Abstract
The levels of interleukin (IL)-7 and its receptor are elevated in the salivary glands of patients with Sjögren’s syndrome (SS). Our previous study indicates that IL-7 plays a critical pathogenic role in the development and onset of SS in a mouse model of this disease. The present study aims at determining whether IL-7 also plays a role in sustaining SS pathologies after the disease onset, by using the non-obese diabetic (NOD) model. Intraperitoneal administration of a blocking antibody against the IL-7 receptor α chain (IL-7Rα) to female NOD mice aged 10 weeks, which exhibited newly onset clinical SS, for the duration of 3 weeks significantly ameliorated characteristic SS pathologies including hyposalivation and leukocyte infiltration of the submandibular glands (SMGs). These changes were accompanied by a decrease in IFN-γ-producing CD4 T- and CD8 T cells, B cells, and lymphocyte chemoattractants CXCL9, -10, -11 and -13 in the SMGs. Anti-IL-7Rα treatment markedly diminished the amount of TNF-α in the SMGs and increased the level of claudin-1 and aquaporin 5, two molecules critical for normal salivary secretion. Furthermore, neutralization of IFN-γ and TNF-α, individually or in combination, considerably improved salivary secretion, reduced leukocyte infiltration and down-regulated CXCL9 and -13 expression in the SMGs. Collectively, the results indicate that endogenous IL-7R signals promote Th1 and Tc1 responses and IFN-γ- and TNF-α production to sustain the persistence of SS-like sialadenitis in NOD mice. These findings suggest that IL-7 and Th1 cytokines could serve as promising therapeutic targets for this prevalent autoimmune disease.
Keywords: Sjögren’s syndrome, salivary gland, autoimmune exocrinopathy, T helper 1 cells, interferon-γ, hyposalivation, autoimmunity
1. Introduction
Sjögren’s syndrome (SS) is a prevalent systemic autoimmune disorder that affects millions of Americans, with a female to male ratio of 9:1 [1–4]. It is characterized by lymphocyte infiltration of exocrine salivary and lacrimal glands, impairment in the salivary and tear secretion and elevation of an array of autoantibodies, with the clinical manifestation as xerostomia (dry mouth) and keratoconjunctivitis sicca (dry eyes)[1, 2, 5, 6]. Accumulating evidence has indicated that autoreactive effector T cells play a vital pathogenic role in SS [7–9]. Multiple cytokines that can affect or mediate effector T cell functions, including IFN-γ, TNF-α, IL-4, IL-17 and type-1 IFNs, critically contribute to the induction and development of this autoimmune inflammatory disease as demonstrated by in vivo studies using mouse models of this disease [1, 10–14].
IL-7 is a pleiotropic cytokine produced by non-hematopoietic cells, such as stromal and epithelial cells, and provides essential signals for the development and homeostasis of T cells under physiological conditions [15–17]. IL-7 signals through IL-7 receptor (IL-7R), which is composed of the IL-7Rα chain and the common γ chain. Elevated levels of both IL-7 and IL-7R are detected in the inflamed tissues of an array of autoimmune and inflammatory disease conditions, including experimental autoimmune encephalomyelitis, systemic erythematosus lupus, rheumatoid arthritis, inflammatory bowel’s disease and type-1 diabetes [18–23]. Moreover, blocking the IL-7-IL-7R pathway with anti-IL-7Rα antibody can prevent, reverse or attenuate the pathologies and clinical manifestations of these disorders in mouse disease models [19, 20, 23–25] and such effect is associated with a preferential reduction in T helper 1 (Th1) and T cytotoxic 1 (Tc1) immune responses characterized by the production of IFN-γ [18, 23, 24].
In SS patients, IL-7 production and IL-7Rα-expressing T cells are markedly increased in salivary gland tissues and the degree of such increase is associated with the severity of tissue immunopathology and the dry mouth symptoms [26, 27]. Our previous study, using the C57BL/6.NOD-Aec1.Aec2 (B6.NOD-Aec) model of SS, has demonstrated that exogenous IL-7 administration accelerates, whereas blockade of endogenous IL-7Rα signals prevents the development and onset of SS [28]. The pathogenic effect of IL-7 at the pre-disease stage is chiefly underlain by enhanced Th1 and Tc1 responses in the target submandibular glands (SMGs) and the augmented production of IFN-γ and TNF-α, two cytokines that are essentially required for the induction and development of SS pathologies [28, 29]. Hence, IL-7 and its target Th1 cytokines play a critical pathogenic role in the developmental phase of SS disease.
In the present study, we sought to determine whether the endogenous IL-7R-mediated signaling plays a role in sustaining the persistence of SS pathologies after the disease onset and whether blockade of this pathway can reverse or attenuates the newly established SS disease, by using the non-obese diabetic (NOD) mouse strain, a spontaneous model of human SS [30–32]. By using anti-IL-7Rα antibody-mediated blockade of endogenous IL-7R signaling in these mice, our study demonstrated an indispensable role of this pathway in sustaining the persistence of SS.
Materials and methods
2.1 Mice
Female non-obese diabetic (NOD) mice were purchased from the Jackson Laboratory and were housed in the specific pathogen-free animal facility at the Forsyth Institution. All the experimental protocols were approved by the Institutional Animal Care and Use Committee of the Forsyth Institute and all the procedures were implemented in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.2 Antibodies
Purified monoclonal rat-anti-mouse IL-7Rα (A7R34) and its isotype control rat-IgG2a (2A3) used for injection were obtained from BioXCell. For flow cytometry, fluorescence conjugated anti-CD4, anti-CD8, anti-CD19, anti-IFN-γ, anti-PD-1, anti-IL-17 and anti-CD16/32 antibodies were purchased from BioLegend, and fluorescence conjugated anti-Foxp3 antibody was obtained from eBioscience. For Immunohistochemical staining, biotin-conjugated anti-CD4 and anti-CD8 antibodies were obtained from eBiosience and biotin-conjugated anti-B220 antibody was obtained from BioLegend; anti-TNF-α, anti-claudin-1 and anti-claudin-2 antibodies were purchased from Abcam. For immunofluorescence staining, anti-aquaporin-5 (AQP5) antibody and Alexa Fluor647-conjugated anti-rabbit IgG were purchased from Abcam.
