Abstract
Regulation of pH is critical for physiological processes. Maintenance of acid-base homeostasis is tightly regulated by the renal and respiratory systems. However, fluctuations in extracellular pH are also sensed by other organ systems. Ion transporter activity to modify the amount of acid (H+ and CO2) and bicarbonate (HCO3−) is therefore actively maintained within the kidney and lung. This review describes acid-base disorders (acidosis and alkalosis) and highlights the importance of pH sensors and ion transporters that may be potential therapeutic targets for treatment of acid-base disorders. Specifically, the renal pH sensors proline-rich tyrosine kinase-2 (Pyk2) and G-protein coupled receptor-4 (GPR4) are discussed here.
Keywords: pH sensor, acid-base disorder, acidosis, alkalosis, Pyk2, GPR4
Respiratory and Renal Regulation of Arterial pH
Regulation of physiological pH is very important since most biochemical processes occur only within a narrow pH range. Typically, intracellular pH is about 7.2 and extracellular (or arterial) pH is between 7.36 and 7.44. pH fluctuation can occur in many organs and triggers a response in multiple cell types. pH can change as a result of ischemia, inflammation, exercise, musculoskeletal pain, and normal metabolic and neuronal activity [1]. Acid-base homeostasis is primarily maintained by the renal and respiratory systems, but pH is also sensed by the epididymis, osteoclast, myocyte, neuron, and acid/base-producing cells in the kidney, gut, and pancreas, among others. Within the cell interior and in the blood, the carbonic acid-bicarbonate buffering system works to maintain pH homeostasis by adjusting the amount of hydrogen and bicarbonate ions present. The reaction of the CO2/ HCO3− buffering system, catalyzed by carbonic anhydrase (CA), is as follows: [2]. CO2 can readily diffuse through the membrane without the aid of a transporter. The lipid bilayer however is impermeable to charged H+ and HCO3−, thereby requiring transport proteins to allow these ions to cross. Regulation of the acid or base transporters occurs through signaling transduction cascades initiated by a pH sensor, which is a protein directly activated by fluctuations in arterial or intracellular pH. This article focuses on the ion transporters that regulate pH and the putative pH sensors that control H+ and HCO3− influx and efflux within a cell.
Acid loads occur regularly due to metabolism of protein from a Western diet. Normal physiology is therefore in place to prevent acidemia after ingestion of dietary acids including compensatory mechanisms of the renal and respiratory systems. Excessive drops in pH (<6.8) is termed acidosis, and can occur as a result of the loss of an alkali load via diarrhea or vomiting [3]. The kidney can compensate for acid loads by increasing proximal tubule bicarbonate reabsorption, increasing distal acid secretion, and/or increasing ammonia excretion [4]. Hyperventilation is another way the body prevents acidosis by decreasing PCO2 [4]. Alkali loads can be metabolic, due to excessive excretion of urinary (or gastric) acid or respiratory; it can also due to hyperventilation, which lowers PCO2. To maintain a normal pH, the proximal tubule compensates for an alkali load by decreasing bicarbonate reabsorption; the pH can also be lowered through hypoventilation [4].
