Abstract
Background
Engaging in health-promoting behaviors (e.g., healthy fruit- and vegetable-rich diet, physical activity) and living in supportive social and built environments are consistently and significantly associated with reductions in cancer, heart disease, diabetes, and other chronic diseases. Interventions to change diet and physical activity behaviors should aim to educate individuals, change the environments in which people live, work and recreate, improve access, availability, and affordability of healthy foods, and create safe places the facilitate active lifestyles. This trial will assess whether community gardening increases fruit and vegetable consumption and physical activity, improves social support and mental health, and reduces age-associated weight gain and sedentary time among a multi-ethnic, mixed-income population.
Methods/design
A randomized controlled trial of community gardening began in Denver, Colorado in January 2017. Over 3 years, we will recruit 312 consenting participants on Denver Urban Gardens’ waitlists and randomize them to garden or remain on the waitlist. At baseline (pre-gardening), harvest time, and post-intervention, study participants will complete three 24-hour dietary recalls, a 7-day activity monitoring period using accelerometry, a health interview and physical anthropometry.
Discussion
This project addresses health-promoting behaviors among a multi-ethnic, mixed-income adult population in a large metropolitan area. If successful, this trial will provide evidence that community gardening supports and sustains healthy and active lifestyles, which can reduce risk of cancer and other chronic diseases.
Trial registration
ClinicalTrials.gov, ID: NCT03089177: Registered on 03/17/17.
Keywords: Community gardens, Cancer prevention, Physical activity, Diet, Weight
1. Background
Improving diets, increasing physical activity, and reducing sedentary time are critical for the primary and secondary prevention of chronic diseases such as obesity, cancer, heart disease, and diabetes, and promoting health more generally [1,2]. Moreover, half of adults in the United States have at least one chronic condition and over a quarter of Americans adults have two or more conditions [3]. Consequently, these chronic conditions are the main drivers of disease, disability, and health care costs in the United States. The American Cancer Society and others consistently report that, in addition to smoking cessation, socioeconomic disadvantage, access to fruits and vegetables, and access to supportive environments for physical activity are critical areas for reducing cancer risk [4] and for improving survival [5–14]. People of color living in communities with higher levels of poverty (> 30%) experience neighborhood conditions that can negatively affect their likelihood to engage in health promoting behaviors and overall health status [15,16]. This is due, in part, to exposure to health-compromising conditions such as lack of access to healthy food and outdoor activity spaces [16–24]. Additionally, people who perceive their neighborhoods as unsafe and aesthetically unpleasing report greater levels of stress [25] and poorer sleep quality [26]. In turn, individuals who report high stress are less likely to engage in healthy behaviors such as physical activity and healthy eating [27].
Given the burden of cancer and other chronic diseases and the costs that could be prevented by changes in health-promoting behaviors, a primary goal in chronic disease prevention is to identify interventions that are sensitive to social, cultural, and economic factors and encourage all people to make healthy lifestyle choices that reduce their risk of chronic disease [28–30]. Addressing health behavior change require theory-informed, socio-ecological approaches that are sensitive to cultural preferences and diverse social contexts [29].
Community gardens, defined as green spaces where individuals from more than one family grow food communally or side-by-side, are an example of an environmental and social intervention at the neighborhood level. Across North America and beyond, municipalities are experiencing a renewed interest from residents to grow food themselves in their own communities. Yet, in an era of increasingly constrained budgets for public amenities, local governments are weighing the costs and benefits of such an investment with other community priorities. Current evidence about the effectiveness of gardens in promoting health and wellbeing among adults is based largely on results from qualitative and cross-sectional studies [31,32]. Past studies have shown that community gardeners eat fruits and vegetables more often [33,34] and report lower body mass index (BMI) [35,36]. Gardeners engage in more physical activity than non-gardeners [37,38]. The effect on fruit and vegetable consumption is partially explained by the finding that gardeners are more socially involved and feel more social support than non-gardeners [33,34,39,40].
The aim of this report is to describe the design of a randomized controlled trial to test the effects of community gardening on diet and physical activity behaviors, weight status, waist circumference, and the mechanisms by which these changes may occur. We hypothesize that community gardens represent a place-based social and environmental strategy for reducing cancer risk through increasing health-promoting behaviors. Our randomized controlled trial of community gardening takes advantage of a community setting to rigorously test our hypothesis. This trial will allow us to study cancer and chronic disease risk reduction strategies in mixed income, multi-ethnic populations.
2. Methods/design
2.1. Study design and objectives
A randomized controlled trial of community gardening began in January 2017 at the University of Colorado (ClinicalTrials.gov, ID: NCT03089177) in partnership with Michigan State University, the University of South Carolina, and Colorado State University. The study protocol was reviewed and approved by the University of Colorado Boulder Human Subjects Institutional Review Board (Protocol # 16-0644). The purpose of this study is to evaluate whether community gardening increases fruit and vegetable consumption, physical activity and social support, and reduces age-associated weight gain, and sedentary time among a multi-ethnic, low-income population of adults from before gardening to two follow-up time points: harvest and nine-month follow up. The secondary outcomes of interest include anxiety, perceived stress, and general well-being. We will conduct mediation analyses in order to assess how factors, such as self-efficacy, perceived barriers, social involvement, and social support, affect the relationships between community gardening and our study outcomes.