2.3 In vivo administration of anti-IL-7Rα, anti-TNF-α and anti-IFN-γ antibodies
10 week-old female NOD mice received intraperitoneal (i.p.) administration of 200 μg anti-mouse IL-7Rα, or anti-mouse TNF-α or anti-mouse IFN-γ, or anti-TNF-α plus anti-IFN-γ antibodies or the corresponding isotype control IgG 3 times weekly for 3 weeks. All the analyses were performed 2 days after the last injection.
2.4 Histological analysis
SMG tissues were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned to 5 μm thickness. Routine histology was carried out on these sections with hematoxylin and eosin (H&E) to determine the degree of inflammation. The numbers of leukocytic foci (comprising more than 50 mononuclear cells per 4 mm2) in each of the three non-consecutive sections of SMG samples were counted, and the average number of the three was used for further statistical analysis.
2.5 Immunohistochemical staining
The de-paraffinized sections were subjected to antigen retrieval process and dehydration procedure. Then, they were stained with anti-CD4, anti-CD8, anti-B220, anti-TNF-α, anti-claudin-1 or anti-claudin-2 antibody, at 4°C overnight using VECTASTAIN Elite ABC Kit (Vector Laboratories) according to the manufacturer’s instructions. Images were recorded at 400× magnification under a light microscope. Quantification of positively-stained cells or areas in the samples was performed using Image J 1.50i software. Briefly, images were saved as RGB Tiff files and appropriate segmentation of stained area was performed using color thresholding. The percentage of thresholded area in each image was measured and calculated.
2.6 Immunofluorescence staining
The de-paraffinized sections were subjected to antigen retrieval process and dehydration procedure. After that, they were incubated with anti-AQP5 antibody at 4°C overnight followed by Alexa Fluor 647 fluorescent dye conjugated anti-rabbit IgG. Images were captured using a Zeiss LSM 780 laser scanning confocal microscope at 400× magnification and processed with Zeiss software (ZEN black edition). Quantification of the fluorescence intensity was carried out by ImageJ 1.50i software. Briefly, Images with single stained color were converted into grayscale (8-bit) and the grey color was segmented properly using thresholding. Integrated density was analyzed to determine the fluorescence intensity of the staining.
2.7 Flow cytometry
Freshly isolated single cells from SMGs or submandibular lymph nodes (smLNs) were incubated with anti-CD16/32 antibody to prevent unspecific binding. The cells were then stained with a combination of fluorescence-conjugated antibodies to surface markers CD4, CD8, CD19 and PD-1 at 4°C for 30 min. Intracellular and nuclear staining with anti-IFN-γ, anti-IL-17A and anti-Foxp3 antibodies was subsequently performed following fixation and permeabilization. The stained samples were then washed, processed and analyzed with a FACS Arial II flow cytometer (BD) and the FlowJo V10 software.
2.8 Detection of serum antinuclear antibodies (ANA)
Sera collected from the mice were diluted 1:40 and subjected to ANA measurements using HEp-2 human epithelial cell substrate slides (INOVA Diagnostics) following the manufacturer’s instructions. The slides were observed and imaged under an inverted wide-field fluorescence microscope (Zeiss) at 400× magnification. The images were further processed with the Zeiss software (ZEN blue edition), and the fluorescence intensity of the staining was quantified with the ImageJ 1.50i software as described in 2.6.
2.9 Enzyme-linked immunosorbent assay (ELISA)
The peptide that contains 15 amino acids (AILFWQYFVGKRTVP) of the second extracellular loop of the M3 muscarinic acetylcholine receptor (M3R) protein (synthesized by Biomatic Corporation) was dissolved in PBS. After further diluted in 1×BioLegend ELISA coating buffer, this M3R peptide solution (2μg/ml) was used to coat Nunc™ MaxiSorp™ flat-bottom 96 well plates (BioLegend). Non-specific binding sites on the plates were blocked with ELISA Assay Diluent Buffer (BioLegend), and 1:6-diluted serum samples were added to the plates and incubated at 4°C overnight. The serum samples bound to the plates were then incubated with 1:300 diluted biotinylated goat anti-mouse IgG antibody (Vector Laboratories) for 1 h, followed by avidin-HRP solution for 30 min, and finally with the TMB substrate. The absorbance was measured at 450 nm using a BioTek microplate reader.
2.10 Measurement of stimulated salivary flow rate
Female NOD mice received an i.p.-injection of 100 μl PBS-based secretagogue solution containing pilocarpine (2 mg/ml) and isoproterenol (1 mg/ml). One min after the injection, saliva was collected continuously for 5 min and the volume of the saliva from each mouse was measured and normalized to the body weight.
2.11 Real-time PCR
Total RNA was isolated using RNeasy Micro kit (Qiagen) and transcribed into cDNA with MLV reverse transcriptase (Promega). SYBR Green-based real-time PCR amplification (Qiagen) was performed for 40 cycles with annealing and extension temperature at 60°C on a LightCycler 480 Real-Time PCR System (Roche). Primer sequences are as follows: IFN-γ forward, 5′-GGATGCATTCATGAGTATTGC-3′, reverse, 5′-CTTTTCCGCTTCCTGAGG-3′; T-bet forward, 5′-CCAACAACCCCTTTGCCAAAG-3′, reverse, 5′-TCCCCCAAGCAGT TGACAGT-3′; CXCL9, forward, 5′-CCCTCAAAGACCTCAAACAGT-3′; reverse, 5′-AGTCCGGATCTA GGCAGGTT-3′; CXCL10 forward, 5′-CCAGTGAGAATGAGGGCCAT-3′, reverse, 5′-CCGGATTCAGACATCTCTGC-3′; CXCL11 forward, 5′-GCAGAGATCG AGAAAGCTTCT-3′, reverse, 5′-GTCCAGGCACCTTTGTCGTT-3′; CXCL13 forward, 5′-AGATCGGATTCAAGTTACGCC -3′, reverse, 5′-TTTGGCACGAGGATTCACACA-3′. Other sequences will be provided upon request. All transcript levels were normalized to β-actin.
2.12 Urine glucose test
Urine glucose from the mice was measured at the end-point of the experiments using Diastix® Reagent Strips following the manufacturer’s guidelines.
2.13 Statistical analysis
The statistical comparisons were carried out using a two-tailed Student’s t test or ANOVA as appropriate. P values smaller than 0.05 were considered as statistically significant.
