL mice are already elevated (in comparison to baseline EPSCs in naïve STTn or SNL-contralateral STTn) and fall in the same range of LTP state EPSCs in STTn from naïve mice. Figure 3C shows CS-induced LTP in STTn from naïve mice (n=7, N=7). When the same CS was applied to STTn in the ipsilateral and contralateral side of SNL, LTP was occluded in the former but normally developed in the latter (Fig. 3D, n=6, N=6). This suggests that STTn in the ipsilateral side of SNL are already in the LTP state and thus cannot be further potentiated by CS.
Figure 3.
Long-term potentiation (LTP) is already established in spinothalamic tract neurons (STTn) in the ipsilateral side of spinal nerve ligation (SNL). (A) STTn labeled with a retrograde tracer DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) in the superficial laminae of the dorsal horn in a live L5 spinal cord slice from FVB-Tg(GAD67-GFP) mice. (B) Box-plot showing medians, ranges between 25th and 75th percentiles (boxes), and whiskers representing minimum and maximum values of raw excitatory postsynaptic current (EPSC) amplitudes (pA) of (a) baseline (pre-CS;CS=conditioning stimulation, n=9, N=9) and (b) LTP state (15 min after CS, n=9, N=9) in naïve mice, compared to baselines in the (c) ipsilateral (Baseline-Ipsi, n=6, N=6) and (d) contralateral (Baseline-Cont, n=6, N=5) sides of SNL mice. Baseline-Ipsi was significantly higher in amplitude than the baseline in naïve STTn and lies in the range of LTP observed in naïve STTn, whereas Baseline-Cont was similar to the baseline in naïve STTn under our stimulation parameters. Individual EPSC traces, designated by lowercase letters show their corresponding areas of collection. Data were analyzed using 1-way ANOVA followed by Holm-Sidak multiple comparison tests (*, different from (a); #, different from (d); P< 0.05). (C) LTP developed in naïve STTn after CS (2 Hz for 40 seconds, holding potential at +30 mV, n=7, N=7). (D) EPSCs were not potentiated in STTn in the ipsilateral side of SNL after CS application (red, n=6, N=6), whereas they developed normally in the contralateral side (blue, n=6, N=5). Stimulation event interval was set at 20 seconds resulting in three EPSC values per minute. Individual EPSC values were normalized to the mean of baseline (before CS). Then each of the three normalized EPSC values (per minute) were averaged and plotted vs. time. All data were obtained from adult 4 weeks old mice.
3.4. LTD develops in GABAn via both electrical stimulation (CS) and nerve injury (SNL)
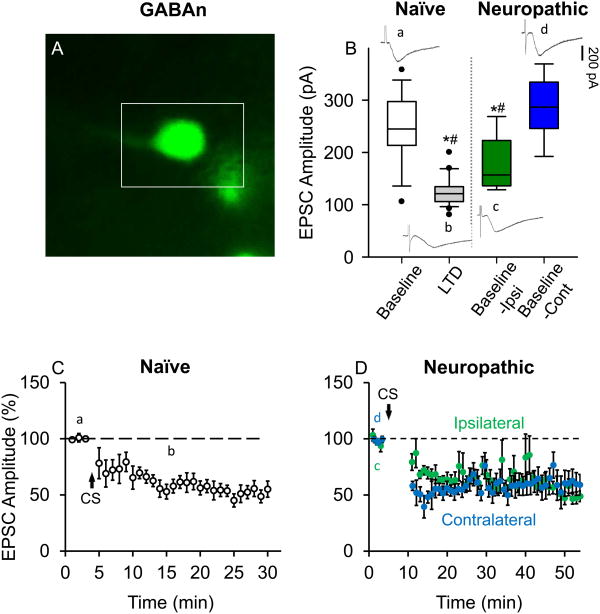
The same experiments done in figure 3 on STTn were repeated on GFP-labeled GABAn (Fig. 4A). Similarly, we compared the averages of raw baseline (pre-CS) EPSC magnitudes recorded from GABAn in (a) naïve mice (n=9, N=9), (c) in the ipsilateral side of SNL mice (n=6, N=6) and (d) in the contralateral side of SNL mice (n=6, N=5) with the magnitude of (b) LTD state EPSCs from GABAn in naïve mice (n=9, N=9). Baseline EPSCs in the ipsilateral side of SNL mice were comparable with LTD state EPSCs in naïve mice, being smaller than baseline EPSCs in naïve or the contralateral side of SNL mice (Fig. 4B). Following CS, GABAn-LTD developed in naïve mice (Fig. 4C, n=7. N=7) as well as in both the ipsilateral and contralateral sides of SNL mice (Fig. 4D, n=6, N=6).