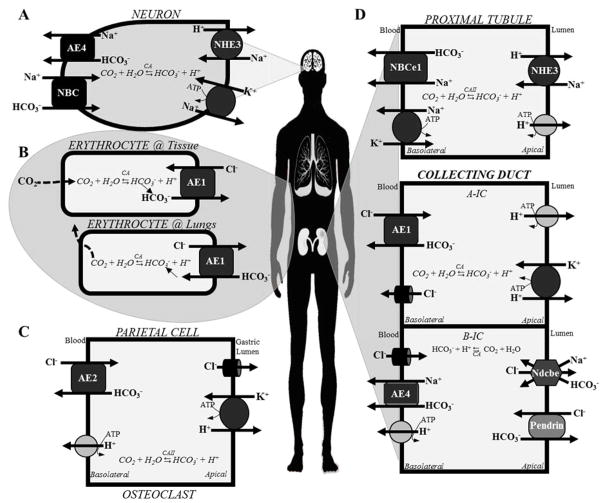
The respiratory system regulates pH by altering the amount of CO2 in the blood versus that exhaled through breathing. There are respiratory compensatory mechanisms triggered by decreased blood pH that results in greater diaphragm contraction, therefore more CO2 gets exhaled [5]. The equilibrium between CO2 (gas) CO2 (dissolved) varies with partial pressure of CO2, temperature, and pH [2]. Upon entering the low CO2 concentration environment of the lungs, erythrocytes control the amount of CO2 exhaled through regulation of the activity of CA and of ion transporter activity, specifically of AE1 (anion exchanger 1), which exchanges intracellular HCO3− for extracellular Cl−. CA converts HCO3− to CO2, which readily diffuses across the membrane and is exhaled [2]. This is shown in the diagram in (Figure 1, Panel B). Anion exchangers (AEs) (AE1, AE2, AE3, and AE4) are important bicarbonate transporters in nearly all acid-sensitive cells, as shown in Figure 1. AE1 is a bicarbonate/chloride exchanger expressed in the erythrocyte and collecting duct (Figure 1, Panel D). AE2 is another important bicarbonate/chloride exchanger expressed in gastric parietal cells and osteoclasts (Figure, 1, Panel C). AE3 is important in myocyte acidification. AE4, a sodium/bicarbonate cotransporter, is important in the brain (Figure 1, Panel A) and in B-IC cells of the collecting duct (Figure 1, Panel D). Other important bicarbonate transporters include NBCe1, Ndcbe, and Pendrin (described in more detail in Table 1).
Figure 1. Cell-specific transporters involved in regulation of acid-base homeostasis.
The diagram shows the main six cell types involved in pH sensing and/or regulation of arterial pH and the acid (H+) and base (HCO3−) transporters expressed on the plasma membrane of each cell type. (A) The neuron senses and responds to changes in pH through regulation of bicarbonate transporters AE4 (HCO3− efflux) and NBC (HCO3− influx). (B) Respiratory regulation of pH occurs primarily through AE1 in erythrocytes, which interestingly reverses the direction of its normal HCO3−/Cl− transport when in the lungs. Bicarbonate secretion occurs when the blood is at the body’s tissues. When at the lungs, bicarbonate absorption occurs through AE1. Once inside the cell, bicarbonate is converted to CO2, diffuses through the membrane and is exhaled. (C) The gastric parietal cell and osteoclast exhibit similar ion transporter expression, with basolateral bicarbonate absorption through AE2 and proton extrusion through the basolateral H+-ATPase and apical H+,K+-ATPase activity. (D) The three polarized epithelial cells diagramed on the right show renal acid- and base-secreting cells of the proximal tubule (top) and collecting duct (middle and bottom). Two types of intercalated cells are shown, Type A acid-secreting (A-IC) cells (middle) and type B bicarbonate-secreting (B-IC) cells (bottom). In the proximal tubule, basolateral bicarbonate reabsorption occurs via NBCe1 and apical acid secretion occurs via NHE3 and the H+-ATPase. In A-ICs of the collecting duct, basolateral bicarbonate reabsorption occurs via AE1 and apical acid secretion occurs via H+,K+-ATPase and V-ATPase. In B-ICs of the collecting duct, basolateral bicarbonate reabsorption occurs via AE4 and apical bicarbonate transport occurs via Ndcbe and Pendrin. Proton reabsorption also occurs in B-ICs across the basolateral membrane via active transport of the V-ATPase.
Table 1. Transport proteins involved in pH regulation.