3. Results
3.1 Blockade of IL-7Rα improves salivary secretion and reduces leukocyte infiltration of SMGs in NOD mice
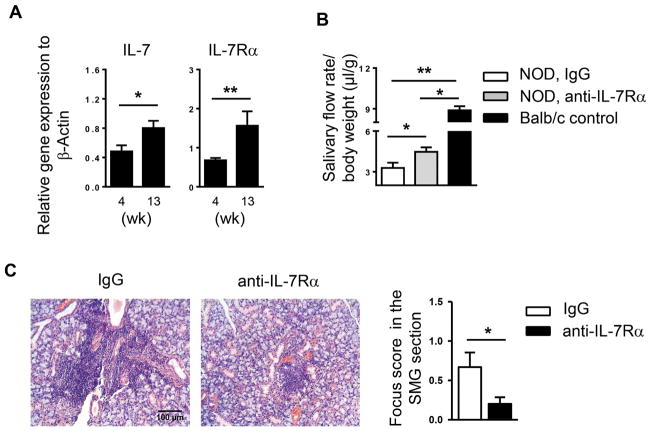
Elevated levels of IL-7 and increased frequency of IL-7Rα-expressing T cells are observed in the salivary glands of SS patients [26, 27]. Our previous study has shown that IL-7 promotes the initial development and onset of SS in B6.NOD-Aec mice [28]. To further determine whether IL-7 is also important in sustaining SS disease after its initial onset, we utilized female NOD mice that spontaneously develop SS-like sialadenitis, with disease onset at around 10 weeks of age based on leukocyte infiltration of SMGs, presence of ANA in the serum, and impaired salivary secretion [29, 33, 34]. Moreover, none of the mice became diabetic at 13 weeks of age, indicated by normal urine glucose levels (data show shown). Real-time PCR analysis showed that the mRNA amount of both IL-7 and IL-7Rα in the SMGs was increased between 4 and 13 weeks of age (Fig. 1A). To determine whether blockade of IL-7Rα signals can reverse newly established SS, we i.p.-administrated 200μg of anti-IL-7Rα antibody or its isotype control, rat IgG2a, to 10-week-old female NOD mice 3 times weekly for a consecutive 3 weeks, and analyzed SS-characteristic pathologies at 13 weeks of age. Anti-IL-7Rα-treated group had markedly higher salivary flow rate compared to the control group, indicating a remission of SS-like exocrine dysfunction (Fig. 1B). Moreover, H&E staining of SMG sections revealed abundant leukocyte infiltrates in IgG-treated control mice, but much fewer in anti-IL-7Rα-treated mice (Fig. 1C). Quantification of leukocyte infiltration showed that the focus score was significantly lower in the anti-IL-7Rα-treated group (Fig. 1C). Hence, endogenous IL-7-IL-7Rα pathway plays a crucial role in the persistence of SS-like hyposalivation and sialadenitis in NOD mice after the disease onset.
Figure 1. Anti-IL-7Rα treatment improves salivary secretion and reduces leukocyte infiltration of submandibular glands (SMGs) in NOD mice.
(A) Real-time PCR analysis of IL-7 and IL-7Rα levels in the SMGs of NOD mice aged 4 and 13 weeks. The results are presented relative to that of β-actin. Data are the average of analyses of 4–7 mice each group. Error bars represent the SEM. (B) Anti-IL-7Rα antibody or control IgG was i.p.-administered to 10-week-old female NOD mice 3 times weekly for 3 weeks. The stimulated salivary flow rate normalized to body weight is shown. (C) H&E staining of SMG sections from mice described in B (scale bar = 100μm). Bar graph shows the mean leukocyte focus score. Data are representative or the average of 7 mice each group.
3.2 Blockade of IL-7Rα reduces T and B cell accumulation in the SMG tissues
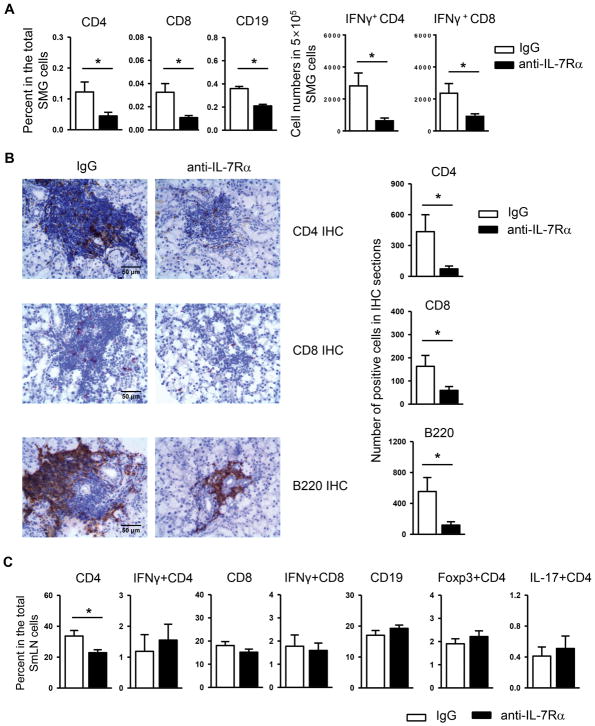
We next assessed the effect of anti-IL-7Rα treatment on lymphocyte populations and subsets in the target tissues. In accordance with the lowered leukocyte focus score, flow cytometric analysis demonstrated that the percentage of CD4+ and CD8+ T cells and that of CD19+ B cells among the total SMG cells were substantially reduced by IL-7Rα blockade (Fig. 2A, left panels). Moreover, anti-IL-7Rα treatment significantly decreased the frequency of IFN-γ producing-CD4+ and CD8+ T cells in the SMGs, as shown by the reduced number of these cells among the 5×105 total SMG cells analyzed (Fig. 2A, right panels), indicating reduced T helper 1 (Th1) and T cytotoxic 1 (Tc1) responses. In comparison, IL-7Rα blockade did not significantly alter the proportion of IL-17- or Foxp3-expressing CD4 T cells (data not shown). The marked decrease in CD4 T, CD8 T and B cells was further confirmed by the immunohistochemical staining of SMG sections (Fig. 2B). In the smLNs, anti-IL-7Rα decreased the percentage of total CD4 T cells but did not affect that of CD8 T cell and B cell populations and that of IFN-γ-producing CD4 and CD8 T cells (Fig. 2C). IL-7Rα blockade also did not alter the proportion of IL-17- and Foxp3-expressing CD4 T cells in the smLNs (Fig. 2C). Hence, anti-IL-7Rα treatment attenuates Th1 and Tc1 cell responses in the SMG tissues in the NOD mice with newly established SS disease. We also investigated whether anti-IL7Rα treatment could affect the expression of coinhibitory receptor PD-1, a negative regulator of Th1 and Tc1 responses. Flow cytometric analysis showed that the proportion of PD-1-expressing CD4 and CD8 T cells in the SMGs were comparable between anti-IL-7Rα-treated and the control mice (data not shown). In accordances, the PD-1 expression level on CD4 and CD8 T cells in the SMGs was not altered by anti-IL-7Rα treatment, as indicated by comparable geometric mean of fluorescence intensity (MFI) values (data not shown). These findings suggest that the inhibitory effect of anti-IL-7Rα on Th1 and Tc1 responses in the SMGs was not a consequence of an increase in PD-1 pathway.
