Abstract
The objective of this trial was to evaluate the effects of feed restriction (FR) on serum glucose, nonesterified fatty acids, progesterone (P4), insulin, and milk production in dairy cows. Eight multiparous Holstein cows, 114 ± 14 d pregnant and 685 ± 39 kg of body weight, were randomly assigned to a replicated 4 × 4 Latin square design with 14-d periods. During the first 8 d of each period, cows in all treatments were fed for ad libitum feed intake. Beginning on d 9 of each period, cows received 1 of 4 treatments: ad libitum (AL), 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). Daily feed allowance was divided into 3 equal portions allocated every 8 h with jugular blood samples collected immediately before each feeding through d 14. In addition, on d 12 of each period, blood samples were collected before and at 60, 120, 180, 240, 300, 360, 420, and 480 min after morning feeding. The conventional total mixed ration and total mixed ration with straw averaged 15.1 and 10.8%, 32.1 and 50.5%, and 26.8 and 17.0% for concentrations of crude protein, neutral detergent fiber, and starch, respectively. Cows that were feed and energy restricted had reduced dry matter intake, net energy for lactation intake, circulating glucose concentrations, and milk production, but greater body weight and body condition score losses than AL cows. Circulating concentrations of insulin were lower for cows fed 50FR (8.27 μIU/mL) and 50ST (6.24 μIU/mL) compared with cows fed AL (16.65 μIU/mL) and 25FR (11.16 μIU/mL). Furthermore, the greatest plasma nonesterified fatty acids concentration was observed for 50ST (647.7 μEq/L), followed by 50FR (357.5 μEq/L), 25FR (225.3 μEq/L), and AL (156.3 μEq/L). In addition, serum P4 concentration was lower for cows fed AL than cows fed 50ST and 25FR. Thus, FR reduced circulating glucose and insulin but increased P4 concentration, changes that may be positive in reproductive management programs.
Keywords: feed restriction, reproductive hormones, dairy cow
INTRODUCTION
Improved genetic selection and nutritional management of lactating dairy cows have dramatically enhanced average milk production over the past few decades, resulting in an increased demand for high DM and energy intake (Harrison et al., 1990; Butler, 2003; Ferraretto et al., 2013). Conversely, a decline in reproductive efficiency has been temporally associated with this increased milk production for the overall dairy industry (Lucy, 2001; Washburn et al., 2002; Butler, 2003) although this association does not signify causality (LeBlanc and Campbell, 2010; Chapinal et al., 2012). At the individual cow level, higher milk production can decrease expression of estrus (Lopez et al., 2004), increase double ovulation (Lopez et al., 2005), and decrease pregnancies per AI (Faust et al., 1988; Santos et al., 2001; Wiltbank et al., 2006). The level of milk production for individual cows is highly related to DMI and energy intake (Ferraretto et al., 2013), which is, in turn, correlated with blood flow through the portal vein (Huntington et al., 1981; Wieghart et al., 1986), and overall liver blood flow (Parr et al., 1993; Sangsritavong et al., 2002). Because the steroid hormones progesterone (P4) and estradiol-17β (E2) are primarily metabolized in the liver, increases in liver blood flow lead to increases in metabolism of these hormones and therefore decreased circulating P4 and E2 concentrations (Parr et al., 1993; Freetly and Ferrell, 1994; Sangsritavong et al., 2002). It appears that either acute or chronic changes in feed intake can produce these changes in liver blood flow and circulating P4 and E2 concentrations (Nolan et al., 1998; Sangsritavong et al., 2002; Vasconcelos et al., 2003). Reduced circulating concentrations of P4 and E2 seem to underlie some of the changes in reproductive physiology, expression of estrus, twinning, and fertility that have been observed in lactating dairy cows (Wiltbank et al., 2006, 2011). From a practical standpoint, programs that can optimize circulating P4 and E2 by hormonal, management, or nutritional approaches could lead to improved reproductive management of dairy cows.
Increased DMI and energy intake is also associated with elevated plasma insulin concentration (Harmon, 1992; Adamiak et al., 2006). Increased circulating insulin during the early postpartum period can lead to earlier first ovulation and potential positive effects on reproduction (Butler and Butler, 2004; Butler et al., 2004; Garnsworthy et al., 2009a,b; Galvao et al., 2010). Conversely, high insulin during the period of final follicle growth and ovulation can reduce fertility potentially because of the negative effects on the oocyte (Yaakub et al., 1999; Lozano et al., 2003; Adamiak et al., 2005). In addition, insulin may directly decrease hepatic expression of cytochrome P450 2C and P450 3A, which can metabolize P4, potentially leading to increased circulating P4 concentrations in the presence of high insulin (Lemley et al., 2010a,b). Thus, manipulation of circulating insulin concentrations could be used to improve reproductive management programs of dairy cattle.
Thus, this study was designed to determine the effects of acute feed restriction on circulating P4 and insulin, to provide key background information necessary to develop practical methods to manipulate these hormones during reproductive management programs. We hypothesized that a small, acute decrease in feed intake would produce a substantial decrease in circulating insulin but an increase in circulating P4, hormonal changes that could be compatible with increased fertility during reproductive management protocols. Further, we hypothesized that decreasing the energy content of the diet would result in reduced energy intake, potentially without a major reduction in DMI, and perhaps leading to decreased circulating insulin. We speculated that the magnitude of any changes in circulating P4 would depend on whether liver blood flow was primarily driven by DMI or by energy intake. Three feed restriction procedures (25 or 50% feed restriction and energy restriction by substituting 50% of the TMR with wheat straw) were utilized. Cows were evaluated for changes in DMI, BW, and milk production as well as circulating P4, insulin, glucose, and NEFA concentrations. A companion paper (Bender et al., 2014) reports the practical application of one of these feed restriction programs during superovulation of dairy cows.
MATERIALS AND METHODS
Animals and Management
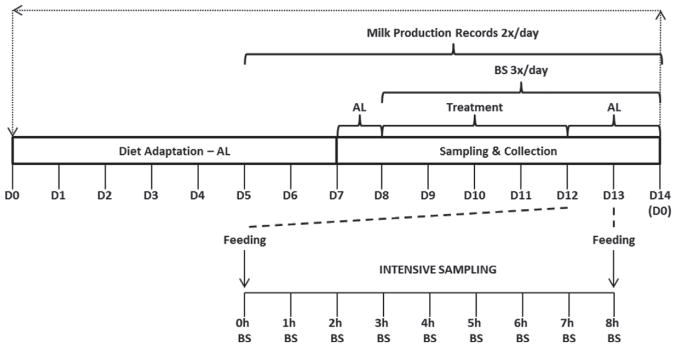
Eight multiparous Holstein cows 114 ± 14 d pregnant, 204 ± 29 DIM, and 685 ± 39 kg of BW at trial initiation were randomly assigned to a replicated 4 × 4 Latin square design with 14-d periods. Squares had similar DIM at trial initiation (DIM = 202 or 205). A schematic of the experimental treatments and sampling is provided in Figure 1. The first 7 d of each period were for adaptation, followed by 7 d of sampling and data collection. During the first 8 d of each period, all cows were fed ad libitum. Beginning on d 9 of each period, cows were exposed to 1 of 4 treatments: ad libitum (AL), 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). Feed-restricted groups were fed 25 and 50% of the average daily DMI from previous ad libitum feeding period. On d 12, all cows were fed for ad libitum intake (Figure 1). Cows were fed alfalfa silage, corn silage, concentrate mixture, vitamins, and minerals to meet or exceed NRC recommendations (NRC, 2001). Ingredient composition of the experimental diets is provided in Table 1, and diet nutrient composition is presented in Table 2. The TMR with straw, on average, contained lower CP and starch content (4.3 and 9.8 percentage units, respectively), and higher DM and NDF content (9.7 and 18.4 percentage units, respectively). This was due to the replacement of 50 percentage units of TMR DM with wheat straw. Consequently, the NEL value was lower for the TMR containing straw (1.65 vs. 1.42 Mcal/kg). Likewise, it was 15.1 and 9.5 percentage units lower, on average, in content of calculated NFC and total digestible nutrients at a maintenance level of intake (TDN1×), respectively.