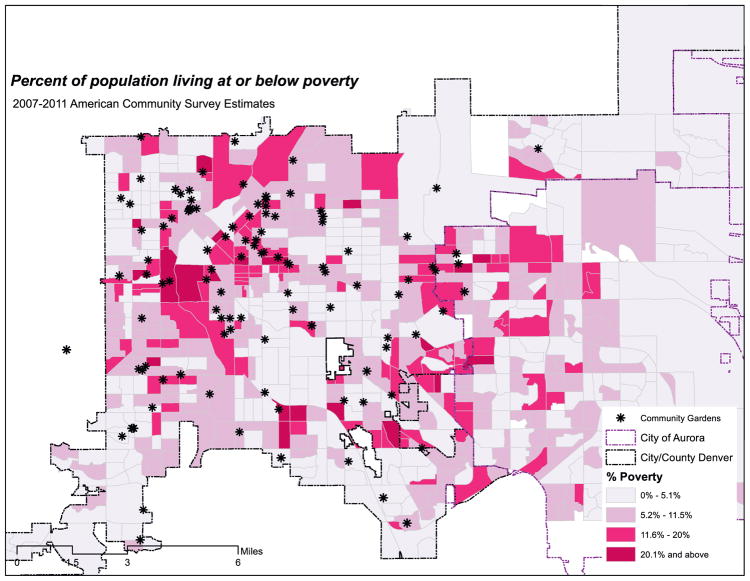
In total, this study will enroll 312 participants over 3 years who will be randomized to receive a community garden plot (156 participants) or remain on a garden plot waitlist (156 participants). The recruitment effort involves three waves of data collection to recruit 104 residents per year for three years (one wave per year). Each participant completes three assessments surrounding the garden season at baseline pre-gardening (T1), mid-season/harvest (T2), and at nine-month follow up (T3). At these assessments, we collect diet, sedentary time, physical activity, and anthropometric data and conduct a health survey. Participants receive three monetary incentives following the completion of each assessment, 7-day physical activity monitoring and diet recalls ($25 (T1), $50 (T2), and $75 (T3). Fig. 1 illustrates the geographic area of Denver and Aurora counties, percent living at or below poverty population, and the overlay of gardens across these census block groups.
Fig. 1.
Community gardens and socioeconomic measures (Denver and Aurora by Block Group) Percent of population living at or below poverty 2007–2011 American Community Survey Estimates.
2.2. Recruitment and eligibility
The Denver Urban Gardens (DUG) network includes over 165 community gardens. Phase 1 of the recruitment process involves the engagement of and commitment from garden leaders from DUG-sponsored gardens to participate in the study. Using Census data, attention will be made to recruit community gardens in a range of neighborhoods along income and socio-demographic characteristics to achieve a mixed-income multi-ethnic sample. Garden leaders, who represent individuals who volunteer to oversee garden membership, assign plots, manage garden fees, and liaise with DUG, are asked to commit 2–6 plots to the study. In return for their cooperation, gardens received payments for plot fees ($25–$65 per plot) and plot commitments ($50 per plot, up to 6 plots). Once a garden agrees to participate, garden leaders provide the study coordinator access to the garden waitlist. Staff canvass in neighborhoods, attend community meetings and health fairs, and distribute fliers at homes within 1 mile from the garden and at recreation centers and churches to encourage residents to join garden waitlists. Once the waitlist is populated, the study coordinator and staff recruit study participants and obtain written consent at the beginning of the first assessment.
All participants are screened for initial study eligibility by study personnel. Eligibility criteria require the participant to be at least 18 years or older, able to give consent in English or Spanish, currently on the waitlist for a garden in the Denver Urban Gardens network, and not have gardened in the past two gardening seasons. Only one person per household is eligible to participate.
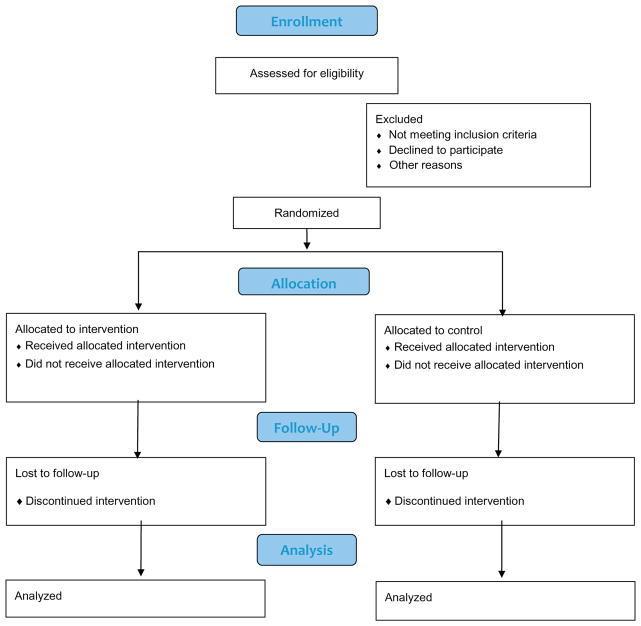
If a person meets eligibility requirements, the study personnel provide a brief description of the study, and asks the prospective gardener if he or she wants to participate. During this process, study personnel explain the intervention and randomization protocols as well as the data collection procedures, risks, and benefits of participating in the study. Informed consent forms are available both in Spanish and in English and are administered at the first assessment. A study design flow diagram is displayed in Fig. 2.