Figure 4.
Long-term depression (LTD) is already established in GABAergic interneurons (GABAn) in the ipsilateral side of spinal nerve ligation (SNL). (A) GABAn GFP (green fluorescent protein)-tagged in FVB-Tg(GAD67-GFP) in the superficial laminae of the dorsal horn in a live L5 spinal cord slice. (B) Box-plot showing medians and ranges between 25th and 75th percentiles (boxes), and whiskers representing minimum and maximum values of raw EPSC amplitudes (pA) of (a) baseline (pre-CS, n=9, N=9) and (b) LTD state (15 min after CS, n=9, N=9) in naïve mice, compared to baselines in the (c) ipsilateral (Baseline-Ipsi, n=6, N=6) and (d) contralateral (Baseline-Cont, n=6, N=5) sides of SNL mice. Baseline-Ipsi was lower in amplitude than the baseline in naïve GABAn and lies in the range of LTD observed in naïve GABAn, whereas Basline-Cont was similar to the baseline in naïve GABAn under our stimulation parameters. Individual EPSC traces, designated by lowercase letters, show their corresponding areas of collection. Data were analyzed using 1-way ANOVA followed by Holm-Sidak multiple comparison tests (*, different from (a); #, different from (d); P< 0.05). (C) LTD developed in GABAn in naïve mice (n=7, N=7) and (D) both in the ipsilateral (n=6, N=6) and contralateral (n=6, N=5) sides of SNL mice after CS (2 Hz for 40 seconds, holding potential at +30 mV). Stimulation event interval was set at 20 seconds resulting in three EPSC values per minute. Individual EPSC values were normalized to the mean of baseline (before CS) EPSC values. Then each of the three normalized EPSC values (per minute) were averaged and plotted vs. time. All data were obtained from adult 4 weeks old mice.
3.5. Superoxide and hydroxyl radicals are differentially involved in synaptic plasticity in STTn vs. GABAn
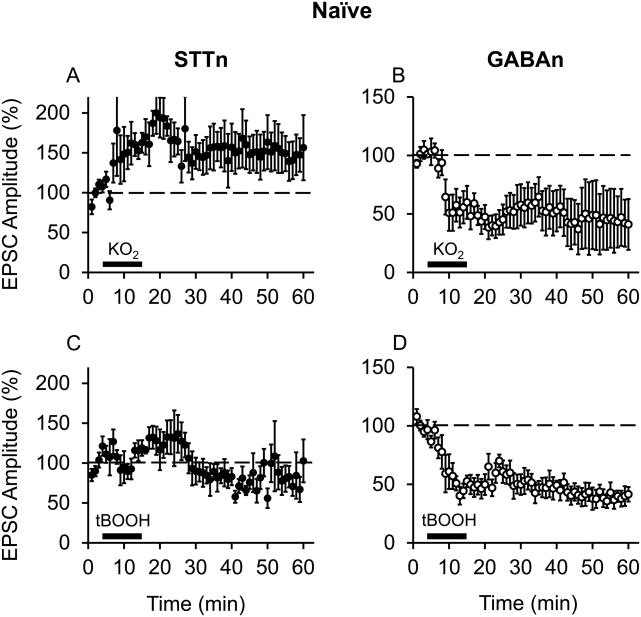
This experiment tested the effects of superoxide and hydroxyl radical donors on synaptic plasticity in STTn and GABAn. Figure 5 shows the effects of two types of ROS donors: a superoxide donor, potassium superoxide (KO2, 1 mM; n=5, N=5), and a hydroxyl radical donor, t-butyl-hydroperoxide (tBOOH, 1 mM; n=5, N=5). Each ROS donor was superfused into the recording chamber after obtaining baseline whole-cell patch clamp recordings on both STTn and GABAn. Following treatment with KO2 (without CS), STTn showed increased amplitude of EPSC resembling CS-induced LTP (Fig. 5A), while GABAn showed LTD-like reduction in EPSC (Fig. 5B). However, tBOOH failed to cause changes in the amplitude of EPSC in STTn (Fig. 5C) though it did induce LTD in GABAn (Fig. 5D). These results indicate that exogenous superoxide radicals, as electrical CS, activate cell type-specific synaptic plasticity mechanisms for both STTn-LTP and GABAn-LTD, whereas exogenous hydroxyl radicals are only able to activate LTD-inducing mechanism in GABAn.