The following transporters in the regulation of acid-base homeostasis are activated by changes in arterial pH. The cells expressing each protein and the function of each transporter are shown.
| Ion Transporter | Gene | Cell type | Ion efflux | Ion influx | Function | Refs | |
|---|---|---|---|---|---|---|---|
| AE1 | Anion exchanger 1; Band 3 protein | SLC4A1 | Collecting duct (A-IC) | Cl− | HCO3− | Bicarbonate reabsorption (Cl− / HCO3− exchanger) | [4] |
| AE2 | Anion exchanger 2 | SLC4A2 | Gastric parietal cells & osteoclasts | Cl− | HCO3− | Cl−/ HCO3− exchanger | [24] |
| AE3 | Anion exchanger 3 | SLC4A3 | Cardiac myocyte | Cl− | HCO3− | Myocyte acidification (Cl− / HCO3− exchanger) | [2] |
| AE4 | Anion exchanger 4 | SLC4A9 | Collecting duct (B-IC) & Neuron | Na+ | HCO3− | Bicarbonate reabsorption | [4] |
| HKα1 | α-subunit of gastric H+,K+-ATPase | Gastric parietal cells & Collecting duct (A-IC) | H+ | K+ | Urinary & intestinal acidification | ||
| HKα2 | α-subunit of colonic H+,K+-ATPase | ATP1A2 | Colonic & Collecting duct (A-IC) | H+ | K+ | Urinary & intestinal acidification | [8, 25] |
| KCC4 | KCl cotransporter | SLC12A7 | Collecting duct | Cl− | K+ | Urinary acidification | [4] |
| NBCe1 | Sodium bicarbonate cotransporter-1 | SLC4A4 | Proximal tubule | HCO3− | Na+ | HCO3− absorption (Electrogenic Na+/ HCO3− cotransporter) | [4] |
| Ndcbe | Sodium-Driven Chloride/Bicarbonate Exchanger | SLC4A8 | Collecting duct (B-IC) | Cl− | Na+ & HCO3− | HCO3− secretion (Na+/ HCO3− cotransporter) | [4] |
| NHE1 | Na+/H+ exchanger 1 | Cardiac myocyte Neuron | H+ | Na+ | Cardiac hypertrophy Epilepsy | [2] [26] |
|
| NHE3 | Na+/H+ exchanger 3 | Proximal tubule & Neuron | H+ | Na+ | Urinary acidification | [19, 26] | |
| NHE6 | Na+/H+ exchanger 6 | Neuron | H+ | Na+ | Epilepsy and brain disorders | [26] | |
| NHE9 | Na+/H+ exchanger 9 | Neuron | H+ | Na+ | ADHD | [26] | |
| Pendrin | Collecting duct (B-IC) | HCO3− | Cl− | Bicarbonate secretion | [4] | ||
| SLC26A7 | Gastric parietal cells, CCD and OMCD | Cl− | HCO3− | Basolateral HCO3− reabsorption (Cl−/ HCO3− exchanger) | [27] | ||
| V-ATPase | Vacuolar-type H+-ATPase | Collecting duct & Epididymal clear cells | H+ | --- | Urinary acidification | [8, 13] |
The kidneys are ultimately responsible for maintenance of acid-base homeostasis through regulation of the HCO3− reabsorption and H+ excretion into the urine. 90% of bicarbonate reabsorption occurs in the proximal tubule via transporters that are often Cl− driven [6]. Acidification of the urine occurs primarily in the distal nephron via proton transporters that are often Na+ or K+ driven, such as the sodium/hydrogen exchangers (NHE) and Na+,(H+),K+-ATPases. The apical membrane of the collecting duct is known to pump protons into the lumen via V-ATPases and H+,K+-ATPases to acidify the urine. The collecting duct (CD) is comprised of three segments, the cortical collecting duct (CCD), outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD), each consisting of a different distribution of principal and intercalated cells. The three types of intercalated (IC) cells are acid-secreting Type A-ICs, bicarbonate-secreting Type B-ICs, and nonAnonB-ICs (not discussed in this article). Figure 1, Panel D diagrams the transporters present in the apical and basolateral membranes of renal proximal tubule and intercalated cells. Many of the transporters responsible for the maintenance of pH are listed in Table 1 along with the localization, physiological function, and ion specificity for each transporter.