Figure 1.
Schematic of the experimental protocol showing the 8 d of adaptation, 4 d of treatment, and 2 d of posttreatment evaluations. Intensive blood sampling (BS) every hour for 8 h was done after the final treatment feeding (d 12). AL = ad libitum feeding.
Table 1.
Ingredient composition of the diets
| Ingredient (% of DM) | TMR | TMR with straw |
|---|---|---|
| Corn silage | 33.3 | 16.7 |
| Alfalfa silage | 16.7 | 8.4 |
| Wheat straw | 0 | 50.0 |
| Dry ground shelled corn | 22.8 | 11.4 |
| Soy hulls | 3.6 | 1.8 |
| Soybean meal, 48% | 9.8 | 4.9 |
| Distillers dried grains | 11.0 | 5.5 |
| Energy Booster 1001 | 0.59 | 0.30 |
| Calcium carbonate | 1.20 | 0.60 |
| Magnesium oxide | 0.27 | 0.14 |
| Mg-K-S2 | 0.11 | 0.06 |
| Trace mineral salt3 | 0.45 | 0.23 |
| Vitamin premix4 | 0.18 | 0.09 |
Minimum 98% total fatty acids (MSC Company, Dundee, IL).
Dynamate (11% Mg, 18% K, 22% S; The Mosaic Co., Plymouth, MN).
Contained 88% NaCl, 0.002% Co, 0.2% Cu, 0.012% I, 0.18% Fe, 0.8% Mn, 0.006% Se, and 1.4% Zn.
Vitamin A, 3,300,000 IU/kg; vitamin D, 1,100,000 IU/kg; vitamin E, 11,000 IU/kg.
Table 2.
Nutrient composition (% of DM unless otherwise noted) of TMR1
| Nutrient | Normal TMR | TMR with straw |
|---|---|---|
| DM (% as fed) | 51.5 ± 1.9 | 61.2 ± 3.6 |
| OM | 93.2 ± 0.6 | 92.4 ± 0.5 |
| CP | 15.1 ± 1.1 | 10.8 ± 1.3 |
| NDF | 32.1 ± 0.7 | 50.5 ± 3.0 |
| ADF | 21.9 ± 0.8 | 35.6 ± 3.0 |
| Lignin | 3.1 ± 0.3 | 4.9 ± 0.5 |
| NFC | 42.1 ± 1.4 | 27.0 ± 3.3 |
| Ether extract | 4.6 ± 0.5 | 4.6 ± 2.7 |
| Starch | 26.8 ± 2.6 | 17.0 ± 1.9 |
| Ash | 6.8 ± 0.6 | 7.6 ± 0.5 |
| TDN1× | 72.3 ± 1.6 | 62.8 ± 2.8 |
| NEL (Mcal/kg) | 1.65 ± 0.0 | 1.42 ± 0.1 |
Treatments were normal TMR diet (no wheat straw added to TMR) and TMR with wheat straw (straw added to TMR at 50% on DM basis).
All animal procedures were approved before the experiment by the Research Animal and Resource Center of the College of Agricultural and Life Sciences (University of Wisconsin-Madison). All cows were injected with bST (Posilac, Monsanto Company, St. Louis, MO) every 14 d. Cows were housed in tiestalls and had free access to water. Cows were fed thrice daily individually at 0700, 1500, and 2300 h. Refusals were weighed daily before commencement of feeding, and DMI was measured on individual cows during the trial. Rations were adjusted for DM content weekly, based on DM content of corn silage, alfalfa silage, wheat straw, and concentrates. Equivalent amount of wheat straw was divided equally in 3 parts and was added to the 50ST TMR using hand mixing at each feeding time.
Body weight and BCS were determined with measurements from 1 to 5 in 0.25 increments (Wildman et al., 1982) every other week throughout the trial. The BW and BCS changes were calculated as differences between BW and BCS measured at the end of each period and BW and BCS measured before the start of each period. Milk yield was recorded daily (DairyComp305, Valley Agricultural Software, Tulare, CA) on individual cows milked twice daily throughout the trial.
Blood Samples and Analyses
Blood samples were collected before feeding via puncture of the jugular vein every 8 h on d 8 and continuing through d 14 of each period. On d 12 of each period, intensive blood samples were collected before morning feeding (0700 h) and at 60, 120, 180, 240, 300, 360, 420, and 480 min after feeding (Figure 1). Blood samples were collected using evacuated tubes without (Vacutainer, 8.5 mL; Becton Dickinson, Franklin Lakes, NJ) or with 8 mg of potassium oxalate 10 mg of sodium fluoride (Vacutainer, 4 mL; Becton Dickinson), for serum and plasma, respectively. All tubes, for serum or plasma, were kept on ice or in the refrigerator before being centrifuged at 2,050 × g at 4°C for 20 min; serum and plasma samples were stored at −20°C until analyzed. Blood serum was analyzed for insulin concentration (Porcine Insulin RIA Kit PI 12K; Linco Research Inc., St. Charles, MO) and NEFA (NEFA-C kit, Wako Fine Chemical Industries USA, Richmond, VA; Johnson and Peters, 1993). Circulating P4 was evaluated from unextracted serum using an antibody-coated tube RIA kit (Diagnostic Products Corp., Los Angeles, CA). Plasma samples were analyzed for glucose (glucose oxidase/peroxidase method; Karkalas, 1985).
Samples of TMR were collected twice a week and dried at 60°C for 48 h in a forced-air oven to determine DM content. Dried samples were ground to pass a 1-mm Wiley mill (Arthur H. Thomas, Philadelphia, PA) screen and composited by period before sending to Dairyland Laboratories Inc. (Arcadia, WI) for analysis. The absolute DM was determined by oven-drying at 105°C for 72 h. All samples were analyzed for DM, OM (method 942.05; AOAC International, 2006), CP (method 990.03; AOAC International, 2006), NDF using α-amylase and sodium sulfite (Van Soest et al., 1991), ether extract (method 2003.05; AOAC International, 2006), and starch content (YSI Biochemistry Analyzer, YSI Inc., Yellow Springs, OH; Knudsen, 1997).