Figure 2. Blockade of IL-7Rα causes a reduce of T and B cell accumulation in the SMGs of NOD mice.
Anti-IL-7Rα antibody or control IgG was i.p.-administered to 10-week-old female NOD mice, 3 times weekly for 3 weeks. (A) Flow cytometric analysis of the percentage of lymphocyte populations among total SMG cells, left panels, and the number of IFN-γ+CD4 and IFN-γ+CD8 T cells among the 5×105 total SMG cells analyzed, right panels. (B) Immunohistochemical staining of SMG sections for CD4, CD8 and B220 (scale bar = 50μm). Bar graph shows the quantification of the number of positively-stained cells. (C) Flow cytometric analysis of the percentage of lymphocyte populations among total submandibular lymph node (smLN) cells. Data are the average of analyses of 7 mice each group.
3.3 Blockade of IL-7Rα decreases expression of Th1 genes and lymphocyte chemoattractants in the SMGs
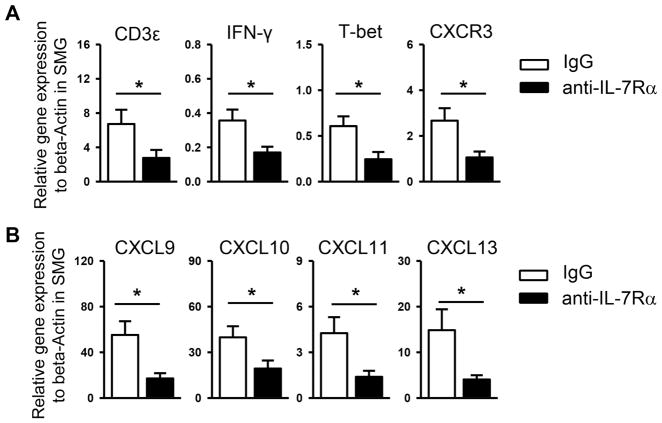
To further determine the effect of IL-7Rα blockade on the autoimmune responses, we analyzed the expression of key factors and cytokines controlling the function of effector T cells. Real time PCR analysis revealed that administration of anti-IL-7Rα significantly decreased the mRNA amount of CD3ε, IFN-γ, and T-bet in the SMGs without significantly altering that of IL-17, foxp3 and IL-4 (Fig. 3A, and data not shown). These results further support that the SS disease-inhibiting effect of anti-IL-7Rα treatment is underlain by a preferential down-modulation of Th1 (and Tc1) immune responses.
Figure 3. Blockade of IL-7Rα reduces expression of Th1 signature molecules and lymphocyte chemoattractants in the SMGs.
Anti-IL-7Rα antibody or control IgG was i.p.-administered to 10-week-old female NOD mice, 3 times weekly for 3 weeks. (A) Real-time PCR analysis of the gene expression of (A) T cell-associated genes and (B) lymphocyte chemoattractant genes, presented relative to that of β-actin. Data are the average of analyses of 7 mice each group.
We next assessed whether anti-IL-7Rα can affect SMG expression of T cell chemoattractants CXCL9, -10 and -11, which can promote the recruitment of Th1/Tc1 cells to inflamed tissues in autoimmune and inflammatory conditions [35, 36]. In accordance with the reduction of IFN-γ producing-CD4 and CD8 T cells, the amount of transcripts of CXCL9, -10 and -11 and their corresponding receptor CXCR3 in the SMGs was substantially lowered by anti-IL-7Rα treatment (Fig. 3B). In addition, blockade of IL-7Rα significantly decreased the mRNA amount of B cell chemoattractant CXCL13 (Fig. 3B), consistent with reduced B cell numbers in the SMGs. Collectively, these results indicate that the Th1-enhancing and sialadenitis-promoting effect of endogenous IL-7-IL-7Rα signaling is associated with an upregulated expression of T- and B cell chemoattractants in the SMGs.
3.4 Blockade of IL-7Rα leads to diminished TNF-α production and enhanced claudin-1 and AQP5 expression in the SMGs
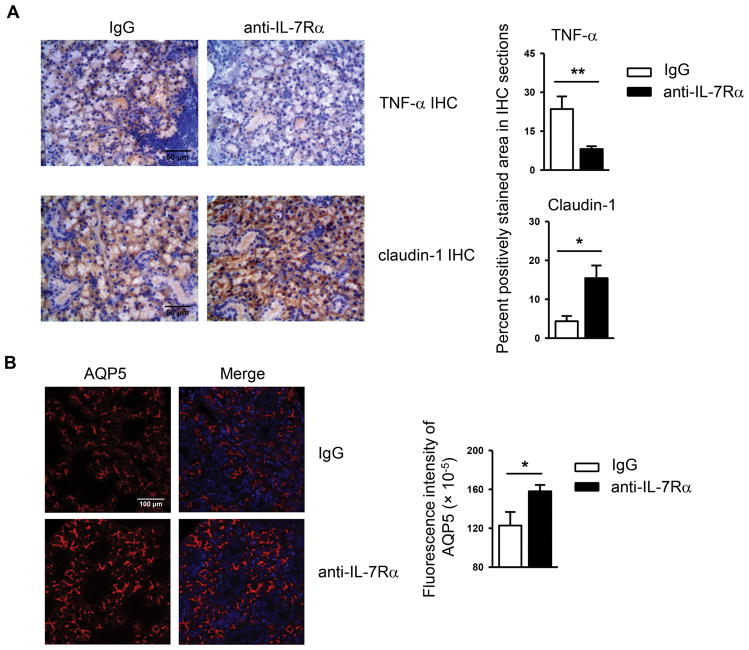
TNF-α expression is elevated in the salivary glands of SS patients and plays a critical pathogenic role in this disease [29, 37, 38]. We thus examined the impact of IL-7Rα blockade on TNF-α production by immunohistochemical staining of SMG sections, which showed that TNF-α levels were reduced by anti-IL-7Rα treatment (Fig. 4A). One of our recent studies has shown that TNF-α downregulates the expression levels of tight junction component claudin-1 and water channel protein aquaporin 5 (AQP5) in the SMGs of NOD mice [29], both of which play vital roles in maintaining normal salivary secretion [39–43]. We hence measured the expression levels of these molecules in the SMG tissues, which demonstrated that blockade of IL-7Rα markedly increased claudin-1 expression levels in the SMGs as determined by immunohistochemical staining, without affecting that of claudin-2 (Fig. 4A and data not shown). In addition, anti-IL-7Rα treatment significantly upregulated AQP5 protein expression as assessed by immunofluorescence staining (Fig. 4B). Hence, IL-7Rα blockade reduces SMG expression of proinflammatory mediator TNF-α and upregulates that of claudin-1 and AQP5, which are critical components of normal salivary gland secretory machinery.