Clinical manifestations and Pharmacological implications of acid-base disorders
pH is indicative of acid-base status using the Henderson-Hasselbalch equation: . The acid dissociation constant is denoted as pK, bicarbonate concentration is in millimoles per liter and PCO2 is in millimeters of mercury [7]. Acid-base disorders involving abnormal CO2 (changes in PCO2) are termed respiratory disorders whereas those involving bicarbonate or acid are termed metabolic disorders [4]. Acidosis and alkalosis can each be either metabolic or respiratory. The methods for assessing acid-base disturbances depend on if the cause is respiratory or metabolic. Clinical evaluation includes analysis of any primary disease (pulmonary, cardiovascular, gastrointestinal) that may cause a secondary acid-base disturbance. Although these acid-base disorders are often due to genetic and/or physiological abnormalities, they can also be side-effects of certain medications or intoxications, as shown in Table 2. Clinical evaluations of acid-base disorders involve measurement of blood pH, bicarbonate levels, and calculation of the urinary anion gap (AG), usually 10 to 12 mEq/L, and defined as follows: AG = Na+ − (Cl− + HCO3− ). The AG is elevated in patients with metabolic acidosis (defined by arterial pH < 7.35), which occurs due to excessive bicarbonate loss or acid loading.
Table 2. Drug-induced acid-base disorders[6].
Acidosis (low pH) and alkalosis (high pH) can be due to dysregulation of H+ or HCO3−, termed Metabolic, or due to abnormal CO2 or PCO2, termed Respiratory. These acid-base disorders can result from medications or intoxications.
| Metabolic | Respiratory | ||
|---|---|---|---|
| Acidosis | Normal Anion Gap | Elevated Anion Gap | Opiates Benzodiazepines Propofol |
| Acetazolamide Topiramate Amphotericin B Ifosfamide |
Linezolid Lorazepam Metformin Nitroprusside Nucleoside reverse transcriptase inhibitors Ethylene glycol Ethanol/Methanol |
||
| Alkalosis | Diuretic therapy Bicarbonate administration Mineralocorticoids |
Salicylates Nicotine Xanthine |
|
Symptoms of acidosis include weakness, headaches, shortness of breath, increased heart rate, nause, vomiting, diarrhea, and sleepiness. Metabolic acidosis contributes to chronic kidney disease progression to end-stage renal disease [8]. Renal tubular acidosis (RTA) is a kidney disease that causes metabolic acidosis. Recent studies have shown that alkali treatment via sodium bicarbonate treatment slows the progression of CKD [9]. There are several types of RTA, including distal RTA (dRTA) and proximal RTA (pRTA), where distal and proximal refer to the segment of the nephron affected. Acid-base disorder treatment usually relies on treating the primary disease or problem that is contributing to the dysregulation of pH. In extreme cases of acidosis, bicarbonate infusions are used to regulate systemic pH.
pH sensors: Regulation of Ion Transport
Regulation of the ion transporters that maintain pH homeostasis typically involves acid- or base- activated signaling transduction mechanisms. Changes in pH may activate ion transporters directly, but more likely are sensed by an upstream pH sensor that regulates transporter activity, either directly or via a signaling cascade. Although a universal pH sensor has not been identified, several pH-sensitive signaling pathways that control acid-base transport have been elucidated. Putative pH sensors include pH-sensitive G-protein coupled receptors (GPCRs) [10], H+-sensing ion channels [11], alkali-sensing receptor tyrosine kinases (RTKs), soluble adenylyl cyclase (sAC) [12], cyclic nucleotides, and intracellular non-RTKs [12, 13].