Statistical Analyses
Data were analyzed as a replicated Latin square using PROC MIXED (SAS Institute, 2004). Blood samples were analyzed with time as repeated measures. The model included treatment, period, day, and treatment × day interaction as fixed effects; and cow within the square as a random effect. The model for intensive measurements of glucose, insulin, and P4 samples included treatment, period, hour, and treatment × hour interaction as fixed effects; and cow as a random effect. Treatment means were determined using the least means square statement. A value of P ≤ 0.05 was considered statistically significant and P > 0.05 to P < 0.10 was considered a statistical trend.
RESULTS AND DISCUSSION
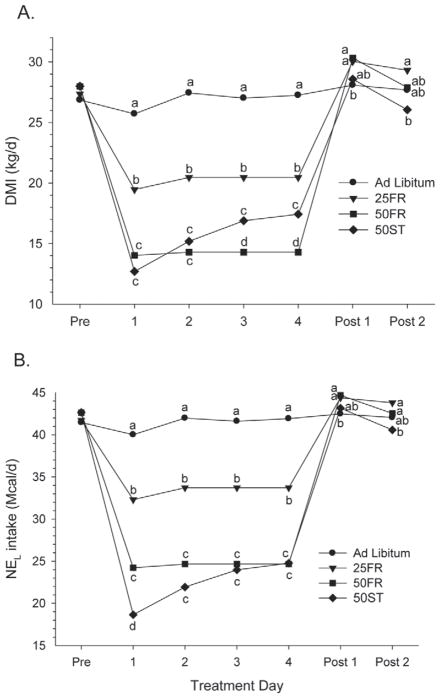
As planned, feed restriction of 25 or 50% resulted in the expected decrease in DMI and NEL intake (P < 0.001; Table 3). During the treatment period, the DMI of cows fed AL was 6.7, 12.7, and 11.3 kg/d greater than for cows fed 25FR, 50FR, and 50ST, respectively. The daily NEL (Mcal/d) consumption for the 3 groups was 80.7, 59.4, and 57.0% of the AL for 25FR, 50FR, and 50ST, respectively. During the periods when cows were fed the TMR with 50% wheat straw (50ST), we observed not only the expected decrease in energy intake due to consumption of a less-energy-dense diet but also a large decrease in DMI. During the first 2 d of treatment, the 50ST cows had a similar 50% decrease in DMI as observed in the 50FR cows, even though the 50ST cows had ad libitum access to feed. Obviously, the palatability of the wheat straw was a primary driver of the substantial reduction in intake. In addition, some of the decrease in DMI could be related to lower fiber digestibility of the wheat straw with a corresponding increase in rumen retention time (Kendall et al., 2009), leading to slow passage rate through the digestive tract (Allen, 1997) and reduced intake due to rumen fill (Mertens, 1987). Kendall et al. (2009) replaced alfalfa and corn silage with wheat straw and reported a decrease in DMI when dietary NDF content was increased from 28 to 32%. Observations that match our results were reported when cows were fed a partial mixed ration with supplemental hay, to reduce energy density of the diet, with more than a 50% decrease in DMI from 22.0 to 10.8 kg/d (Gross et al., 2011). In our study, a treatment × day interaction was observed (P < 0.01; Figure 2a), primarily because of the dramatic decline in DMI on d 1 for 50ST, with a subsequent 36% increase (from 12.7 to 17.4 kg/d) during the next 4 d. In contrast to d 1 and 2 when 50ST intake was similar to 50FR, on d 3 and 4 we observed a greater intake in 50ST compared with 50FR. It appears that cows fed 50ST seemed to overlook the wheat straw palatability issues as the energy deficit became more pronounced. After return to ad libitum intake, cows fed 25FR and 50FR had greater DMI than AL the first day after ad libitum feed was offered to all cows. Likewise, a treatment × day interaction was observed (P < 0.02; Figure 2b) for NEL intake, primarily driven by the DMI pattern observed for 50ST. The NEL intake was 5.5 Mcal/d lower for 50ST than 50FR on d 1, but was not lower on d 2 to 4.
Table 3.
Effect of feed restriction (FR) on least squares means for DMI, milk yield, NEFA, glucose, insulin, progesterone, and FSH during the FR period and BW and BCS change
| Item | Treatment (Trt)1 | SEM | P < | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| AL | 25FR | 50FR | 50ST | Trt | Day | Trt × Day | ||
| DMI (kg/d) | 26.9a | 20.2b | 14.2c | 15.6c | 0.96 | 0.001 | 0.001 | 0.01 |
| NEL intake (Mcal/d) | 41.4a | 33.4b | 24.6c | 22.3c | 1.20 | 0.001 | 0.001 | 0.02 |
| Milk yield (kg/d) | 40.8a | 36.4b | 31.4c | 24.4d | 1.33 | 0.001 | 0.001 | 0.001 |
| NEFA (μEq/L) | 156.3c | 225.3c | 357.5b | 647.7a | 37.5 | 0.001 | 0.001 | 0.001 |
| Glucose (mg/dL) | 53.6a | 51.9b | 50.1c | 48.3d | 0.58 | 0.001 | 0.001 | 0.03 |
| Insulin (μIU/mL) | 16.65a | 11.16b | 8.27c | 6.24c | 1.05 | 0.001 | 0.01 | 0.02 |
| Progesterone (ng/mL) | 6.87c | 7.84ab | 7.42bc | 8.50a | 0.84 | 0.02 | 0.08 | 0.45 |
| FSH (ng/mL) | 0.206 | 0.191 | 0.170 | 0.185 | 0.019 | 0.46 | 0.52 | 0.60 |
| BW change (kg/14 d) | −8.5a | −30.0b | −45.5c | −36.1bc | 5.1 | 0.001 | — | — |
| BCS change | 0.03a | −0.13b | −0.16b | −0.19b | 0.05 | 0.02 | — | — |
Means in the same row with different superscripts differ (P < 0.05).
The dietary treatments were 4 levels of feed intake: ad libitum (AL), 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% feed restriction + 50% wheat straw (50ST).
Figure 2.
(A) Dry matter intake (kg/d) and (B) NEL intake (Mcal/d) on the day before the initiation of treatment (Pre); during treatment (d 1 to 4) while exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST); and the 2 d after all cows returned to ad libitum feeding (Post 1, Post 2). a–dWithin day, means with different letters differ (P < 0.05).