Figure 5.
Superoxide radicals induce LTP in STTn and LTD in GABAn, but hydroxyl radicals are important only for GABAn-LTD. (A and B) Induction of STTn-LTP (n=5, N=5) and GABAn-LTD (n=5, N=5) by a superoxide radical donor (KO2, 1 mM). (C and D) Induction of GABAn-LTD (D, n=5, N=5) but not STTn-LTP (C, n=5, N=5) by a hydroxyl radical donor (tBOOH, 1 mM). ROS donors were superfused into the recording chamber for 10 min (thick black bar) after 3 min of baseline EPSC measurements. All data were obtained from adult 4 weeks old mice.
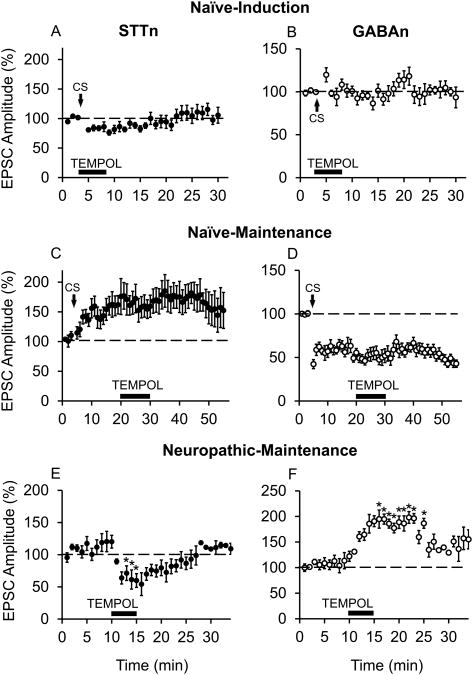
3.6. Superoxide radical scavenger TEMPOL blocked CS-induced induction and altered SNL-induced maintenance of STTn-LTP and GABAn-LTD
To examine the role of different subtypes of endogenous free radicals in cell type-specific synaptic plasticity, free radical subtype-specific scavengers were tested on STTn-LTP and GABAn-LTD induction and maintenance phases. For induction phase experiments, TEMPOL (1 mM) superfusion into the recording chamber was started simultaneously with CS application and lasted for a total of 5 min. TEMPOL completely blocked the induction of both STTn-LTP (Fig. 6A n=6, N=6) and GABAn-LTD (Fig. 6B, n=8, N=6), indicating that superoxide radicals are critical for the induction of cell-type specific synaptic plasticity in the dorsal horn.
Figure 6.
Superoxide radical scavenger blocks CS-induced induction and SNL-induced maintenance of STTn-LTP and GABAn-LTD. A superoxide radical scavenger TEMPOL (1 mM), applied for 5 min during CS, blocked the CS-induced STTn-LTP (A, n=6, N=6) and GABAn-LTD (B, n=8, N=6). The same dose of TEMPOL (1 mM), applied for 10 min during the CS-induced maintenance phase (16 min after CS), failed to reverse STTn-LTP (C, n=7, N=7) or GABAn-LTD (D, n=6, N=6). However, 1 mM of TEMPOL, applied 10 min after obtaining EPSC baseline 7 days post-SNL, successfully decreased evoked EPSC amplitude in STTn in ipsilateral side of SNL (E, n=5, N=5) and increased evoked EPSC amplitude in GABAn in ipsilateral side of SNL (F, n=5, N=5). No CS was applied in E and F. All data were obtained from adult 4 weeks old mice. Data in E and F were obtained from adult 4 weeks old mice, 1 week after SNL. The latter data were analyzed using 1-way ANOVA followed by Holm-Sidak multiple comparison tests (*, different from pre-drug at 10 min; P<0.05).
To investigate the possible role of superoxide radicals in the maintenance phase of LTP and LTD, the same dose of TEMPOL (1 mM) was applied for 10 min, 16 min after CS when STTn-LTP and GABAn-LTD were established. TEMPOL (1 mM) failed to reverse CS-induced LTP in STTn (Fig. 6C, n=7, N=7) and LTD in GABAn (Fig. 6D, n=6, N=6).