It is expected that there must be a pH sensor, a protein activated by changes in extracellular pH, such as a membrane-spanning GPCR, ion channel, or receptor tyrosine kinase. In the cardiovascular system, acid-sensing ion channels (ASICs), relatives of Epithelial Sodium Channel (ENaC), may be responsible for sensing changes in pH in the blood, as well as regulating blood pressure [14]. There are also four known acid-stimulated GPCRs activated by the protonation of extracellular histidine residues at low pH: GPR4 (G-protein coupled receptor-4) [3], OGR1 (ovarian cancer GPR1) aka GPR68 [15], TDAG8 (T Cell death-associated gene) aka GPR65 [16], and G2A aka GPR132 [10], shown in Table 3. In the kidney, GPR4 is required for proper acid secretion [17]. GPR4−/− knockout mice develop metabolic acidosis due to reduced acid secretion, and also exhibit abnormal angiogenesis and increased insulin sensitivity [17]. GPR4 signals through a cAMP/PKA-mediated signaling mechanism and is required for H+,K+-ATPase activation in the CD [8]. Acid-sensing GPCRs have also been shown to be important in cancer cells, which often thrive in an acidic micro-environment [10].
Table 3. Putative Intracellular pH Sensors.
The intracellular pH-sensitive proteins that have been identified are listed along with the particular cell types they are found, the downstream signaling proteins, and downstream targets.
| Gene symbol | Protein Name | pHo or pHi | Localization | Signaling Mechanism | Target | Ref |
|---|---|---|---|---|---|---|
| Pyk2 (FAK) | Proline-rich tyrosine kinase-2 (Focal adhesion kinase) | pHi | Proximal tubule Collecting duct Osteoclast |
Ca++ → ERK1/2 | NHE3 V-ATPase | [18, 19] [13] |
| sAC | Soluble Adenylyl Cyclase | pHi | Epididymis Collecting duct | cAMP | V-ATPase ENaC | [22, 23] |
| GPR4 | G-protein coupled receptor-4 | pHo | Kidney, vasculature, pancreas | Gs →cAMP →PKA | AE1 | [3, 12, 17] |
| OGR1 (GPR68) | Ovarian Cancer G-protein coupled receptor | pHo | Bone, smooth muscle | Gq → PLC→ Ca++ | V-ATPase | [15] |
| TDAG8 (GPR65) | T-Cell Death-associated GPCR | pHo | Immune and lymphoid cells | Gs →tmAC → cAMP →PKA | Inhibits MAPK | [16] |
tmAC – transmembrane adenylyl cyclase
Intracellular pH sensors are localized inside the cell and activated by changes in intracellular pH (pHi). Two potential intracellular pH sensors are proline-rich tyrosine kinase-2 (Pyk2) and sAC. Pyk2, an acid-activated non-RTK, is a putative intracellular pH sensor in the kidney that controls acid secretion through regulation of NHE3 and vacuolar V-ATPase activity in the proximal tubule and collecting duct, respectively [13, 18, 19]. In the case of Pyk2, mitogen-activated protein kinase (MAPK) signaling cascades involving extracellular signal-related kinase-1/2 (ERK1/2) control acid-activation of H+-secretion (via NHE3 and H+-ATPase) during pH recovery after an acid load [13, 20, 21]. V-type H+-ATPase mobilization is regulated by sAC, an intracellular CO2/ HCO3− sensor directly activated by increased intracellular calcium and/or bicarbonate ions [22]. sAC stimulates cAMP/cGMP release via phospholipase C (PLC) and protein kinase C (PKC)-mediated signaling pathways to regulate V-ATPase recycling in the epididymis and Na+ reabsorption in the collecting duct [23]. Mineralocorticoids stimulate H+-ATPase activity in the medullary collecting duct. Mutations in genes that encode the α4 and β1 isoforms of the V-ATPase lead to dRTA [6]. The importance of V-ATPase activity in acid-base balance, especially its regulation in the kidney, makes it a possible therapeutic target for controlling systemic pH. Pharmacologically, the transporters of the distal nephron and the pH sensors that regulate their activity would be good therapeutic targets for the treatment of acidosis.