Reduced DM and energy intake of cows fed 25FR, 50FR, and 50ST depressed milk yield (P < 0.001; Table 3) to 89, 77, and 60% of cows fed AL, respectively. The maximal decrease in milk production was reached by 2 d after FR in 50ST (45% decrease) and 50FR (25% decrease), whereas the 25FR cows were still similar to AL cows after 2 d of FR. The maximal decrease in 25FR was only 14%, which was reached at 4 d after FR. Similar to our results, the milk yield of mid-lactation cows that were feed restricted by 30 or 50% was 83 and 78% of control group (Lapierre et al., 1995; Velez and Donkin, 2005), respectively. In a recent review, milk yield was reported to be reduced 0.9 kg/d per kg of reduced intake (Ferraretto et al., 2013). Milk yield in cows on 25FR, although significantly different from the control on d 3 and 4 of treatment, returned to levels similar to that of the control group 2 d after reestablishment of ad libitum intake (Figure 3). Cows fed 50FR and 50ST had lower milk yield during the entire treatment period compared with AL and had not returned to normal production by 2 d after return to ad libitum intake (Figure 3). Similar results were reported by Velez and Donkin (2005) and Gross et al. (2011) after 5 d of feed restriction or 3 wk of nutrient restriction, respectively. Thus, cows exposed to a moderate (≤25%), short-term restriction of feed and energy have a modest decrease in milk production but return rapidly to normal milk production after the restriction is removed. More extreme restriction of feed (≥50%) leads to a more rapid and marked decrease in milk production and a slower return to normal production after replacement of ad libitum feed.
Figure 3.
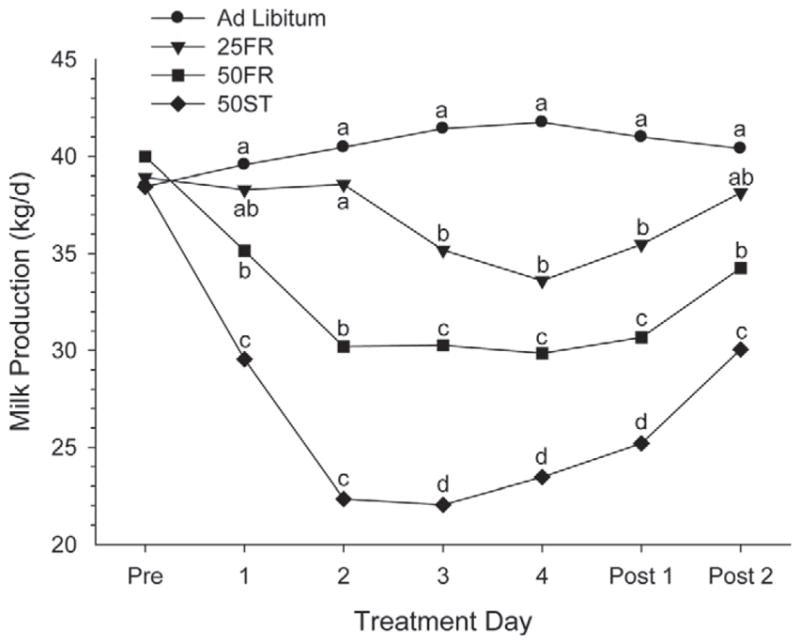
Milk production (kg/d) before (Pre), during, and after (Post) treatment in cows exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). a–dWithin day, means with different letters differ (P < 0.05).
Cows exposed to the negative energy balance (NEB) caused by FR in our study had some physiological responses similar to those of periparturient dairy cows that experience decreased DMI and NEB, in particular the fat mobilization and increase in circulating NEFA (Grummer, 1993). Increase in serum NEFA concentration (P < 0.001) and decreases in BW (P < 0.001) and BCS (P < 0.02) were observed in all the FR groups (Table 3), indicative of NEB and fat mobilization, during our study. Increased serum NEFA concentration was previously observed when FR was applied to nonlactating (Rodrigues et al., 2011), early lactation (Radcliff et al., 2006; Chagas et al., 2008) and mid lactation (Velez and Donkin, 2005; Gross et al., 2011) cows. Cows fed 50ST had the greatest increase in circulating NEFA with a >4-fold increase on the first day after FR, peaking at a 7-fold increase on the second day of FR. The 50FR cows had no detectable increase in NEFA on the first day of FR but had over a 4-fold increase by the second day. The 25FR cows had even a greater delay, with NEFA concentrations similar to those of AL cows on all days of FR. Diets with greater energy density are associated with lower serum NEFA concentrations in early lactation cows, even when cows have similar DMI (Minor et al., 1998). Greater ruminal propionate concentrations (Jenkins and McGuire, 2006), producing greater circulating insulin (Harmon, 1992), could explain this phenomenon, because insulin is antilipolytic and may function to decrease serum NEFA. Circulating NEFA returned to normal concentrations in all groups on the day after cows returned to normal intake (Figure 4). Rapid return of circulating NEFA to control values was also reported by others (Velez and Donkin, 2005; Gross et al., 2011), consistent with circulating NEFA being maintained near basal concentrations except during substantial energy deficits. Our observed losses in BW and BCS after FR are similar to previous observations in lactating (Gross et al., 2011) and nonlactating (Rodrigues et al., 2011) dairy cows during longer periods of FR. The substantial loss of BW could be explained by depressed DMI and loss of gut fill (NRC, 2001), whereas the modest BCS loss is likely related to decreased energy intake leading to fat mobilization.
Figure 4.
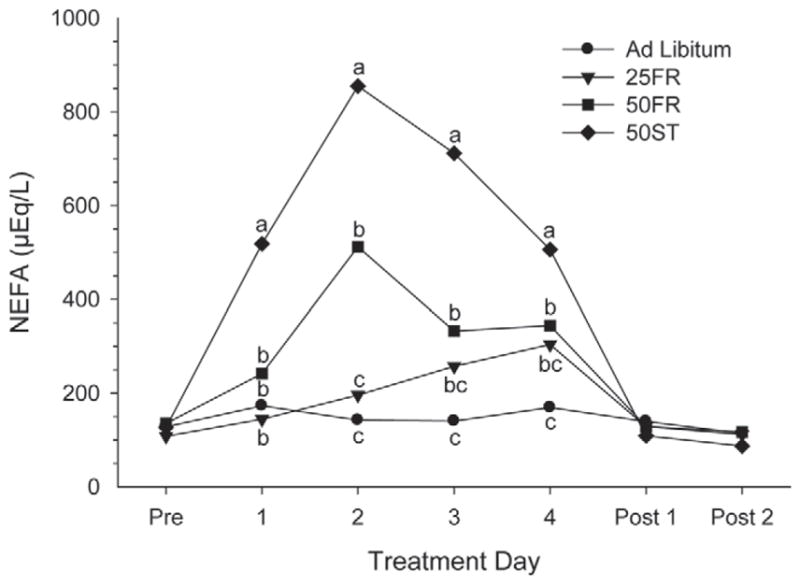
Circulating NEFA concentrations (μEq/L) before (Pre), during, and after (Post) treatment in cows exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). a–cWithin day, means with different letters differ (P < 0.05).