Nevertheless, the lack of effect of TEMPOL on the maintenance phase of CS-induced STTn-LTP and GABAn-LTD does not correlate with the significant analgesic effect of TEMPOL observed behaviorally. It becomes worthy to mention that TEMPOL's analgesic effect was observed in ‘SNL mice’, whereas CS-induced STTn-LTP was done in ‘naïve mice’, therefore not entirely reflecting the pathophysiological environment of a chronic pain model. In addition, EPSCs in STTn were shown to be potentiated and those in GABAn depressed at rest in the ipsilateral side of SNL mice (Figs. 3 and 4). Therefore, we examined the effect of TEMPOL (1 mM) on evoked EPSCs recorded from STTn and GABAn in the ipsilateral side of SNL mice. Baseline (immediately pre-drug in this case) was obtained for 10 min, then TEMPOL was superfused for 5 min. TEMPOL was able to significantly decrease STTn EPSC amplitudes (Fig. 6E, n=5, N=4) and increase GABAn EPSC amplitudes (Fig. 6F, n=5, N=4), indicating that TEMPOL is effective in alleviating the chronic maintenance phase of STTn-LTP and GABAn-LTD in a neuropathic pain condition. These results agree with TEMPOL's analgesic effect observed 7 days after SNL.
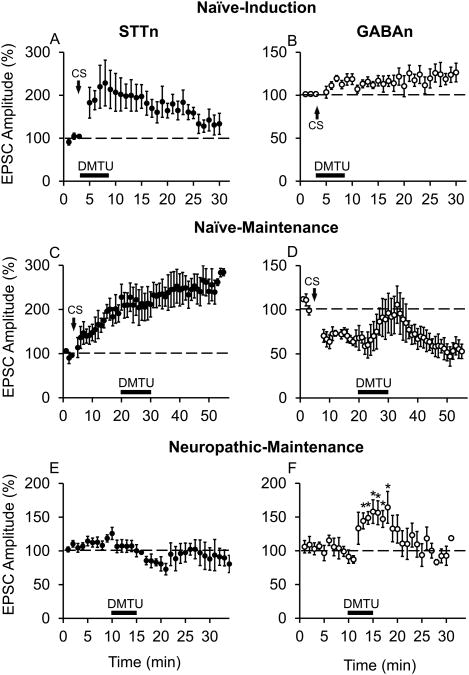
3.7. Hydroxyl radical scavenger affected both the induction and maintenance of GABAn-LTD but not STTn-LTP
We tested the effects of scavenging endogenous hydroxyl radicals on synaptic plasticity in STTn and GABAn. A hydroxyl radical scavenger, DMTU (20 mM), did not interfere with the induction of LTP in STTn (Fig. 7A, n=8, N=7) but did impede the induction of LTD in GABAn (Fig. 7B, n=7, N=6). The same dose of DMTU failed to affect the CS-induced maintenance phase of LTP in STTn (Fig. 7C, n=6, N=6) but fully reversed CS-induced maintenance phase of LTD in GABAn (Fig. 7D, n=5, N=5). Consistent results were obtained when DMTU (20 mM) was applied on STTn and GABAn at immediately pre-drug time-point in the ipsilateral side of SNL mice. DMTU failed to significantly alter evoked EPSC amplitudes in STTn (Fig. 7E, n=5, N=5) but significantly increased evoked EPSC amplitudes in GABAn (Fig. 7F, n=5, N=5) from the neuropathic mice. Results thus suggest that hydroxyl radicals are crucial for GABAn-LTD but not for STTn-LTP induction and maintenance. We also attempted to use DMSO as a hydroxyl radical scavenger in this experiment due to its significant analgesic effect; however, giga-seal integrity was often compromised and no reliable recording could be obtained.
Figure 7.