Physiology of acid-base studies
Renal micropuncture, nephron microperfusion and various molecular and biochemical techniques have greatly contributed to our current understanding on the acid-base regulation. The use of transgenic mice has also allowed us to further comprehend the physiological roles of acid-base transports. A mutation in acid-base transport can result in neonatal death [24, 25,26,27]. However, it is important to note that these mice also have other severe phenotypes in addition to acid-base imbalance. Other phenotypes include growth retardation, pulmonary edema, electrolyte imbalance, anemia, intestinal obstruction, and other organ abnormalities, making it hard to discern the cause of death in these mice. Interestingly, administration of sodium bicarbonate in one of the mouse models prolonged survival rate, indicating that acid-base balance could dampen the progression of organ damage [26].
Physiologically, the acid-base homeostasis is also known to be a redundant process. To better understand this, we will use NHE3 as an example. In NHE3 mutant mice, NHE8 plays a compensatory role in renal acidification [28]. Both NHE2 and NHE3 also share a redundant function [29]. Although NHE3 mutant mice may present mild absorptive defects [30,31], the resulting hypovolemia does not help in understanding the specific effects of NHE3 deficiency on kidney function [32]. Furthermore, there is co-regulation between NHE3 with different NHE isoforms [33, 34], denoting a highly complex acid-base regulation. Given the significance of acid-base regulation in all tissues, it is not surprising that we have a redundant physiological system to compensate any acid-base imbalance in our body. Nonetheless, transgenic mouse models have provided a plethora of knowledge on acid-base transport in vivo. This includes a better understanding that NHE3 plays an important role in fluid and bicarbonate reabsorption in the proximal convoluted tubule but does not play an important role in NH4 excretion [31]. Despite the significant physiological complexity, much has been learned about acid-base regulation in vivo through animal models, including the molecular mechanism of different transporters (Table 1). For more information about animal models used to study acid-base regulation, please refer to a recent comprehensive review [35].
Summary
In summary, the regulation of systemic (or arterial) pH is highly complex and involves different mechanisms in multiple cell types, various transporters, and pH sensors. Recent advances using transgenic animal models suggest no single genetic knockout mouse has caused a major disruption in acid-base homeostasis, indicating it is a highly complex and redundant process. Pharmacological control of pH may be accomplished by targeting ion transporters, pH sensors, or signaling molecules that regulate acid-base homeostasis. We have discussed the putative pH sensors and ion transporters that need to be examined as therapeutic targets, including but not limited to Pyk2, sAC, and GPR4.
Acknowledgments
We would like to thank Rinzhin T. Sherpa for his technical support and contribution. We also thank Dr. Thomas D. DuBose, M.D, M.A.C.P at Wake Forest School of Medicine for his proofreading and advice. This work was supported in part by Congressionally Directed Medical Research Program PR130153.