Figure 4. Blockade of IL-7Rα reduces TNF-α levels and increases claudin-1 and AQP5 amounts in the SMGs.
Anti-IL-7Rα antibody or control IgG was i.p.-administered to 10-week-old female NOD mice 3 times weekly for 3 weeks. (A) Immunohistochemical staining of TNF-α and claudin-1 protein in SMG sections (scale bar = 50μm). Bar graph shows the percentage of positively stained areas in the sections. (B) Immunofluorescence staining of AQP5 protein in SMG sections (scale bar = 100μm). Bar graph shows the fluorescence intensity of AQP5 staining. Data are representative or the average of analyses of 7 mice each group.
3.5 Blockade of IL-7Rα does not affect autoantibody production
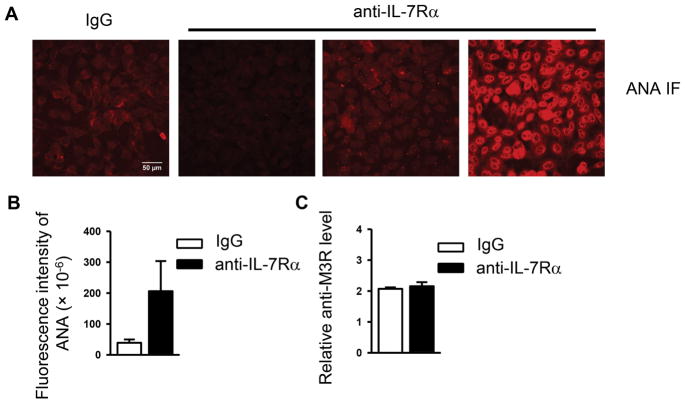
Elevation in serum autoantibody levels is another characteristic manifestation of SS disease. Indirect immunofluorescence staining with human HEp-2 epithelial cells as substrates showed that sera from anti-IL-7Rα treated mice displayed varying levels of ANA, which on average were not significantly different from those in the IgG-treated control group (Fig. 5A and 5B). Accordingly, ELISA assay demonstrated that the level of autoantibodies against muscarinic acetylcholine receptor M3 (M3R) in the sera of anti-IL-7Rα treated mice was comparable to that of the control group (Fig. 5C). Therefore, blockade of IL-7Rα in newly established SS does not affect autoantibody production levels.
Figure 5. Anti-IL-7Rα treatment does not significantly affect autoantibody production.
Anti-IL-7Rα antibody or control IgG was i.p.-administered to 10-week-old female NOD mice 3 times weekly for 3 weeks. (A) Detection of serum ANA (scale bar = 50μm). (B) Bar graph shows the fluorescence intensity of ANA staining. (C) ELISA assay for serum anti-M3R autoantibody levels. Data are representative or the average of analyses of 7 mice each group.
3.6 Neutralization of either IFN-γ or TNF-α attenuates newly established SS-like sialadenitis in NOD mice
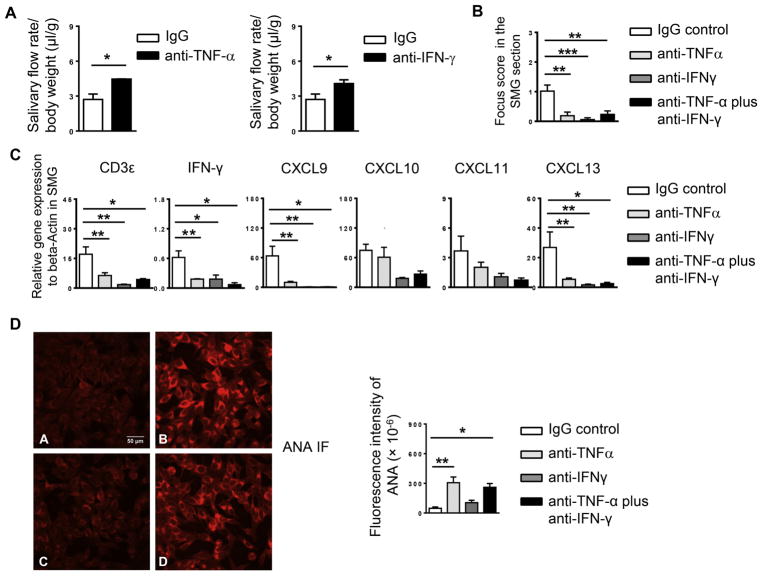
We and other groups have shown that both IFN-γ and TNF-α are required for the development and onset of SS disease [11, 29]. Since the disease-attenuating effect of IL-7Rα blockade in newly established SS was accompanied by a reduction in IFN-γ-producing CD4 and CD8 T cells and TNF-α levels in the SMGs, we postulated that such downregulation may account for, at least partially, the disease-attenuating effect of anti-IL-7Rα, and that neutralization of TNF-α and IFN-γ will achieve some of the same outcomes as anti-IL-7Rα treatment. We i.p.-administration of neutralizing anti-IFN-γ, anti-TNFα, or the combination of the two antibodies to NOD mice aged 10 weeks, 3 times weekly for a consecutive 3 weeks. Analyses of the disease profiles showed that neutralization of either IFN-γor TNF-α considerably increased the salivary flow rate (Fig. 6A) and decreased the amount of SMG-infiltrating leukocytes (Fig. 6B), and significantly reduced the gene expression levels of CD3ε, IFN-γ, CXCL9 and -13 in the SMGs without affecting those of CXCL10 or CXCL11 (Fig. 6C). The combination of anti-IFN-γ and anti-TNF-α exhibited overall similar degree of effects on salivary secretion and tissue inflammation as the individual antibody (Fig. 6B–C, and data not shown). Therefore, neutralization of either IFN-γ or TNF-α exerts similar attenuating effect as IL-7Rα blockade on salivary gland inflammation and dysfunction, indicating their contributions to the persistence of these SS pathologies as downstream effectors of IL-7R signals. However, unlike anti-IL-7Rα or anti-IFN-γ treatment, which did not significantly alter serum ANA levels, anti-TNFα, alone or in combination with anti-IFN-γ, caused a significant increase in the serum ANA levels (Fig. 6D). The autoantibody enhancing-effect of anti-TNFα treatment has been observed in SS-disease setting at the pre-disease stage as we recently reported and has also been widely reported in patients receiving anti-TNFα therapies for other inflammatory diseases [ 44, 45].