Fig. 2.
Study design flow diagram.
2.3. Allocation
Participants are randomized to two groups: gardening and waitlist. Individuals randomized to the garden group receive a garden resource package modeled on existing resources provided by DUG, which includes the following:
A garden plot: Intervention participants are assigned a plot in a DUG garden
Seeds and plant seedling starts: Intervention participants select vegetable seeds and plant starts provided by DUG’s Grow a Garden program
Introductory gardening workshop: DUG offers beginner gardening classes
Social events: Participants are invited to garden-based social events, and one-to-one and one-to-many garden mentoring available through DUG’s Master Community Gardener Program
The non-gardening group remains on DUG waitlists and does not receive these resources during the trial. As recompense for not being randomized to a garden, and for remaining a non-gardener for the year of the trial, DUG staff and garden leaders have promised to make efforts to place non-gardening participants in DUG community gardens the following year, when they have completed the study. As part of the compensation offered to the non-gardening group, the study covers plot fees and provides seeds and transplants to non-gardening participants who decide to garden the following season. Those who participate in the trial as part of the non-gardening group are excluded from the trial for any subsequent waves of the study.
2.4. Randomization
The team statistician uses a pseudo random-number generator (RANUNI, SAS Version 9.4, SAS Institute, Cary North Carolina) with a fixed seed to choose participant assignments. The randomization is stratified by garden. That means that participants are randomized using a a one-to-one allocation within each garden waitlist, so an an equal number of participants from each waitlist are either randomized to garden or not to garden. The approach involves a permuted block randomization with varying block sizes. The goal of the permuted randomization is to prevent study personnel or gardeners from guessing what the next treatment assignment would be, thus breaking the blind for study staff. Given the size of each garden waitlist, the algorithm randomly selects blocks of size 2, 4 or 6, so that the total number of participants in all blocks is equal to the number of the people on the waitlist for the garden. Within each block, there is equal allocation to the gardening and not gardening arm. Following randomization, participants receive a phone call from the project coordinator to notify them of their allocation assignment.
2.5. Blinding
Health surveys, accelerometry, and dietary interviews are conducted at T1 before random allocation. An analyst with sole access to a unique study identification number and a code for the strata implements the randomization assignment. Once the treatment group is assigned, only data collectors who are blinded to treatment assignment can administer questionnaires or collect participant data. All health visits will be conducted at either Denver Urban Garden’s central office or at a community center near the participant homes. No interviews will be done at the community gardens. Upon treatment assignment, participants are strongly reminded not to disclose their allocation status to the data collector at follow up assessments. To minimize bias at T2 and T3, the study coordinator assigns staff members to specific participants whose allocation assignment is unknown. For each interview, the participants are welcomed into their health visit room by the study coordinator or staff not blinded to the allocation assignment. They will welcome the participant and answer any questions they may have. After allowing the participant to settle in and ask questions specific to the trial, the staff member will remind the participant to refrain from mentioning the allocation assignment. At this point, the blinded study assistant is able to complete the health visit.
In the event data collectors become aware of the participant’s treatment assignment, they are asked to record the point in the evaluation when the blind was broken. Study data are stored in a separate database from other identifying information with treatment group recorded as A or B. The statistician is blinded to the allocation assignment. A data analyst blinded to the allocation assignment performs data checks and prepares data reports for the research team to evaluate. A data quality committee blinded to allocation assignment conducts periodic reviews of data completeness and quality.
2.6. Measures
To assess outcomes, all participants undergo 1.5-hour assessments performed by trained study personnel blinded to allocation assignment. The outcome assessor collects data on primary and secondary outcome variables and mediators.
2.6.1. Anthropometric measurement
Participant height is measured with a portable stadiometer to the nearest 0.1 cm (Seca 213 Portable Stadiometer; Seca). Weight is measured with a digital platform scale to the nearest 0.23 kg (0.5 lb) (Seca 876 Digital Scale; Seca). Waist circumference measurements are measured to the nearest 0.1 cm following the method detailed by the Diabetes Prevention Program (DPP) [41]. Two measurements for each anthropometric variable are taken and the average of the measurements are used for the analysis.