Hydroxyl radical scavenger blocks both the induction and maintenance of GABAn-LTD but does not interfere with STTn-LTP. A hydroxyl radical scavenger DMTU (20 mM), applied for 5 min during CS, blocked the CS-induced GABAn-LTD (B, n=7, N=6) but not STTn-LTP (A, n=8, N=7). The same dose of DMTU (20 mM), applied for 10 min during the maintenance phase (16 min after CS), reversed GABAn-LTD (D, n=5, N=5) but not STTn-LTP (C, n=6, N=6). Consistent selectivity to GABAn was observed when DMTU (20 mM) was applied 10 min after obtaining baseline EPSCs in SNL mice. DMTU significantly increased EPSC amplitude in GABAn in the ipsilateral side of SNL (F, n=5, N=5), whereas it failed to affect EPSC amplitude in STTn in the ipsilateral side of SNL (E, n=5, N=5). No CS was applied in E and F. All data were obtained from adult 4 weeks old mice. Data in E and F were obtained from adult 4 weeks old mice, 1 week after SNL. The latter data were analyzed using 1-way ANOVA followed by Holm-Sidak multiple comparison tests (*, different from pre-drug at 10 min; P<0.05).
3.8. Conceptual representation of the possible role of specific ROS subtypes in cell type-specific synaptic plasticity
Figure 8 shows a conceptual box diagram representing a hypothesis formulated from the obtained results and previous studies in our laboratory. This hypothesis suggests a specific role of ROS subtypes in pain-related synaptic plasticity14. In more details, excitatory STTn and inhibitory GABAn receive excitatory inputs. Following nerve injury and aberrant afferent stimulation, calcium enters the cells through NMDA and AMPA receptor channels. This, in turn, leads to downstream production of secondary messengers including ROS. Our interpretation of the present results is that superoxide radicals are the main form of ROS accumulating in STTn and driving LTP. However, in GABAn, both superoxide radicals and hydroxyl radicals accumulate and lead to the induction and maintenance of LTD. LTP in STTn will increase STTn excitability. Moreover, LTD in GABAn will inhibit GABAn inhibition of STTn and further increase STTn excitability. Mechanisms of the differential increase in ROS subtypes in STTn vs. in GABAn are currently under investigation.
Figure 8.
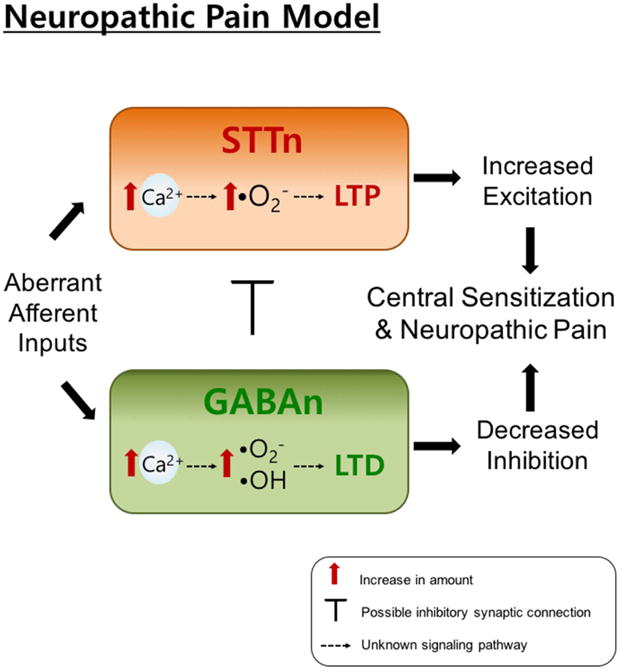
Hypothesized/conceptual role of specific ROS subtypes in cell type-specific synaptic plasticity. We hypothesize that following aberrant afferent activities caused by nerve injury, calcium influx through N-Methyl-D-aspartic acid (NMDA) receptors will lead to an increase of superoxide radicals in both STTn and GABAn. In GABAn, hydroxyl radicals are also generated in addition to superoxide radicals. Superoxide radicals in STTn will then lead to LTP, whereas both superoxide and hydroxyl radicals in GABAn will cause LTD. These two opposing cell-type specific synaptic plasticity will cause increased excitation of STTn and decreased inhibition by GABAn, which results in central sensitization that contributes to neuropathic pain.
Discussion
The polarity of synaptic plasticity (LTP or LTD) in the brain is believed to be dependent on stimulation parameters (frequency of stimulation)10,11,26,27. In the spinal cord, however, the situation seems to be different in that LTP and LTD in the spinal dorsal horn are cell type-specific: LTP develops in excitatory STTn, whereas LTD develops in inhibitory GABAn14. The results of the present study suggest that two different biologically active ROS subtypes—superoxide and hydroxyl radicals—might be contributing to this cell type-specific synaptic plasticity. The results further indicate that superoxide radicals are crucial for the induction and chronic pathological maintenance of both STTn-LTP and GABAn-LTD, whereas hydroxyl radicals are only involved in GABAn-LTD induction and maintenance.