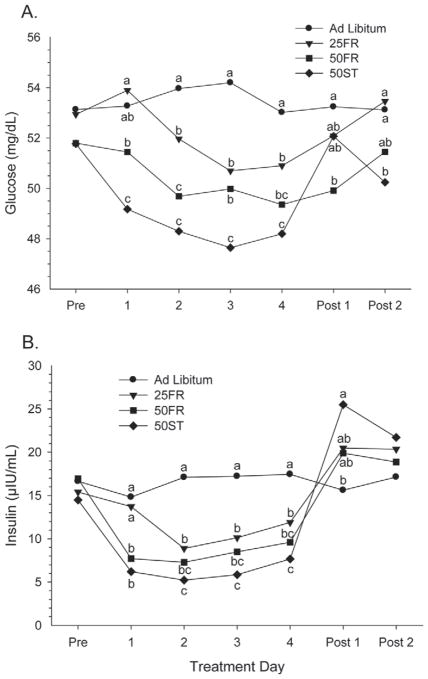
Plasma glucose concentration decreased (P < 0.001) with feed and energy restriction (Table 3). The decline was greatest in the 50ST cows, with less striking and delayed declines in circulating glucose in the 50FR and 25FR cows. Previous studies have reported that FR cows had lower (Chagas et al., 2008; Gross et al., 2011) or similar (Lapierre et al., 1995; Velez and Donkin, 2005; Carlson et al., 2006) glucose concentrations. Discrepancy in results could be related to timing of blood sampling in relation to feeding in the different studies. All of our samples, except during the intensive period, were collected 8 h after the previous feeding to minimize effects of acute feed intake on glucose and insulin results. In addition, a decline in circulating glucose may be attenuated in some studies due to increased production of glucose from propionate, the major precursor of glucose (Reynolds et al., 1994, 2003; Reynolds, 2005), or by gluconeogenesis from lactate, glycerol, and amino acids when animals are in NEB (Lomax and Baird, 1983).
Serum insulin concentrations followed the same patterns as DMI, milk yield, and glucose concentration and decreased (P < 0.001) with feed and energy restriction (Table 3). This is consistent with our first hypothesis: that even a relatively minor, short-term FR would produce a substantial decline in circulating insulin. The maximal decline in insulin (>50%) was observed during the first day of FR with consistently low circulating insulin throughout for 50FR and 50ST. In contrast, 25FR did not have a detectable decline in insulin until the second day of FR but maintained lower insulin (33% lower on average) on the next 3 d. Previous studies have reported that FR decreased (Lapierre et al., 1995; Carlson et al., 2006; Chagas et al., 2008) or did not change (Velez and Donkin, 2005; Radcliff et al., 2006; Guinard-Flament et al., 2007) circulating insulin concentrations. Differences between studies could be due to the timing of blood sampling, dietary composition, length and severity of FR, or physiological differences between cows. After return to ad libitum intake, a return to normal insulin, and possibly even higher circulating insulin, was observed, particularly in the 50ST cows (Figure 5B).
Figure 5.
Circulating (A) glucose (mg/dL) and (B) insulin (μIU/ mL) concentrations before (Pre), during, and after (Post) treatment in cows exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). a–cWithin day, means with different letters differ (P < 0.05).
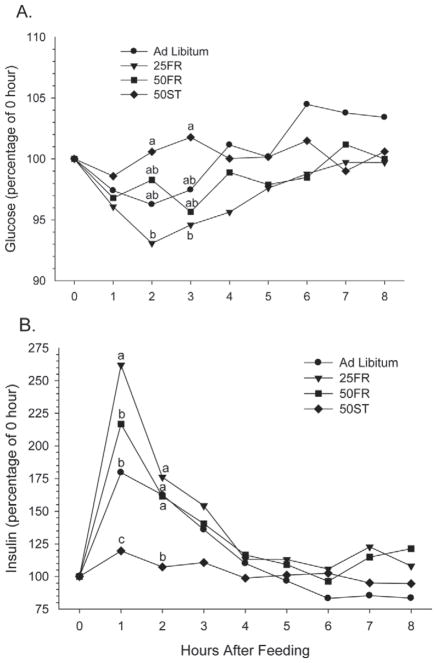
During intensive sampling, glucose and insulin values were evaluated as percentage of initial values (Figure 6) because of significant differences between groups at 0 h (glucose = 52.00, 51.73, 50.24, and 48.46 mg/dL for AL, 25FR, 50FR, and 50ST, respectively; insulin = 17.88, 11.50, 8.75, and 8.13 μIU/mL for AL, 25FR, 50FR, and 50ST, respectively). As graphically demonstrated in Figure 6, there was a treatment × hour interaction for circulating insulin (P < 0.001) and glucose (P < 0.06) concentrations. The 25FR group had the greatest elevation in circulating insulin, observed by 1 h after feeding, and the greatest decrease in plasma glucose, which was not different at 1 h after feeding but was lower at 2 and 3 h after feeding (P < 0.01) compared with the 50ST diet. The timing is logical because the postprandial increase in insulin drives glucose into insulin-responsive cells, such as skeletal muscle, leading to a decline in circulating glucose despite an anticipated increase in nutrient absorption from the gut. Obviously, the increase in circulating insulin is not primarily driven by elevations in circulating glucose concentrations. The relatively constant insulin and glucose in cows fed 50ST is likely due to a slow eating rate as well as slow absorption of nutrients, producing a metabolic pattern that differed substantially from the AL and FR groups. Previous literature (Stone, 2004) has indicated that fasted cows have an elevated eating rate after reintroduction of feed, leading to a spike in insulin (as also observed in our study; Figure 6B) and a subsequent decrease in glucose at 2 to 3 h after feeding. Although eating rate was not measured in our study, all cows are likely to increase eating rate at the time of feed introduction, particularly FR cows. The greater quantity of feed available to 25FR likely led to a greater eating rate (g of DM/min) in 25FR than 50FR. The acute elevations in insulin and depression in glucose had returned to normal by 4 h after feeding.
Figure 6.
Intensive measurements of circulating (A) glucose and (B) insulin concentrations following feeding as a percentage of the 0-h sample while exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). a–cWithin day, means with different letters differ (P < 0.05).