2.7. Primary outcomes
2.7.1. Physical activity
Estimates of sedentary time and physical activity are measured objectively using thigh-mounted activPAL™ accelerometers (PAL Technologies) [42,43], which has been validated for quantifying physical activity when worn on the thigh [44]. During the health survey, participants complete a self-report questionnaire and an activity log during the week that accelerometers are worn. Accelerometry has become an objective means for monitoring activity in free-living conditions and can be worn on the wrist, hip or leg. Thigh-mounted accelerometers have similar accuracy compared to the hip [44–46] and result in excellent compliance [47–49]. Acceleration data can provide an objective measure of the types of activity (e.g. sit vs. walk), as well as the intensity, duration and frequency of activity throughout the day [49,50]. Participants wear the accelerometer for seven consecutive days, except when engaging in water activities that submerge the body in water such as swimming and bathing. Participants can wear the device while showering. Self-report physical activity and sedentary time data are collected using the validated Global Physical Activity Questionnaire (GPAQ) [51,52]. The GPAQ collects participants’ activity and time spent sitting over the previous 7 days across 4 domains, including occupation, transportation, home and recreation/leisure-time. Finally, participants are asked to maintain a log of physical activity during the period in which they are wearing the accelerometer. The log collects sleep and wear time for all participants and additionally, time spent in garden for those in the intervention group.
2.7.2. Diet
Nutrient and food intake data are collected using 3 telephone-administered 24-hour recall interviews, which, although not devoid of measurement error, are considered the “gold standard” for evaluating a nutrition intervention and generate a reliable estimation of macro-nutrient and food group intake [53] [54,55]. The University of South Carolina’s Arnold School of Public Health conducts the 24-hour recalls. The Nutrient Data System for Research software licensed from the Nutrition Coordinating Center at the University of Minnesota is employed to conduct the dietary interviews. Interviewers are bilingual (English/Spanish) experienced registered dietitians specifically trained in using the multi-pass approach to conduct telephone interviews. Portion estimation is facilitated by the use of a validated, 2-dimensional, food portion visual [56].
Prior to data collection, study participants undergo a brief training (10 min) on how to use the FPV to estimate portion sizes of commonly eaten foods at the T1 assessment when accelerometers are mounted on the thigh and when height and weight are measured. The training incorporates life-sized plates, glasses and utensils and food models, in a hands-on experiential interchange [57]. Interviews are scheduled such that information is collected on 2 weekdays and 1 weekend day to help balance known cyclical differences in intake patterns [58]. Interviews are assigned on randomly selected days, and cold calls are made to the study subject to minimize preparation that could bias recall [53]. The sampling window is adequately large (2 weeks in length) to allow multiple attempts on multiple days to contact participants for interviews, which maximizes the likelihood of completing an interview.
2.8. Secondary outcomes
Perceived Stress Scale is widely used for measuring psychological distress because it is linked to cancer and a variety of other chronic diseases [59] [60]. The Perceived Stress Scale (PSS) is a self-reported questionnaire designed to measure how individuals appraise situations in their lives as stressful. The PSS-10 represents a valid and reliable shortened version of the original 14-item instrument [61,62].
General anxiety disorder (GAD) is the most common anxiety disorder observed in general medical practice. The GAD-7 is a valid and reliable self-report scale (score scale range from 0 to 21) used to identify probable cases of GAD [63].
2.9. General well being
The Warwick-Edinburgh Mental Wellbeing Scale (WEMWBS) is used to measure the impact of gardening on mental wellbeing. It is a 14-item scale with 5 response categories, summed to provide a single score ranging from 14 to 70. The items are worded positively and cover feeling and functional aspects of mental wellbeing. It has been shown to be sensitive to change in general lifestyle interventions [64].
2.10. Intrapersonal and interpersonal mediators
Mediators of interest include measures of self-efficacy, social support and perceived barriers to fruit and vegetable intake and vegetable preferences [65]; self-efficacy, social support and perceived barriers to physical activity [66,67]; and intrinsic motivation for physical activity [68], nature relatedness [69].
2.10.1. Process evaluation
The goals of the quality control protocol will be to 1) standardize the measurement techniques, 2) monitor the quality of measurements and intervention delivery over the course of the study, and 3) to document the validity and precision of the measurements. Interviews, surveys and audits of garden and surrounding neighborhood environments are used to assess the intervention across three levels of participation: gardeners, garden leaders (i.e. leaders at each community garden) and garden organizations (i.e. Denver Urban Gardens). The evaluation components, informed by Linnan and others, are described below [70–74].
2.10.1.1. Reach, dose and fidelity
Intervention delivery is assessed through 1) reach (e.g. percent eligible who enroll, who drops out and why, percent who completed the T1-T3 assessments); 2) dose delivered and received (e.g. intensity of garden use, and uptake of garden programming including new gardener workshops, leadership training frequency); and 3) fidelity (percent of people assigned to each arm who did not cross over to the other arm and whether the intervention was delivered in the way it was intended). Through interviews with Denver Urban Gardens staff and garden leaders, we assess whether new gardener workshops are offered, whether seeds and plant starts are available, the level of communication with gardeners, garden leaders, and DUG staff, whether social events are scheduled, and the range of barriers and supports related to garden participation. Garden participant interviews assess level of satisfaction and actual participation in gardening as well as garden-specific workshops, tactics deployed by gardeners, and activities for gardeners. Additionally, the interviews assess likelihood of participants integrating gardening into their future lifestyle. Semi-structured interviews, which are conducted on a subset of participants, elicit more in-depth knowledge about participants’ perceptions of the quality of the garden experience and their level of satisfaction from the perspective of people new to gardening as well as any potential perceived shortcomings of the intervention and potential ways to strengthen future community garden trials. Direct observations assess plot use and productivity, using previously developed audit instruments of garden plots and amenities [75].