Our behavioral data show that a superoxide radical scavenger TEMPOL induces greater analgesic effect than a hydroxyl radical scavenger DMTU and DMSO in neuropathic mice. Similarly, the superoxide radical donor KO2 induces higher levels of hyperalgesia than the hydroxyl radical donor tBOOH at the peak time point in naïve mice. In addition, when spinal cord GABA function is blocked with GABA-R antagonists, DMSO loses its analgesic effect, while TEMPOL stays partially effective. While it is possible that the GABA-R antagonists induced pain-like behavior through a separate non-ROS mediated mechanism, one explanation of the results propose that hydroxyl radicals might downregulate mainly the GABA-mediated pain modulatory system, whereas superoxide radicals’effects extend to more than the GABA modulatory system. This assumption is further supported by a study done by Yowtak et al. showing that tBOOH (hydroxyl radical donor) suppresses GABAergic inhibitory transmission without affecting either glycinergic or excitatory transmission in substantia gelatinosa (SG) neurons39. This suggests the significance of hydroxyl radicals in GABAergic inhibitory transmission more so than in excitatory transmission. No other studies have investigated the distinct involvement of different ROS subtypes in the excitatory-inhibitory balance in the pain circuitry of the dorsal horn at the behavioral level.
We were able to clearly show the differential involvement of superoxide and hydroxyl radicals in synaptic plasticity induction in STTn and GABAn of the dorsal horn. Knowing that the maintenance phase of STTn-LTP and GABAn-LTD could be more relevant to the chronic nature of neuropathic pain, we tested the effects of ROS scavengers on the maintenance of STTn-LTP and GABAn-LTD in naïve mice. The doses of TEMPOL and DMTU, which blocked the induction phase, were tested on the maintenance phase of CS-induced LTP and LTD. TEMPOL, which exerted the highest analgesic effect in neuropathic mice, failed to reverse both CS-induced STTn-LTP and GABAn-LTD maintenance, and DMTU was exclusively effective in reversing CS-induced GABAn-LTD maintenance. However, when these compounds were tested in neuropathic mice, different results were obtained. TEMPOL and DMTU were able to attenuate the SNL-induced changes in excitatory synaptic strength on STTn and GABAn in the same differential manner observed in the induction phase in naïve mice. These results confirm the significance of both superoxide radicals and hydroxyl radicals in dorsal horn neuropathic pain-induced synaptic plasticity and reaffirm the exclusive influence of hydroxyl radicals on GABAn. These results also shed light on possible variances between CS-induced and nerve injury-induced synaptic plasticity in the spinal dorsal horn.
As was suggested earlier, not much has been uncovered concerning the differential involvement of ROS subtypes in excitatory vs. inhibitory synaptic plasticity in the spinal cord. Superoxide and hydrogen peroxide are the most studied reactive oxygen species because of their high abundancy and stability in comparison to other ROS types like hydroxyl radicals34. Where superoxide induced and maintained LTP, hydrogen peroxide, which is generated from superoxide and is potentially a hydroxyl radical precursor24, was shown to attenuate LTP and even facilitate LTD by exploiting LTD signaling machinery—such as calcineurin— in the brain2. Along similar lines, hydrogen peroxide was also shown to increase both amplitude and frequency of miniature inhibitory postsynaptic currents of GABAergic interneurons in the substantia gelatinosa (SG) of the spinal cord. This last study suggests that hydrogen peroxide acts both post- and pre-synaptically to regulate IP3 receptor-regulated calcium pools, leading to prolonged GABAergic inhibitory depression34. A hydroxyl radical donor tBOOH produced similar effects and was, above all, selectively effective in depressing SG inhibitory and not excitatory transmission39. Together, these findings support the notion suggested in our study stating that hydroxyl radicals are important for GABAergic inhibitory more than for excitatory function of dorsal horn circuitry.