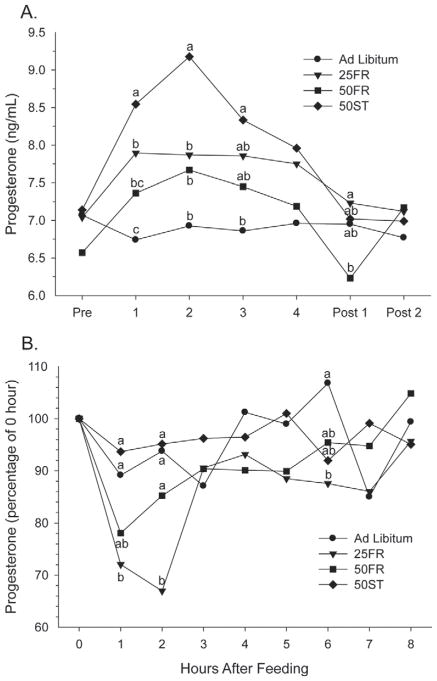
Circulating P4 concentrations during the complete treatment period were affected (P < 0.02) by feed and energy restriction (Figure 7A). Cows in AL had lower P4 concentrations than 25FR and 50ST but similar to 50FR, and 50FR had lower P4 than 50ST (Table 3). For an unknown reason, cows in 50FR had circulating P4 ~0.5 ng/mL lower than the other 3 groups before beginning treatment, which may have confounded treatment effects for circulating P4 in this particular group. Our primary finding of an elevation in circulating P4 in cows with lower DMI is consistent with previous studies. For example, greater circulating P4 (~25%) was found in beef heifers consuming 33 compared with 13 kg/d of an as-fed diet (Nolan et al., 1998). In addition, Sangsritavong et al. (2002) observed increased liver blood flow, increased metabolic clearance rate for P4 and E2, and decreased circulating P4 in cows with greater DMI or greater milk production. Our results did not allow us to distinguish whether increases in circulating P4 were due to reductions in DMI or energy intake. However, the more dramatic increase in circulating P4 on d 1 and 2 in 50ST cows, when energy intake was lower than in the other groups, is consistent with energy having a critical role in determining liver blood flow, resultant P4 metabolism, and circulating P4 concentrations. Nevertheless, another possible explanation for the elevation in circulating P4 during FR is that FR may cause release of P4 that is stored in adipose tissue, due to fat mobilization (Hamudikuwanda et al., 1996; Rodrigues et al., 2011). In addition, some research is consistent with circulating insulin regulating expression of key hepatic enzymes involved in P4 metabolism (Lemley et al., 2010a,b). However, our findings of the greatest circulating P4 in cows with the lowest insulin concentrations are opposite of what would be predicted by those studies (elevated insulin decreases P4 metabolism). It is unclear whether energy status of the cows may have affected our results because a previous study observed the positive effect of insulin on circulating P4 only in cows with positive energy balance and not in cows with NEB (Vieira et al., 2010). Two days after reestablishment of normal intakes, all cows reached control P4 concentrations (Figure 7A).
Figure 7.
Circulating progesterone concentrations (A) during the entire experimental period (ng/mL) and (B) during the intensive measurements calculated as a percentage of the 0 h sample in cows exposed to 1 of 4 treatments: ad libitum, 25% feed restriction (25FR), 50% feed restriction (50FR), and 50% of TMR replaced with wheat straw (50ST). a–cWithin day, means with different letters differ (P < 0.05).
As done for glucose and insulin, the acute changes in circulating P4 after feeding were evaluated as percentage change from 0 h (Figure 7B). Progesterone values were numerically different at 0 h (7.01, 8.12, 6.98, and 7.95 ng/mL for AL, 25FR, 50FR, and 50ST, respectively). We observed a treatment × hour interaction (P < 0.03) for serum P4 concentrations (Figure 7b). Interestingly, the 2 groups that had unrestricted feed available throughout the 8-h period, AL and 50ST, had no change in circulating P4. In contrast, the 25FR cows are likely to have the greatest rate of DMI following feeding (Stone, 2004), and they also had the greatest acute decrease in P4. The acute decline in P4 after rapid feeding is probably caused by an acute elevation in blood flow to the gastrointestinal tract in response to high nutrient intake, resulting in increased liver blood flow with subsequent increases in P4 metabolism and an acute decrease in circulating P4 (Sangsritavong et al., 2002; Vasconcelos et al., 2003). Thus, the 25FR cows had an overall increase in circulating P4 during the treatment period, as measured at 8 h after feeding, but had a rapid, short-lived 30% decline in circulating P4, in response to acute feeding. How these 2 conflicting P4 signals might affect reproductive traits cannot be determined from our study. Feed restriction did not influence FSH concentrations (P > 0.10), in agreement with previous data in fasted ewes (Kiyma et al., 2004) and postpartum dairy cows fed 2 dietary energy densities (de Feu et al., 2009).
CONCLUSIONS
Short-term DMI and energy intake restriction had a substantial effect on the physiological measures that were evaluated in this study. As little as 4 d of FR was sufficient to produce lower milk production, losses of BW and BCS, reduced glucose and insulin concentrations, and elevated insulin, NEFA, and P4 concentrations. It remains to be determined whether short-term FR could be practically utilized in reproductive programs to acutely elevate P4 and reduce insulin at key stages in the reproductive process.
Acknowledgments
The authors are grateful to the staff at the University of Wisconsin-Madison Campus Dairy Cattle Center for animal care, undergraduates Joseph Binversie, Ann-Marie Beine, and Rudi Meyer for their contribution to the project, and Peter Crump of the University of Wisconsin CALS Computer Consulting Laboratory for assistance with statistical analysis of the data.
References
- Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol Reprod. 2005;73:918–926. doi: 10.1095/biolreprod.105.041483. [DOI] [PubMed] [Google Scholar]
- Adamiak SJ, Powell K, Rooke JA, Webb R, Sinclair KD. Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction. 2006;131:247–258. doi: 10.1530/rep.1.00871. [DOI] [PubMed] [Google Scholar]
- Allen MS. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J Dairy Sci. 1997;80:1447–1462. doi: 10.3168/jds.S0022-0302(97)76074-0. [DOI] [PubMed] [Google Scholar]
- AOAC International. Official Methods of Analysis. 18. AOAC International; Arlington, VA: 2006. [Google Scholar]
- Bender RW, Hackbart KS, Dresch AR, Carvalho PD, Vieira LM, Crump PM, Guenther JN, Fricke PM, Shaver RD, Combs DK, Wiltbank MC. Effects of acute feed restriction combined with targeted use of increasing luteinizing hormone content of follicle-stimulating hormone preparations on ovarian superstimulation, fertilization, and embryo quality in lactating dairy cows. J Dairy Sci. 2014;97:764–778. doi: 10.3168/jds.2013-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler ST, Butler WR. Energy balance, dry matter intake, and hormone profiles of cows with ovulatory and non-ovulatory follicles during the first postpartum follicle wave. J Anim Sci. 2004;82:296–297. [Google Scholar]