2.10.1.2. Attrition
Study results could be adversely affected by poor adherence to the study protocol and could threaten the internal validity (e.g. differential attrition across intervention and control groups), although perfect compliance is not likely to be necessary to establish benefit. We recognize a few areas where poor adherence may be an issue. Participants randomized to remain on waitlist may decide to garden elsewhere. Before randomization, staff will share expectations with participants about study conditions. Participants are asked whether they believe they can refrain from gardening elsewhere. For this study, if individuals do not think they can keep this commitment, they will be excluded; if they respond positively, they are enrolled.
2.10.1.3. Retention program
Once randomization occurs, the study personnel maintains contact with both intervention and control participants through scheduled assessment periods (T1-T3) and interim phone calls (e.g., July, October, January) to minimize dropout and loss to follow-up.
2.11. Sample size
The total planned sample size for the study is 312 people in 30 neighborhood gardens. This estimate reflects an adjustment for a potential 30% loss to follow-up. The sample size was chosen by conducting a separate power analysis for each of the three primary outcomes: physical activity, fruit and vegetable intake, and body mass index. The final sample size chosen for the study was the maximum of the three sample sizes found to provide power of at least 80% for each outcome. Power and sample size estimates for the fruit and vegetable and physical activity outcomes were performed using the method of Muller and others (glimmpse.samplesizeshop.org) [76] and for the psychosocial mediators using Monte Carlo simulation in Mplus [77–79].
Secondary outcomes of perceived stress, anxiety, and general well-being are included in this study but are included as exploratory components of the study. Thus, a formal power analysis was not conducted.
2.12. Analysis plan
We will assess balance between randomization arms by comparing the arms for gender, socio-economic status, race/ethnicity and age.
For the outcomes of physical activity and fruit and vegetable intake, we will use similar analyses. We describe the analysis for fruit and vegetable intake as an exemplar. Using an intent to treat analysis and a general linear mixed model to allow for missing data, we will fit the 3-day average recall measurements of fruit and vegetable intake as the outcome. The modeling approach will use garden as the independent sampling unit, and account for correlation between gardeners within garden. For fixed predictors, we will use time of measurement, an indicator variable for gardening, and the time by treatment interaction. We will evaluate the distribution of the jackknifed, studentized residuals of fruit and vegetable intake measures for normality. If the distribution is significantly skewed, an appropriate Box-Cox transformation will be applied. We will test for year by effect homogeneity, and combine responses across years if, as we expect, little difference occurs year by year. The model will include random effects terms for neighborhood, which allows the model to account for differential, unmeasured differences for each neighborhood, which may include race, socio-economic status, language and culture. We will compare study participants with no missing data to those with missing data in an effort to see if differential response rates may have biased the study.
Because gardeners may see each other in the garden each day, their dietary intake may show a higher correlation than that of wait-listers. Thus, two possible nested variance models include the model with a neighborhood random effect only, and a model with an extra random term for garden effect. We will compare the fit of two models using a chi-squared log likelihood test, and by comparing the Akaike information criteria.
In the model with the most appropriate variance, we will conduct hypothesis testing using the Kenward-Roger test. We will use sensitivity analysis to examine the potential confounding, mediating, or moderating activity of variables such as age, BMI, gender, language, race, and socio-economic status. We will provide group means, confidence intervals, and graphical descriptions of the data.
Both within-participant and between-participant effects are of interest. The within-participant effect is the effect of time. The between-participant effects are due to the gardening intervention. The planned sequence of hypothesis tests is as follows: 1) Test the time-by-treatment interaction at a Type I error rate of 0.04; 2) If there is no time-by-treatment interaction, then we will test the main effect of gardening at a Type I error rate equal 0.005. If the main effect of gardening is significant, we will stop testing; 3) If there is no main effect of treatment, then we will test the main effect of time using orthogonal polynomials to examine the linear and quadratic trends each at a Type I error rate of 0.0025. The hypothesis testing uses an alpha spending approach to assure a total experiment-wise error rate of 0.05.
Psychosocial outcomes will be analyzed using Mplus [78] following well-established statistical methods for testing mediation [80], particularly in a multilevel framework where individuals are nested within gardens/neighborhoods [81]. The potential mediators will be examined individually, as well as together in a multiple mediator model to account for relationships among mediators. The regression of the potential mediators on community gardening will be estimated simultaneously with tests of the effect of the mediators on dietary intake and physical activity, controlling for gardening. Multilevel mediation analysis is feasible in Mplus [81]; depending on the magnitude of the garden-level interclass coefficients (ICCs) observed in this study, the between and within components will either be modeled explicitly, or at least the standard errors will be corrected for the non-zero ICCs. Effects are expected to be mediated by changes to the theoretical constructs from baseline to the first follow-up assessment.