The polarity of synaptic plasticity has been mostly correlated with the size of calcium influx into the neuron. In hippocampal neurons, high calcium influx leads to the activation of kinases like protein kinase C, protein kinase A, Ca2+/calmodulin-dependent protein kinase II, and extracellular signal-regulated protein kinases, and thus activates LTP-inducing machinery3,6,8,14, whereas low calcium influx activates phosphatases such as calcineurin and induces LTD30. This idea has been recently challenged by a study showing that high calcium influx in the cerebellum induces LTD. They explained this phenomenon by appealing to the different spatial and temporal aspects of calcium signaling in the cerebellum: timing, source, and location of calcium influx can greatly influence the type of signaling molecules and thus the machinery activated1. Correspondingly, similar differences in spatial and temporal calcium influx can exist between excitatory neurons (STTn) vs. inhibitory interneurons (GABAn), leading to the accumulation of different types of ROS and thus inducing opposing synaptic plasticity polarity. Experiments are warranted to investigate in depth the spatial and temporal aspects of calcium signaling in STTn vs. GABAn.
A question emerges as to how neurons respond to endogenous vs. exogenous sources of ROS. Exogenous and endogenous ROS applications are not well characterized in terms of their effect on cellular signaling and synaptic plasticity. Exogenous ROS donors like H2O2 or tBOOH are widely used to induce intracellular oxidative stress. On the other hand, TEMPOL and DMTU are commonly used, well characterized, cell permeable endogenous ROS scavengers. However, it is not clear whether endogenous and exogenous ROS exposure influence the cellular proteins and signaling cascades similarly or differently2. In our study, we investigated the effect of both ROS donors and scavengers on STTn and GABAn synaptic excitability. Our results show high consistency in terms of the specific ROS donors' and scavengers' effects on cell type-specific synaptic plasticity (refer to figures 5, 6, 7). Without going into the details of ROS-mediated signaling cascades, our results indicate that exogenous and endogenous ROS may play similar roles in synaptic plasticity mechanism. In addition, other studies have shown that exogenous ROS species induce similar patterns of protein activation (e.g., protein kinase C, Ca2+/calmodulin-dependent protein kinase II, AMPA, cAMP response element-binding protein, extracellular signal-regulated kinases) to that induced by nerve injury- or conditioning stimulus-increased ROS2,8. Furthermore, our previous study showed that CS- and tBOOH-induced synaptic plasticity cancel each other supporting that endogenous and exogenous ROS might share a signaling cascade or converge at a certain step along the pathway when it comes to synaptic plasticity22. Further experimentation is warranted to investigate the specific roles of endogenous versus exogenous ROS in synaptic plasticity.
In summary, the results of the present study suggest that superoxide and hydroxyl radicals regulate the polarity of synaptic plasticity in a cell type-specific manner; superoxide radicals drive both STTn-LTP and GABAn-LTD, whereas hydroxyl radicals only drive GABAn-LTD. Therefore, the full anti-hyperalgesic effect of ROS scavengers can only be achieved by reducing excitatory drive to STTn as well as restoring GABA inhibition. In other words, recovering GABA function alone is not sufficient for maximum pain reduction. This study highlights the important roles of specific subtypes of ROS in synaptic plasticity in the spinal cord and points the way to new treatment targets and tools for managing neuropathic pain.
Acknowledgments
This study was supported by NIH R01 grant NS031680. There are no financial or other relationships that will lead to a conflict of interest. We thank Dr. Kyungsoon Chung for providing scientific vision and expertise that greatly aided the project. We also thank Mrs. Denise Broker and Mr. David Bayless for their great help in improving the manuscript.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Yowtak J, Wang J, Kim HY, Lu Y, Chung K, Chung JM. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain. 2013;154(11) doi: 10.1016/j.pain.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckhauser TF, Francis-Oliveira J, Pasquale RD. Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity. J Exp Neurosci. 2016;10(S1):23–48. doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33(Pt 6):1354–6. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira N, Attal J, Fermanian H, Alchaar M, Gautron E, Masquelier S, Rostaing M, Lanteri-Minet E, Collin J, Grisart F, Boureau Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 5.Chung J, Kim HK, Chung K. Segmental Spinal Nerve Ligation Model of Neuropathic Pain. Methods in Molecular Medicine. 2004;99:35–45. doi: 10.1385/1-59259-770-X:035. [DOI] [PubMed] [Google Scholar]
- 6.Elgersma Y, Silva AJ. Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol. 1999;9(2):209–213. doi: 10.1016/s0959-4388(99)80029-4. [DOI] [PubMed] [Google Scholar]