Figure 6. Blockade of IFN-γ or TNF-α attenuates SS-like sialadenitis in NOD mice.
Anti-IL-IFN-γ, anti-TNF-α, or the combination of the two antibodies was i.p.-administered to 10-week-old female NOD mice 3 times weekly for 3 weeks. (A) Stimulated salivary flow rate normalized to body weight. (B) Mean leukocyte focus score as determined based on the H&E staining of SMG tissue sections (C) Real-time PCR analysis of the gene expression of T cell-associated genes and lymphocyte chemoattractant genes, presented relative to that of β-actin. (D) Detection of serum ANA (scale bar = 50μm). Bar graph shows the fluorescence intensity of ANA staining. All data are the average of analyses of 6 mice each group.
4. Discussion
The study was untaken to examine the effects of anti-IL-7Rα antibody on newly established SS-like sialadenitis using NOD mice and the results demonstrate the importance of IL-7R signaling and its targeted Th1 cytokines in the persistence of this disease.
We have previously reported that IL-7 is essential for the development and onset of SS disease in a mouse model [28]. Here we showed that IL-7Rα also plays an essential role in sustaining the disease after its onset in the NOD mice and its effect is accompanied by reduced Th1 and Tc1 responses at the inflamed sites, consistent with the reported effects of IL-7/IL-7R blockade in a number of autoimmune and inflammatory diseases [18, 19, 23, 24]. Furthermore, neutralization of either IFN-γ or TNF-α indeed can achieve several major effects of IL-7R blockade, providing supporting evidence that downregulation of these cytokines is one of the mechanisms by which IL-7R blockade ameliorate SS pathologies, albeit not the direct proof. Moreover, anti-IFN-γ and anti-TNF-α treatments lead to very similar changes without exhibiting synergistic or additive effects, suggesting that both IFN-γ and TNF-α are critically and non-redundantly required for the same pathological events.
Both IL-7 and IL-7Rα in the SMGs of the NOD mice increase over time as we have shown in this study. The main sources of IL-7 are non-immune tissue cell types, including epithelial and stromal cells. We have previously shown that proinflammatory cytokines IFN-γ and type 1 IFNs can enhance IL-7 production from salivary gland epithelial cells in vitro and in vivo [28, 46]. We therefore postulate that the increase in SMG IL-7 levels in NOD mice over time likely results from the progressing SMG inflammation in these mice. IL-7Rα is mainly expressed on immune cells, including T cells, which are the major targets of IL-7. It is conceivable that the increase in IL-7Rα levels in the SMGs of NOD mice over time is a consequence of increased T cell infiltration of these glands as the autoimmune inflammation progresses.
The Th1- and Tc1-enhancing effect of IL-7 in type-1 diabetes and chronic viral infections has been shown to be closely linked to the downregulation of T cell expression of PD-1 molecule, an immune-suppressive coinhibitory receptor [47, 48]. In this study however, IL-7Rα blockade did not decrease the level of PD-1 expression on T cells or the percentage of PD-1-expressing T cells in NOD mice with newly established SS. Hence, the disease-ameliorating effect of anti-IL-7Rα is not mediated through downregulation of T cell PD-1 expression, which also further supports the notion that the functional mechanisms employed by IL-7R signaling to influence T cell responses are disease context- and disease stage-dependent.
B cell autoantibody production is a characteristic pathological change in SS. We previous reported that IL-7 administration exacerbates, whereas IL-7Rα blockade suppresses ANA production during the development of SS [28]. In this study, we did not observe a significant effect of anti-IL-7Rα treatment on the production of ANA or anti-M3R antibodies after the disease onset, in newly established disease, indicating that IL-7 promotes sustained secretory dysfunction without enhancing the production of these autoantibodies. Our future studies will more comprehensively examine the effect of anti-IL-7Rα treatment on SS-associated serological changes and systemic pathologies. It is well documented that anti-TNF-α therapies, employed to treat inflammatory diseases such as rheumatic arthritis and inflammatory bowel disease, cause a lupus-like condition termed as anti-TNF-α-induced lupus erythematosus in some of the patients, which is characterized by elevated serum ANA and anti-double-stranded DNA antibody levels [44, 45]. Consistent with these reports, our previous study has shown that TNF-α blockade in NOD mice prior to SS onset impedes the development of salivary gland hypofunction and inflammation but induces production of ANA and anti-M3R autoantibodies [29]. Here we showed that neutralization of TNF-α in newly established SS disease also induced similar elevation in serum ANA. By contrast, anti-IFN-γ treatment did not cause such increase. The mechanisms underlying anti-TNF-α-induced enhancement of autoantibody production, in both in the developing SS and in established SS disease, require further investigation and delineation, and our recent finding that blockade of CXCR3 in NOD mice achieved similar enhancement of ANA production will facilitate such effort [49].
Our study shows that IL-7Rα blockade increased the level of tight junction protein claudin-1 and water channel protein aquaporin 5 (AQP5), which are crucial for normal salivary secretion [39–43], suggesting two potential molecular mechanisms by which IL-7Rα blockade ameliorates hyposalivation. We did not detect a change in claudin-2 and occludin, another two key components of tight junction. Apart from claudin-1, claudin-2 and occludin, multiple other tight junction components, such as ZO-1 and other claudins also play vital roles in the tight junction integrity and salivary secretion [50, 51]. Whether the expression and cellular distribution of these tight junction proteins are also affected by IL-7Rα blockade will be addressed in more details in our future studies.
Some female NOD mice start to develop clinical SS become diabetic around 12–16 weeks of age [52]. We therefore monitored the urine glucose levels in the NOD mice used in this study at 13 weeks of age, the end-point of the study, and found that none of the mice exhibited abnormal urine glucose levels. Hence, the SS disease-attenuating effect of anti-IL-7Rα treatment in the NOD mice is not likely an indirect result of its effect on diabetes.