3. Discussion
Health behavior changes, such as improved diets, increased physical activity, and reduced sedentary time, are critical for the primary and secondary prevention of chronic diseases such as cancer, heart disease, and diabetes, and promoting health more generally. Despite this recognition about impacting chronic disease and promoting health, few Americans participate in regular physical activity and consume recommended levels of fruits and vegetables [7], too many Americans engage in unhealthy levels of sedentary behavior [82,83], and many experience poor mental health. Supportive physical and social environments where people live, work and recreate are necessary for achieving and sustaining these changes [84].
Community gardens represent one neighborhood-based strategy through which we can examine the ways in which behaviorally based interventions impact health. The trial described in this report, to the investigators’ knowledge, is the first randomized controlled blinded study on health behaviors and weight outcomes as a result of participating in a community garden and the mediating factors that may influence these changes. Moreover, this study aims to explore a community-level intervention that is available and accessible to people across different social and economic groups and from different cultural backgrounds.
This study will help provide the highest level of scientific evidence about whether community gardening improves and sustains health-promoting behaviors in a mixed-income, multi-ethnic adult population. If successful, the evidence generated through this project will inform ways in which planning, housing and public health professionals, and non-profit leaders can create, increase and sustain access to beautiful, social places that promote health, particularly in disadvantaged areas.
Trial status
The study began in March 2017. Data collection for T1 and T2 are complete. Planning for wave 2 recruitment is underway.
References
- 1.Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM, Kim S, Rusev ER, Du S, Zizza C. Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes Rev. 2006;7(3):271–293. doi: 10.1111/j.1467-789X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 3.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey 2010. Prev Chronic Dis. 2013;10:E65. doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer. 2008;113(3):582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs – United States, 1995–1999. Morbidity and Mortality Weekly Report. 2002;51(14):300–303. [PubMed] [Google Scholar]
- 6.Hayman L, Hughes S. Cardiovascular health for all. J Cardiovasc Nurs. 2006;21(2):154–155. doi: 10.1097/00005082-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Kushi L, Doyle C, McCullough M. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clin. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 8.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 9.Mensah GA. Eliminating disparities in cardiovascular health. Circulation. 2005;111(10):1332–1336. doi: 10.1161/01.CIR.0000158134.24860.91. [DOI] [PubMed] [Google Scholar]
- 10.Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26(1):1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Pate RR, Pratt M, Blair SN. Physical activity and public health. A recommendation from the centers for disease control and prevention and the American college of sports medicine. J Am Med Assoc. 1995;273(5):402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics: 2011 update. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in health care, Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Institute of Medicine; Washington, DC: 2003. pp. 1–432. [Google Scholar]
- 14.Smedley BD, Syme SL. A social environmental approach to health and health interventions, Promoting Health: Intervention Strategies from Social and Behavioral Research. 1. National Academy Press; Washington, DC: 2000. [Google Scholar]
- 15.Winkleby M, Cubbin C. Racial/ethnic disparities in health behaviors: a challenge to current assumptions. In: Anderson N, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press (US); Washington, DC: 2004. [PubMed] [Google Scholar]
- 16.Institute of Medicine, Institute of Medicine, editor. How Far Have We Come in Reducing Health Disparities? Progress since 2000: Workshop summary. The National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- 17.Burke GL, Savage PJ, Manolio TA, Sprafka JM, Wagenknecht LE, Sidney S, Perkins LL, Liu K, Jacobs DR., Jr Correlates of obesity in young black and white women: the CARDIA study. Am J Public Health. 1992;82(12):1621–1625. doi: 10.2105/ajph.82.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duelberg SI. Preventive health behavior among black and white women in urban and rural areas. Soc Sci Med. 1992;34(2):191–198. doi: 10.1016/0277-9536(92)90096-9. [DOI] [PubMed] [Google Scholar]