- Butler ST, Pelton SH, Butler WR. Insulin increases 17β-estradiol production by the dominant follicle of the first post-partum follicle wave in dairy cows. Reproduction. 2004;127:537–545. doi: 10.1530/rep.1.00079. [DOI] [PubMed] [Google Scholar]
- Butler WR. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livest Prod Sci. 2003;83:211–218. [Google Scholar]
- Carlson DB, Litherland NB, Dann HM, Woodworth JC, Drackley JK. Metabolic effects of abomasal 3-carnitine infusion and feed restriction in lactating Holstein cows. J Dairy Sci. 2006;89:4819–4834. doi: 10.3168/jds.S0022-0302(06)72531-0. [DOI] [PubMed] [Google Scholar]
- Chagas LM, Gore PJS, Graham G, Macdonald KA, Blache D. Effect of restricted feeding and monopropylene glycol postpartum on metabolic hormones and postpartum anestrus in grazing dairy heifers. J Dairy Sci. 2008;91:1822–1833. doi: 10.3168/jds.2007-0339. [DOI] [PubMed] [Google Scholar]
- Chapinal N, LeBlanc SJ, Carson ME, Leslie KE, Godden S, Capel M, Santos JEP, Overton MW, Duffield TF. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J Dairy Sci. 2012;95:5676–5682. doi: 10.3168/jds.2011-5132. [DOI] [PubMed] [Google Scholar]
- de Feu MA, Evans ACO, Lonergan P, Butler ST. The effect of dry period duration and dietary energy density on milk production, bioenergetic status, and postpartum ovarian function in Holstein-Friesian dairy cows. J Dairy Sci. 2009;92:6011–6022. doi: 10.3168/jds.2009-2374. [DOI] [PubMed] [Google Scholar]
- Faust MA, McDaniel BT, Robison OW, Britt JH. Environmental and yield effects on reproduction in primiparous Holsteins. J Dairy Sci. 1988;71:3092–3099. doi: 10.3168/jds.S0022-0302(89)79097-4. [DOI] [PubMed] [Google Scholar]
- Ferraretto LF, Crump PM, Shaver RD. Effect of cereal grain type and corn grain harvesting and processing methods on intake, digestion, and milk production by dairy cows through a meta-analysis. J Dairy Sci. 2013;96:533–550. doi: 10.3168/jds.2012-5932. [DOI] [PubMed] [Google Scholar]
- Freetly HC, Ferrell CL. Net uptakes of estradiol-17-beta and progesterone across the portal-drained viscera and the liver of ewes. J Endocrinol. 1994;141:353–358. doi: 10.1677/joe.0.1410353. [DOI] [PubMed] [Google Scholar]
- Galvão KN, Frajblat M, Butler WR, Brittin SB, Guard CL, Gilbert RO. Effect of early postpartum ovulation on fertility in dairy cows. Reprod Domest Anim. 2010;45:e207–e211. doi: 10.1111/j.1439-0531.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- Garnsworthy PC, Fouladi-Nashtaa AA, Mann GE, Sinclair KD, Webb R. Effect of dietary-induced changes in plasma insulin concentrations during the early postpartum period on pregnancy rate in dairy cows. Reproduction. 2009a;137:759–768. doi: 10.1530/REP-08-0488. [DOI] [PubMed] [Google Scholar]
- Garnsworthy PC, Gong JG, Armstrong DG, Mann GE, Sinclair KD, Webb R. Effect of site of starch digestion on metabolic hormones and ovarian function in dairy cows. Livest Sci. 2009b;125:161–168. [Google Scholar]
- Gross J, van Dorland HA, Bruckmaier RM, Schwarz FJ. Performance and metabolic profile of dairy cows during a lactational and deliberately induced negative energy balance with subsequent realimentation. J Dairy Sci. 2011;94:1820–1830. doi: 10.3168/jds.2010-3707. [DOI] [PubMed] [Google Scholar]
- Grummer RR. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J Dairy Sci. 1993;76:3882–3896. doi: 10.3168/jds.S0022-0302(93)77729-2. [DOI] [PubMed] [Google Scholar]
- Guinard-Flament J, Delamaire E, Lamberton P, Peyraud JL. Adaptations of mammary uptake and nutrient use to once-daily milking and feed restriction in dairy cows. J Dairy Sci. 2007;90:5062–5072. doi: 10.3168/jds.2007-0259. [DOI] [PubMed] [Google Scholar]
- Hamudikuwanda H, Gallo G, Block E, Downey BR. Adipose tissue progesterone concentrations in dairy cows during late pregnancy and early lactation. Anim Reprod Sci. 1996;43:15–23. [Google Scholar]
- Harmon DL. Impact of nutrition on pancreatic exocrine and endocrine secretion in ruminants—A review. J Anim Sci. 1992;70:1290–1301. doi: 10.2527/1992.7041290x. [DOI] [PubMed] [Google Scholar]
- Harrison RO, Ford SP, Young JW, Conley AJ, Freeman AE. Increased milk production versus reproductive and energy status of high producing dairy cows. J Dairy Sci. 1990;73:2749–2758. doi: 10.3168/jds.S0022-0302(90)78960-6. [DOI] [PubMed] [Google Scholar]
- Huntington GB, Prior RL, Britton RA. Glucose and lactate absorption and metabolic interrelationships in steers changed from low to high concentrate diets. J Nutr. 1981;111:1164–1172. doi: 10.1093/jn/111.7.1164. [DOI] [PubMed] [Google Scholar]
- Jenkins TC, McGuire MA. Major advances in nutrition: Impact on milk composition. J Dairy Sci. 2006;89:1302–1310. doi: 10.3168/jds.S0022-0302(06)72198-1. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Peters JP. An improved method to quantify nonesterified fatty acids in bovine plasma. J Anim Sci. 1993;71:753–756. doi: 10.2527/1993.713753x. [DOI] [PubMed] [Google Scholar]
- Karkalas J. An improved enzymatic method for the determination of native and modified starch. J Sci Food Agric. 1985;36:1019–1027. [Google Scholar]
- Kendall C, Leonardi C, Hoffman PC, Combs DK. Intake and milk production of cows fed diets that differed in dietary neutral detergent fiber and neutral detergent fiber digestibility. J Dairy Sci. 2009;92:313–323. doi: 10.3168/jds.2008-1482. [DOI] [PubMed] [Google Scholar]
- Kiyma Z, Alexander BM, Van Kirk EA, Murdoch WJ, Hallford DM, Moss GE. Effects of feed restriction on reproductive and metabolic hormones in ewes. J Anim Sci. 2004;82:2548–2557. doi: 10.2527/2004.8292548x. [DOI] [PubMed] [Google Scholar]
- Knudsen KEB. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67:319–338. [Google Scholar]
- Lapierre H, Pelletier G, Abribat T, Fournier K, Gaudreau P, Brazeau P, Petitclerc D. The effect of feed-intake and growth hormone-releasing factor on lactating dairy-cows. J Dairy Sci. 1995;78:804–815. doi: 10.3168/jds.S0022-0302(95)76692-9. [DOI] [PubMed] [Google Scholar]
- LeBlanc SJ, Campbell MS. Is high production compatible with good reproductive performance in dairy cattle? Updates on ruminant production and medicine. Proc. XXVI World Buiatrics Congr; Santiago, Chile. Santiago, Chile: World Buiatrics Association; 2010. pp. 103–113. [Google Scholar]
- Lemley CO, Vonnahme KA, Tager LR, Krause KM, Wilson ME. Diet-induced alterations in hepatic progesterone (P4) catabolic enzyme activity and P4 clearance rate in lactating dairy cows. J Endocrinol. 2010a;205:233–241. doi: 10.1677/JOE-10-0042. [DOI] [PubMed] [Google Scholar]