Apart from IL-7 and Th1 cytokines, a number of other cytokines that are elevated in SS patients, including IL-17, IL-4, IL-13 and type-1 IFNs, play critical pathogenic roles in the induction and development of SS disease as demonstrated by functional studies using mouse models [1, 8, 12, 13, 53–56]. Whereas recent evidence has shown that IL-17 and IL-21 have critical contributions to the late stage SS disease pathologies [13, 57], the functional importance of the other cytokines in the chronic persistence of this disease awaits further delineation. In addition, while previous studies have indicated a role of IL-7, Th1 cytokines and several other inflammatory cytokines such as IL-1 in the induction of lacrimal gland inflammation and dysfunction, the significance of these cytokines in the persistence of SS-like lacrimal gland disorder requires further investigations [14, 58]. These studies will generate new knowledge for the design of effective cytokine-targeted therapies, especially combination therapies targeting multiple pathogenic cytokines, for patients diagnosed with SS.
5. Conclusions
Endogenous IL-7R signals play a critical role in sustaining the pathogenic Th1 and Tc1 responses and the characteristic SS pathologies in NOD mice with newly established SS disease, suggesting the potential of targeting IL-7-IL-7R pathway and Th1/Tc1 effector cytokines as future therapeutic strategies for treating this chronic and prevalent autoimmune condition.
Supplementary Material
Highlights.
Blockade of IL-7Rα in NOD mice with newly established SS disease ameliorated salivary gland inflammation and secretory dysfunction
Blockade of IL-7Rα decreased the amount of Th1, Tc1 and B cells and that of lymphocyte-attracting chemokines in the salivary glands
Anti-IL-7Rα treatment reduced the amount of Th1 cytokines IFN-γ and TNF-α, and increased the level of claudin-1 and aquaporin 5 in the salivary glands
Neutralization of IFN-γ and TNF-α, individually or in combination, attenuated salivary gland inflammation and secretory dysfunction in NOD mice with newly established SS
Acknowledgments
We thank Drs. Toshihisa Kawai and Atsushi Ikeda for the assistance in M3R ELISA.
Funding: This work was supported by the National Institutes of Health to QY [R01 DE023838].
This study was supported by grants from NIH/NIDCR (R01 DE023838) to QY.
Footnotes
Disclosure
The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jin JO, Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. Journal of clinical and cellular immunology, S! 2013 doi: 10.4172/2155-9899.S1-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox PC. Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Annals of the New York Academy of Sciences. 2007;1098:15–21. doi: 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 3.Patel R, Shahane A. The epidemiology of Sjogren’s syndrome. Clinical epidemiology. 2014;6:247–255. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodus NL. Sjogren’s syndrome. Quintessence international. 1999;30:689–699. [PubMed] [Google Scholar]
- 5.Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: an old tale with a new twist. Archivum immunologiae et therapiae experimentalis. 2009;57:57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nature reviews Rheumatology. 2010;6:529–537. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clinical reviews in allergy and immunology. 2007;32:252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Cohen PL. The T cell in Sjogren’s syndrome: force majeure, not spectateur. Journal of autoimmunity. 2012;39:229–233. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin H, Vosters JL, Roescher N, D’Souza A, Kurien BT, Tak PP, Chiorini JA. Location of immunization and interferon-gamma are central to induction of salivary gland dysfunction in Ro60 peptide immunized model of Sjogren’s syndrome. PloS one. 2011;6:e18003. doi: 10.1371/journal.pone.0018003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, Peck AB. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scandinavian journal of immunology. 2004;60:552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 12.Brayer JB, Cha S, Nagashima H, Yasunari U, Lindberg A, Diggs S, Martinez J, Goa J, Humphreys-Beher MG, Peck AB. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjogren’s syndrome. Scandinavian journal of immunology. 2001;54:133–140. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen CQ, Gao JH, Kim H, Saban DR, Cornelius JG, Peck AB. IL-4-STAT6 signal transduction-dependent induction of the clinical phase of Sjogren’s syndrome-like disease of the nonobese diabetic mouse. Journal of immunology. 2007;179:382–390. doi: 10.4049/jimmunol.179.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoukhri D, Macari E, Kublin CL. A single injection of interleukin-1 induces reversible aqueous-tear deficiency, lacrimal gland inflammation, and acinar and ductal cell proliferation. Experimental eye research. 2007;84:894–904. doi: 10.1016/j.exer.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. Journal of immunology. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Arbelaez CA, Glatigny S, Duhen R, Eberl G, Oukka M, Bettelli E. IL-7/IL-7 Receptor Signaling Differentially Affects Effector CD4+ T Cell Subsets Involved in Experimental Autoimmune Encephalomyelitis. Journal of immunology. 2015;195:1974–1983. doi: 10.4049/jimmunol.1403135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawson BR, Gonzalez-Quintial R, Eleftheriadis T, Farrar MA, Miller SD, Sauer K, McGavern DB, Kono DH, Baccala R, Theofilopoulos AN. Interleukin-7 is required for CD4(+) T cell activation and autoimmune neuroinflammation. Clinical immunology. 2015;161:260–269. doi: 10.1016/j.clim.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez-Quintial R, Lawson BR, Scatizzi JC, Craft J, Kono DH, Baccala R, Theofilopoulos AN. Systemic autoimmunity and lymphoproliferation are associated with excess IL-7 and inhibited by IL-7Ralpha blockade. PloS one. 2011;6:e27528. doi: 10.1371/journal.pone.0027528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Annals of the rheumatic diseases. 2006;65(Suppl 3):iii69–74. doi: 10.1136/ard.2006.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, Watanabe M. IL-7 Is essential for the development and the persistence of chronic colitis. Journal of immunology. 2007;178:4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 23.Lee LF, Axtell R, Tu GH, Logronio K, Dilley J, Yu J, Rickert M, Han B, Evering W, Walker MG, Shi J, de Jong BA, Killestein J, Polman CH, Steinman L, Lin JC. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Science translational medicine. 2011;3:93ra68. doi: 10.1126/scitranslmed.3002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, van Roon JA. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis and rheumatism. 2010;62:2716–2725. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 25.Willis CR, Seamons A, Maxwell J, Treuting PM, Nelson L, Chen G, Phelps S, Smith CL, Brabb T, Iritani BM, Maggio-Price L. Interleukin-7 receptor blockade suppresses adaptive and innate inflammatory responses in experimental colitis. Journal of inflammation. 2012;9:39. doi: 10.1186/1476-9255-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, Bijlsma JW, Lafeber FP, van Roon JA. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjogren’s syndrome correlates with increased inflammation. Arthritis and rheumatism. 2010;62:969–977. doi: 10.1002/art.27318. [DOI] [PubMed] [Google Scholar]