- 19.Kimmons J, Gillespie C, Seymor J, Serdula M, Blanck HM. Fruit and vegetable intake among adolescents and adults in the United States: percentage meeting individualized recommendations. Medscape J Med. 2009;11(1) [PMC free article] [PubMed] [Google Scholar]
- 20.McPherson M, Smith-Lovin L, Brashears M. Social isolation in America: changes in Core discussion networks over two decades. Am Sociol Rev. 2006;71:353–375. [Google Scholar]
- 21.Ogden C, Carroll MD, Fryar CD, Flegal KM. U.S. DHHS CDC, editor. NCHS Data Brief. National Center for Health Statistics; Atlanta, GA: 2014. Prevalence of obesity among adults and youth: United States, 2011–2014; pp. 1–8. [Google Scholar]
- 22.Winkleby MA, Cubbin C, Ahn DK, Kraemer HC. Pathways by which SES and ethnicity influence cardiovascular disease risk factors. Ann N Y Acad Sci. 1999;896:191–209. doi: 10.1111/j.1749-6632.1999.tb08116.x. [DOI] [PubMed] [Google Scholar]
- 23.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey 1988–1994. JAMA. 1998;280(4):356–362. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 24.Willett WCKJ, Nugent R, et al. Prevention of chronic disease by means of diet and lifestyle Changes. In: Jamison DTBJ, Measham AR, editors. Disease Control Priorities in Developing Countries. 2. The International Bank for Reconstruction and Development/The World Bank; Oxford University Press; Washington, DC: 2006. [PubMed] [Google Scholar]
- 25.Henderson H, Child S, Moore S, Moore JB, Kaczynski AT. The influence of neighborhood aesthetics, safety, and social cohesion on perceived stress in disadvantaged communities. Am J Community Psychol. 2016;58(1–2):80–88. doi: 10.1002/ajcp.12081. [DOI] [PubMed] [Google Scholar]
- 26.Hale L, Hill TD, Friedman E, Javier Nieto F, Galvao LW, Engelman CD, Malecki KMC, Peppard PE. Perceived neighborhood quality, sleep quality, and health status: evidence from the survey of the health of Wisconsin. Soc Sci Med. 2013;79(Supplement C):16–22. doi: 10.1016/j.socscimed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouchacca J, Abbott GR, Ball K. Associations between psychological stress, eating, physical activity, sedentary behaviours and body weight among women: a longitudinal study. BMC Public Health. 2013;13(1):828. doi: 10.1186/1471-2458-13-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertoia ML, Mukamal KJ, Cahill LE, Hou T, Ludwig DS, Mozaffarian D, Willett WC, Hu FB, Rimm EB. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12(9):e1001878. doi: 10.1371/journal.pmed.1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint Onge JM, Krueger PM. Health lifestyle behaviors among U.S. adults. SSM Popul Health. 2017;3:89–98. doi: 10.1016/j.ssmph.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Cancer Research Fund, Food, Nutrition, Physical Activity, and the Prevention of Cancer. A Global Perspective. American Institute for Cancer Research; Washington, DC: 2007. [Google Scholar]
- 31.Omitted for review.
- 32.Guitart D, Pickering C, Byrne J. Past results and future directions in urban community gardens research. Urban For Urban Green. 2012;11(4):364–373. [Google Scholar]
- 33.Omitted for review.
- 34.Omitted for review.
- 35.Zick CD, Smith KR, Kowaleski-Jones L, Uno C, Merrill BJ. Harvesting more than vegetables: the potential weight control benefits of community gardening. Am J Public Health. 2013;103(6):1110–1115. doi: 10.2105/AJPH.2012.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omitted for review.
- 37.Park S, Kwan SL, Ki-Cheol S. Determining exercise intensities of gardening tasks as a physical activity using metabolic equivalents in older adults. Hortscience. 2011;46(12):1706–1710. [Google Scholar]
- 38.Park S, Shoemaker CA, Haub MD. Physical and psychological health conditions of older adults classified as gardeners or nongardeners. Hortscience. 2009;44(1):206–210. [Google Scholar]
- 39.Omitted for review.
- 40.Omitted for review.
- 41.The Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas. 2014;35:129–138. doi: 10.1088/0967-3334/35/2/129. [DOI] [PubMed] [Google Scholar]
- 43.Trost SG, Zheng Y, Wong WK. Machine learning for activity recognition: Hip versus wrist data. Physiol Meas. 2014;35:2183–2189. doi: 10.1088/0967-3334/35/11/2183. [DOI] [PubMed] [Google Scholar]
- 44.Esliger D, Rowlands A, Hurst T, Catt M, Murray P, Eston R. Validation of the GENEA accelerometer. Med Sci Sports Exerc. 2011;43(6):1085–1093. doi: 10.1249/MSS.0b013e31820513be. [DOI] [PubMed] [Google Scholar]
- 45.Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1S):S68–76. doi: 10.1249/MSS.0b013e3182399e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Rowlands AV, Murray P, Hurst TL. Physical activity classification using the GENEA wrist-worn accelerometer. Med Sci Sports Exerc. 2012;44(4):742–748. doi: 10.1249/MSS.0b013e31823bf95c. [DOI] [PubMed] [Google Scholar]
- 47.Omitted for review.