- Lemley CO, Wilmoth TA, Tager LR, Krause KM, Wilson ME. Effect of a high cornstarch diet on hepatic cytochrome P450 2C and 3A activity and progesterone half-life in dairy cows. J Dairy Sci. 2010b;93:1012–1021. doi: 10.3168/jds.2009-2539. [DOI] [PubMed] [Google Scholar]
- Lomax MA, Baird GD. Blood-flow and nutrient exchange across the liver and gut of the dairy cow—Effects of lactation and fasting. Br J Nutr. 1983;49:481–496. doi: 10.1079/bjn19830057. [DOI] [PubMed] [Google Scholar]
- Lopez H, Caraviello DZ, Satter LD, Fricke PM, Wiltbank MC. Relationship between level of milk production and multiple ovulations in lactating dairy cows. J Dairy Sci. 2005;88:2783–2793. doi: 10.3168/jds.S0022-0302(05)72958-1. [DOI] [PubMed] [Google Scholar]
- Lopez H, Satter LD, Wiltbank MC. Relationship between level of milk production and estrous behavior of lactating dairy cows. Anim Reprod Sci. 2004;81:209–223. doi: 10.1016/j.anireprosci.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Lozano JM, Lonergan P, Boland MP, O’Callaghan D. Influence of nutrition on the effectiveness of superovulation programmes in ewes: Effect on oocyte quality and post-fertilization development. Reproduction. 2003;125:543–553. [PubMed] [Google Scholar]
- Lucy MC. Reproductive loss in high-producing dairy cattle: Where will it end? J Dairy Sci. 2001;84:1277–1293. doi: 10.3168/jds.S0022-0302(01)70158-0. [DOI] [PubMed] [Google Scholar]
- Mertens DR. Predicting intake and digestibility using mathematical models of ruminal function. J Anim Sci. 1987;64:1548–1558. doi: 10.2527/jas1987.6451548x. [DOI] [PubMed] [Google Scholar]
- Minor DJ, Trower SL, Strang BD, Shaver RD, Grummer RR. Effects of nonfiber carbohydrate and niacin on periparturient metabolic status and lactation of dairy cows. J Dairy Sci. 1998;81:189–200. doi: 10.3168/jds.S0022-0302(98)75566-3. [DOI] [PubMed] [Google Scholar]
- Nolan R, O’Callaghan D, Duby RT, Lonergan P, Boland MP. The influence of short-term nutrient changes on follicle growth and embryo production following superovulation in beef heifers. Theriogenology. 1998;50:1263–1274. doi: 10.1016/s0093-691x(98)00225-8. [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle. 7. Natl. Acad. Sci; Washington, DC: 2001. [Google Scholar]
- Parr RA, I, Davis F, Miles MA, Squires TJ. Liver blood flow and metabolic clearance rate of progesterone in sheep. Res Vet Sci. 1993;55:311–316. doi: 10.1016/0034-5288(93)90100-t. [DOI] [PubMed] [Google Scholar]
- Radcliff RP, McCormack BL, Keisler DH, Crooker BA, Lucy MC. Partial feed restriction decreases growth hormone receptor 1a mRNA expression in postpartum dairy cows. J Dairy Sci. 2006;89:611–619. doi: 10.3168/jds.S0022-0302(06)72124-5. [DOI] [PubMed] [Google Scholar]
- Reynolds CK. Glucose balance in cattle. [Accessed Nov. 14, 2005];Florida Ruminant Nutrition Symposium. 2005 http://dairy.ifas.ufl.edu/rns/2005/Reynolds.pdf.
- Reynolds CK, Aikman PC, Lupoli B, Humphries DJ, Beever DE. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J Dairy Sci. 2003;86:1201–1217. doi: 10.3168/jds.S0022-0302(03)73704-7. [DOI] [PubMed] [Google Scholar]
- Reynolds CK, Harmon DL, Cecava MJ. Absorption and delivery of nutrients for milk protein-synthesis by portal-drained viscera. J Dairy Sci. 1994;77:2787–2808. doi: 10.3168/jds.S0022-0302(94)77220-9. [DOI] [PubMed] [Google Scholar]
- Rodrigues RO, Trevisanuto C, Cooke RF, Vasconcelos JLM. Effects of body weight loss on serum progesterone concentrations of non-lactating dairy cows. Theriogenology. 2011;75:131–137. doi: 10.1016/j.theriogenology.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Sangsritavong S, Combs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17 beta in dairy cattle. J Dairy Sci. 2002;85:2831–2842. doi: 10.3168/jds.S0022-0302(02)74370-1. [DOI] [PubMed] [Google Scholar]
- Santos JEP, Thatcher WW, Pool L, Overton MW. Effect of human chorionic gonadotropin, on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J Anim Sci. 2001;79:2881–2894. doi: 10.2527/2001.79112881x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT 9.1 User’s Guide. Version 9.1. SAS Institute Inc; Cary, NC: 2004. [Google Scholar]
- Stone WC. Nutritional approaches to minimize subacute ruminal acidosis and laminitis in dairy cattle. J Dairy Sci. 2004;87(E. Suppl):E13–E16. [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vasconcelos JLM, Sangsritavong S, Tsai SJ, Wiltbank MC. Acute reduction in serum progesterone concentrations after feed intake in dairy cows. Theriogenology. 2003;60:795–807. doi: 10.1016/s0093-691x(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Velez JC, Donkin SS. Feed restriction induces pyruvate carboxylase but not phosphoenolpyruvate carboxykinase in dairy cows. J Dairy Sci. 2005;88:2938–2948. doi: 10.3168/jds.S0022-0302(05)72974-X. [DOI] [PubMed] [Google Scholar]
- Vieira FVR, Lopes CN, Cappellozza BI, Scarpa AB, Cooke RF, Vasconcelos JLM. Effects of intravenous glucose infusion and nutritional balance on serum concentrations of nonesterified fatty acids, glucose, insulin, and progesterone in nonlactating dairy cows. J Dairy Sci. 2010;93:3047–3055. doi: 10.3168/jds.2010-3061. [DOI] [PubMed] [Google Scholar]
- Washburn SP, Silvia WJ, Brown CH, McDaniel BT, McAllister AJ. Trends in reproductive performance in southeastern Holstein and Jersey DHI herds. J Dairy Sci. 2002;85:244–251. doi: 10.3168/jds.S0022-0302(02)74073-3. [DOI] [PubMed] [Google Scholar]
- Wieghart M, Slepetis R, Elliot JM, Smith DF. Glucose-absorption and hepatic gluconeogenesis in dairy-cows fed diets varying in forage content. J Nutr. 1986;116:839–850. doi: 10.1093/jn/116.5.839. [DOI] [PubMed] [Google Scholar]
- Wildman EE, Jones GM, Wagner PE, Boman RL, Troutt HF, Lesch TN. A dairy-cow body condition scoring system and its relationship to selected production characteristics. J Dairy Sci. 1982;65:495–501. [Google Scholar]
- Wiltbank M, Lopez H, Sartori R, Sangsritavong S, Gumen A. Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology. 2006;65:17–29. doi: 10.1016/j.theriogenology.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Souza AH, Carvalho PD, Bender RW, Nascimento AB. Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle. Reprod Fertil Dev. 2011;24:238–243. doi: 10.1071/RD11913. [DOI] [PubMed] [Google Scholar]
- Yaakub H, O’Callaghan D, Boland MP. Effect of type and quantity of concentrates on superovulation and embryo yield in beef heifers. Theriogenology. 1999;51:1259–1266. doi: 10.1016/S0093-691X(99)00070-9. [DOI] [PubMed] [Google Scholar]