- 7.Fidanboylu M, Griffiths LA, Flatters SJL. Global Inhibition of Reactive Oxygen Species (ROS) Inhibits Paclitaxel-Induced Painful Peripheral Neuropathy. PLoS ONE. 2011;6(9):e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwak YS, Hassler SE, Hulsebosch CE. Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 2013;154(9):1699–708. doi: 10.1016/j.pain.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Gwak YS, Hulsebosch CE. GABA and Central Neuropathic Pain following Spinal Cord Injury. Neuropharmacol. 2011;60(5):799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda H, Asai T, Murase K. Robust changes of afferent-induced excitation in the rat spinal dorsal horn after conditioning high-frequency stimulation. J Neurophysiol. 2000;83(4):2412–2420. doi: 10.1152/jn.2000.83.4.2412. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda H, Kusudo K, Ryu PD, Murase K. Effects of corticotropin-releasing factor on plasticity of optically recorded neuronal activity in the substantia gelatinosa of rat spinal cord slices. Pain. 2003;106(1-2):197–207. doi: 10.1016/j.pain.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Kallenborn-Gerhardt W, Schro¨der K, Del Turco D, Lu R, Kynast K, Kosowski J, Niederberger E. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci. 2012;32:10136–10145. doi: 10.1523/JNEUROSCI.6227-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keizer D. Quantitative sensory testing with Von Frey monofilaments in patients with allodynia: what are we quantifying? Clinical Journal of Pain. 2008;24(5):463–466. doi: 10.1097/AJP.0b013e3181673b80. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, Jun J, Wang J, Bittar A, Chung K, Chung JM. Induction of long-term potentiation and long-term depression is cell type-specific in the spinal cord. Pain. 2015;156(4):618–25. doi: 10.1097/01.j.pain.0000460354.09622.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HY, Lee KY, Lu Y, Wang J, Cui L, Kim SJ, Chung JM, Chung K. Mitochondrial Ca(2+) uptake is essential for synaptic plasticity in pain. J Neurosci. 2011;31(36):12982–12991. doi: 10.1523/JNEUROSCI.3093-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: the effect of mitochondrial electron transport complex inhibitors. Neurosci Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111(1-2):116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21(12):505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113(2):200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 20.Klann E. Cell-permeable scavengers of superoxide prevent long-term potentiation in hippocampal area CA1. J Neurophysiol. 1998;80:452–457. doi: 10.1152/jn.1998.80.1.452. [DOI] [PubMed] [Google Scholar]
- 21.Latremoliere A, Woolf CJ. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103(1):382–391. doi: 10.1152/jn.90906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lejeune D. The superoxide scavenger TEMPOL induces urokinase receptor (uPAR) expression in human prostate cancer cells. Mol Cancer. 2006;5:21. doi: 10.1186/1476-4598-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Medicine. 2010;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74(2-3):257–268. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 26.Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78(4):535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 27.Malenka RC, Bear MF. LTP and LTD: An Embarrassment of Riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 29.Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Meth. 1999;87(2):185–193. doi: 10.1016/s0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 32.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5541. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–68. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi A, Mikami M, Yang J. Hydrogen peroxide increases GABAergic mIPSCs through presynaptic release of calcium from IP3 receptor-sensitive stores in spinal cord substantia gelatinosa neurons. Eur J Neurosci. 2007;25(3):705–16. doi: 10.1111/j.1460-9568.2007.05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tena B, Escobar B, Arguis MJ, Cantero C, Rios J, Gomar C. Reproducibility of Electronic von Frey and von Frey monofilaments testing. Clin J Pain. 2012;28:318–323. doi: 10.1097/AJP.0b013e31822f0092. [DOI] [PubMed] [Google Scholar]
- 36.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vander-Heide RS, Sobotka PA, Ganote CE. Effects of the free radical scavenger DMTU and mannitol on the oxygen paradox in perfused rat hearts. J Mol Cell Cardiol. 1987;19(6):615–625. doi: 10.1016/s0022-2828(87)80367-x. [DOI] [PubMed] [Google Scholar]
- 38.Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R, Torebjork E. Towards a mechanism based classification of pain? Pain. 1998;77:227–229. doi: 10.1016/S0304-3959(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 39.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152(4):844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bading H. Nuclear calcium signaling in the regulation of brain function. Nat Rev Neurosci. 2013;14(9):593–608. doi: 10.1038/nrn3531. [DOI] [PubMed] [Google Scholar]