- 48.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 49.Ward V, Schaefer C, Lampe S, Nace H, Kutchman E, Brink L, Browning RC. Achieving excellent compliance in multi-day free-living activity monitoring in elementary school students: the IPLAY study. Med Sci Sports Exerc. 2011;42(5) [Google Scholar]
- 50.Butte NF, Ekelund U, Westerterp KR. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc. 2012;44(1S):S5–S12. doi: 10.1249/MSS.0b013e3182399c0e. [DOI] [PubMed] [Google Scholar]
- 51.Craig C, Marshall A, Sjostrom M. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 52.Matthews C, Keadle SK, Sampson J, Lyden K, Bowles H, Moore S, Libertine A, Freedson P, Fowke J. Validation of a previous-day recall measure of active and sedentary behaviors. Med Sci Sports Exerc. 2013;45(8):1629–1638. doi: 10.1249/MSS.0b013e3182897690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged Women’s estimates of energy intake. Ann Epidemiol. 2002;12(8):577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 54.Resnicow K, Odom E, Wang T, Dudley W, Mitchell D, Vaughan R, Jackson A, Baranowski T. Validation of three food frequency questionnaires and 24-hour recalls with serum carotenoid levels in a sample of African-American adults. Am J Epidemiol. 2000;152(11):1072–1080. doi: 10.1093/aje/152.11.1072. [DOI] [PubMed] [Google Scholar]
- 55.Yaroch A, Tooze J, Thompson F. Evaluation of three short dietary instruments to assess fruit and vegetable intake: the national cancer institute’s food attitudes and behaviors survey. J Acad Nutr Diet. 2012;112(10):1570–1577. doi: 10.1016/j.jand.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Posner BM, Martin-Munley SS, Smigelski C, Cupples LA, Cobb JL, Schaefer E, Miller DR, D’Agostino RB. Comparison of techniques for estimating nutrient intake: the Framingham study. Epidemiology. 1992;3(2):171–177. doi: 10.1097/00001648-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Wilcox S, Sharpe P, Parra-Medina D, Granner M, Hutto B. A randomized trial of a diet and exercise intervention for overweight and obese women from economically disadvantaged neighborhoods: Sisters Taking Action for Real Success (STARS) Contemp Clin Trials. 2011;32(6):931–945. doi: 10.1016/j.cct.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basiotis PP, Welsh SO, Cronin FJ, Kelsay JL, Mertz W. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J Nutr. 1987;117(9):1638–1641. doi: 10.1093/jn/117.9.1638. [DOI] [PubMed] [Google Scholar]
- 59.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hébert JR, Braun KL, Kaholokula JKa, Armstead CA, Burch JB, Thompson B. Considering the role of stress in populations of high-risk, underserved community networks program centers. Prog Community Health Partnerships. 2015;9(0):71–82. doi: 10.1353/cpr.2015.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen S, Kamarch T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 62.Lee E. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. 2012;6:121–127. doi: 10.1016/j.anr.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Spitzer RLKK, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder. Arch Inern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 64.Stewart-Brown SL, Platt S, Tennant A, Maheswaran H, Parkinson J, Weich S, Tennant R, Taggart F, Clarke A. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): a valid and reliable tool for measuring mental well-being in diverse populations and projects. J Epidemiol Community Health. 2011;65(Suppl 2):A38. [Google Scholar]
- 65.Erinosho TO, Pinard CA, Nebeling LC, Moser RP, Shaikh AR, Resnicow K, Oh AY, Yaroch AL. Development and implementation of the national cancer institute’s food attitudes and behaviors survey to assess correlates of fruit and vegetable intake in adults. PLoS One. 2015;10(2):e0115017. doi: 10.1371/journal.pone.0115017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 67.Sallis JF, Saelens BE, Frank LD, Conway TL, Slymen DJ, Cain KL, Chapman JE, Kerr J. Neighborhood built environment and income: examining multiple health outcomes. Social Sci Med (1982) 2009;68(7):1285–1293. doi: 10.1016/j.socscimed.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan R. Control and information in the intrapersonal sphere: an extension of cognitive evaluation theory. J Pers Soc Psychol. 1982;43:450–461. [Google Scholar]
- 69.Nisbet E, Zelenski J. The NR-6: a new brief measure of nature relatedness. Front Psychol. 2013;4:1–11. doi: 10.3389/fpsyg.2013.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lichstein K, Riedel BW, Grieve R. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Ther. 1994;16(1):1–29. [Google Scholar]
- 72.Linnan LTB, Kobetz E. Process evaluation results from the working well trial. In: Steckler A, Linnan L, editors. Process Evaluation for Public Health Interventions and Research. Jossey-Bass; San Francisco: 2002. pp. 155–183. [Google Scholar]
- 73.Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research. Jossey-Bass; San Francisco: 2002. [Google Scholar]
- 74.Saunders R, Evans M, Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract. 2005;6(2):134–147. doi: 10.1177/1524839904273387. [DOI] [PubMed] [Google Scholar]
- 75.Omitted for review.
- 76.Muller KE, LaVange LM, Ramey SL, Ramey CT. Power calculations for general linear multivariate models including repeated measures applications. J Am Stat Assoc. 1992;87:1209–1226. doi: 10.1080/01621459.1992.10476281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthén LK, Muthén BO. How to use a Monte Carlo study to decide on sample size and determine power. Struct Equ Model. 2002;(4):599–620. [Google Scholar]
- 78.Muthén LK, Muthén BO, Muthén M, editors. Mplus User’s Guide. 6. Los Angeles, CA: 1998. [Google Scholar]
- 79.Thoemmes F, MacKinnon DP, Reiser MR. Power analysis for complex mediational designs using Monte Carlo methods. Struct Equ Model Multidiscip J. 2010;17(3):510–534. doi: 10.1080/10705511.2010.489379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Preacher K, Zyphur M, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–233. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- 82.Rhodes RE, Mark RS, Temmel CP. Adult sedentary behavior a systematic review. Am J Prev Med. 2012;42 doi: 10.1016/j.amepre.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies 1996–2011. Am J Prev Med. 2011;41(2):207–215. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Pellmar TC, Brandt EN, Baird MA. Health and behavior: the interplay of biological, behavioral, and social influences: summary of an Institute of Medicine Report. Am J Health Promot. 2002;16(4):206–219. doi: 10.4278/0890-1171-16.4.206. [DOI] [PubMed] [Google Scholar]