References
- 1.Abdelhamid RE, Sluka KA. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology. 30(6):449–459. doi: 10.1152/physiol.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey JR. Why bicarbonate?This paper is one of a selection of papers published in this Special Issue, entitled CSBMCB Membrane Proteins in Health and Disease. Biochemistry and Cell Biology. 2006;84(6):930–939. doi: 10.1139/o06-184. [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Stephens L, DuBose TD, Petrovic S. Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. American Journal of Physiology - Renal Physiology. 309(2):F120–F136. doi: 10.1152/ajprenal.00507.2014. [DOI] [PubMed] [Google Scholar]
- 4.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clinical Journal of the American Society of Nephrology : CJASN. 10(12):2232–2242. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy J, Andrew M. Intermediate and long-term regulation of acid-base homeostasis. British Journal of Nursing. 2007;16(17):1076–1079. doi: 10.12968/bjon.2007.16.17.27253. [DOI] [PubMed] [Google Scholar]
- 6.Ayers P, Dixon C, Mays A. Acid-Base Disorders: Learning the Basics. Nutrition in Clinical Practice. 30(1):14–20. doi: 10.1177/0884533614562842. [DOI] [PubMed] [Google Scholar]
- 7.Berend K, de Vries APJ, Gans ROB. Physiological Approach to Assessment of Acid-Base Disturbances. New England Journal of Medicine. 371(15):1434–1445. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 8.Codina J, Opyd TS, Powell ZB, Furdui CM, Petrovic S, Penn RB, DuBose TD. pH-dependent regulation of the α-subunit of H+-K+-ATPase (HKα2) American Journal of Physiology - Renal Physiology. 301(3):F536–F543. doi: 10.1152/ajprenal.00220.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scialla JJ. The balance of the evidence on acid-base homeostasis and progression of chronic kidney disease. Kidney International. 88(1):9–11. doi: 10.1038/ki.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Justus CR, Dong L, Yang LV. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Frontiers in Physiology. 4:354. doi: 10.3389/fphys.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Cheng X-Y, Wang F, Liu C-F. Acid-sensing ion channels: potential therapeutic targets for neurologic diseases. Translational Neurodegeneration. 4:10. doi: 10.1186/s40035-015-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown D, Wagner CA. Molecular Mechanisms of Acid-Base Sensing by the Kidney. Journal of the American Society of Nephrology. 23(5):774–780. doi: 10.1681/ASN.2012010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher KD, Codina J, Petrovic S, DuBose TD. Pyk2 regulates H+-ATPase-mediated proton secretion in the outer medullary collecting duct via an ERK1/2 signaling pathway. American Journal of Physiology - Renal Physiology. 303(9):F1353–F1362. doi: 10.1152/ajprenal.00008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FoM. The Ion Channel ASIC2 is Required for Baroreceptor and Autonomic Control of the Circulation. Neuron. 2009;64(6):885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig M-G, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425(6953):93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 16.Ishii S, Kihara Y, Shimizu T. Identification of T Cell Death-associated Gene 8 (TDAG8) as a Novel Acid Sensing G-protein-coupled Receptor. Journal of Biological Chemistry. 2005;280(10):9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S. Deletion of the pH Sensor GPR4 Decreases Renal Acid Excretion. Journal of the American Society of Nephrology : JASN. 2009;21(10):1745–1755. doi: 10.1681/ASN.2009050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Sato S, Yang X, Preisig PA, Alpern RJ. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. Journal of Clinical Investigation. 2004;114(12):1782–1789. doi: 10.1172/JCI18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preisig PA. The acid-activated signaling pathway: Starting with Pyk2 and ending with increased NHE3 activity. Kidney Int. 2007;72(11):1324–1329. doi: 10.1038/sj.ki.5002543. [DOI] [PubMed] [Google Scholar]
- 20.Odunewu A, Fliegel L. Acidosis-mediated regulation of the NHE1 isoform of the Na+/H+ exchanger in renal cells. American Journal of Physiology - Renal Physiology. 305(3):F370–F381. doi: 10.1152/ajprenal.00598.2012. [DOI] [PubMed] [Google Scholar]
- 21.Guan J, Wu X, Arons E, Christou H. The p38 Mitogen-Activated Protein kinase pathway is involved in the regulation of Heme Oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. Journal of cellular biochemistry. 2008;105(5):1298–1306. doi: 10.1002/jcb.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastor-Soler N, Beaulieu Vr, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated Adenylyl Cyclase (sAC) Is a Sensor That Regulates pH-dependent V-ATPase Recycling. Journal of Biological Chemistry. 2003;278(49):49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, Buck J, Levin LR, Johnson JP, Pastor-Soler NrM. Regulation of Epithelial Na(+) Transport by Soluble Adenylyl Cyclase in Kidney Collecting Duct Cells. The Journal of Biological Chemistry. 2009;284(9):5774–5783. doi: 10.1074/jbc.M805501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hummler E, Barker P, Talbot C, Wang Q, Verdumo C, Grubb B, Gatzy J, Burnier M, Horisberger J-D, Beermann F, Boucher R, Rossier BC. A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(21):11710–11715. doi: 10.1073/pnas.94.21.11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic Anion Secretory Defects and Metabolic Acidosis in Mice Lacking the NBC1 Cotransporter. Journal of Biological Chemistry. 2007;282(12):9042–9052. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- 26.Lo Y-F, Yang S-S, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai J-D, Yu IS, Lin S-W, Lin S-H. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney International. 79(7):730–741. doi: 10.1038/ki.2010.523. [DOI] [PubMed] [Google Scholar]
- 27.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal Renal Tubular Acidosis in Mice Lacking the AE1 (Band3) Cl−/HCO3− Exchanger (slc4a1) Journal of the American Society of Nephrology. 2007;18(5):1408–1418. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- 28.Baum M, Twombley K, Gattineni J, Joseph C, Wang L, Zhang Q, Dwarakanath V, Moe OW. Proximal tubule Na+/H+ exchanger activity in adult NHE8−/−, NHE3−/−, and NHE3−/−/NHE8−/− mice. American Journal of Physiology - Renal Physiology. 303(11):F1495–F1502. doi: 10.1152/ajprenal.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey MA, Giebisch G, Abbiati T, Aronson PS, Gawenis LR, Shull GE, Wang T. NHE2-mediated bicarbonate reabsorption in the distal tubule of NHE3 null mice. The Journal of Physiology. 2004;561(Pt 3):765–775. doi: 10.1113/jphysiol.2004.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na +/H+ exchanger. Nat Genet. 1998;19(3):282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 31.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na(+)/H(+) exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. Journal of Molecular Medicine (Berlin, Germany) 91(8):951–963. doi: 10.1007/s00109-013-1015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2005;288(3):R685–R691. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 33.Good DW, Watts BA, George T, Meyer JW, Shull GE. Transepithelial HCO3− absorption is defective in renal thick ascending limbs from Na+/H+ exchanger NHE1 null mutant mice. American Journal of Physiology - Renal Physiology. 2004;287(6):F1244–F1249. doi: 10.1152/ajprenal.00176.2004. [DOI] [PubMed] [Google Scholar]
- 34.Watts BA, George T, Good DW. The Basolateral NHE1 Na+/H+ Exchanger Regulates Transepithelial Absorption through Actin Cytoskeleton Remodeling in Renal Thick Ascending Limb. Journal of Biological Chemistry. 2005;280(12):11439–11447. doi: 10.1074/jbc.M410719200. [DOI] [PubMed] [Google Scholar]
- 35.Eladari D, Kumai Y. Renal acid-base regulation: new insights from animal models. Pflügers Archiv - European Journal of Physiology. 467(8):1623–1641. doi: 10.1007/s00424-014-1669-x. [DOI] [PubMed] [Google Scholar]
- 36.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a Targeted Disruption of the AE2 Exchanger Are Achlorhydric. Journal of Biological Chemistry. 2004;279(29):30531–30539. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 37.Bobulescu IA, Moe OW. Na(+)/H(+) Exchangers in Renal Regulation of Acid-Base Balance. Seminars in nephrology. 2006;26(5):334–344. doi: 10.1016/j.semnephrol.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Carney KE, Falgoust L, Pan JW, Sun D, Zhang Z. Emerging roles of Na+/H+ exchangers in epilepsy and developmental brain disorders. Progress in Neurobiology. 138–140:19–35. doi: 10.1016/j.pneurobio.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic S, Barone S, Xu J, Conforti L, Ma L, Kujala M, Kere J, Soleimani M. SLC26A7: a basolateral Cl−/HCO3− exchanger specific to intercalated cells of the outer medullary collecting duct. American Journal of Physiology - Renal Physiology. 2004;286(1):F161–F169. doi: 10.1152/ajprenal.00219.2003. [DOI] [PubMed] [Google Scholar]