- 27.Bikker A, Kruize AA, Wenting M, Versnel MA, Bijlsma JW, Lafeber FP, van Roon JA. Increased interleukin (IL)-7Ralpha expression in salivary glands of patients with primary Sjogren’s syndrome is restricted to T cells and correlates with IL-7 expression, lymphocyte numbers and activity. Annals of the rheumatic diseases. 2012;71:1027–1033. doi: 10.1136/annrheumdis-2011-200744. [DOI] [PubMed] [Google Scholar]
- 28.Jin JO, Kawai T, Cha S, Yu Q. Interleukin-7 enhances the Th1 response to promote the development of Sjogren’s syndrome-like autoimmune exocrinopathy in mice. Arthritis and rheumatism. 2013;65:2132–2142. doi: 10.1002/art.38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Kawai T, Yu Q. Pathogenic role of endogenous TNF-alpha in the development of Sjogren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Laboratory investigation. 2017;97:458–467. doi: 10.1038/labinvest.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson MV, Delaleu N, Jonsson R. Animal models of Sjogren’s syndrome. Clinical reviews in allergy and immunology. 2007;32:215–224. doi: 10.1007/s12016-007-8012-7. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. The American journal of physiology. 1992;263:E607–614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 32.Tran SD, Kodama S, Lodde BM, Szalayova I, Key S, Khalili S, Faustman DL, Mezey E. Reversal of Sjogren’s-like syndrome in non-obese diabetic mice. Annals of the rheumatic diseases. 2007;66:812–814. doi: 10.1136/ard.2006.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjogren’s syndrome. Journal of biomedicine and biotechnology. 2011;2011:549107. doi: 10.1155/2011/549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Jin JO, Kawai T, Yu Q. Endogenous programmed death ligand-1 restrains the development and onset of Sjgren’s syndrome in non-obese diabetic mice. Scientific reports. 2016;6:39105. doi: 10.1038/srep39105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Annals of the New York Academy of Sciences. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 37.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren’s syndrome. Journal of immunology. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 38.Vosters JL, Yin H, Roescher N, Kok MR, Tak PP, Chiorini JA. Local expression of tumor necrosis factor-receptor 1:immunoglobulin G can induce salivary gland dysfunction in a murine model of Sjogren’s syndrome. Arthritis research and therapy. 2009;11:R189. doi: 10.1186/ar2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ewert P, Aguilera S, Alliende C, Kwon YJ, Albornoz A, Molina C, Urzua U, Quest AF, Olea N, Perez P, Castro I, Barrera MJ, Romo R, Hermoso M, Leyton C, Gonzalez MJ. Disruption of tight junction structure in salivary glands from Sjogren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis and rheumatism. 2010;62:1280–1289. doi: 10.1002/art.27362. [DOI] [PubMed] [Google Scholar]
- 40.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. American journal of physiology Cell physiology. 2008;295:C1191–1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Z, Yin H, Cabrera-Perez J, Guimaro MC, Afione S, Michael DG, Glenton P, Patel A, Swaim WD, Zheng C, Nguyen CQ, Nyberg F, Chiorini JA. Aquaporin gene therapy corrects Sjogren’s syndrome phenotype in mice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:5694–5699. doi: 10.1073/pnas.1601992113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culp DJ, Quivey RQ, Bowen WH, Fallon MA, Pearson SK, Faustoferri R. A mouse caries model and evaluation of aqp5−/− knockout mice. Caries research. 2005;39:448–454. doi: 10.1159/000088179. [DOI] [PubMed] [Google Scholar]
- 43.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. The Journal of biological chemistry. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 44.Zhu LJ, Yang X, Yu XQ. Anti-TNF-alpha therapies in systemic lupus erythematosus. Journal of biomedicine and biotechnology. 2010;2010:465898. doi: 10.1155/2010/465898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almoallim H, Al-Ghamdi Y, Almaghrabi H, Alyasi O. Anti-Tumor Necrosis Factor-alpha Induced Systemic Lupus Erythematosus. The open rheumatology journal. 2012;6:315–319. doi: 10.2174/1874312901206010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin JO, Shinohara Y, Yu Q. Innate immune signaling induces interleukin-7 production from salivary gland cells and accelerates the development of primary Sjogren’s syndrome in a mouse model. PloS one. 2013;8:e77605. doi: 10.1371/journal.pone.0077605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee LF, Logronio K, Tu GH, Zhai W, Ni I, Mei L, Dilley J, Yu J, Rajpal A, Brown C, Appah C, Chin SM, Han B, Affolter T, Lin JC. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12674–12679. doi: 10.1073/pnas.1203795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Yu Q. Disruption of CXCR3 function impedes the development of Sjogren’s syndrome-like xerostomia in non-obese diabetic mice. Laboratory investigation. 2018 doi: 10.1038/s41374-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delaleu N, Nguyen CQ, Peck AB, Jonsson R. Sjogren’s syndrome: studying the disease in mice. Arthritis research and therapy. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang LW, Cong X, Zhang Y, Wei T, Su YC, Serrao AC, Brito AR, Jr, Yu GY, Hua H, Wu LL. Interleukin-17 Impairs Salivary Tight Junction Integrity in Sjogren’s Syndrome. Journal of dental research. 2016;95:784–792. doi: 10.1177/0022034516634647. [DOI] [PubMed] [Google Scholar]
- 52.Giarratana N, Penna G, Adorini L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods in molecular biology. 2007;380:285–311. doi: 10.1007/978-1-59745-395-0_17. [DOI] [PubMed] [Google Scholar]
- 53.Voigt A, Esfandiary L, Nguyen CQ. Sexual dimorphism in an animal model of Sjogren’s syndrome: a potential role for Th17 cells. Biology open. 2015;4:1410–1419. doi: 10.1242/bio.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belle I, Mahlios J, McKenzie A, Zhuang Y. Aberrant production of IL-13 by T cells promotes exocrinopathy in Id3 knockout mice. Cytokine. 2014;69:226–233. doi: 10.1016/j.cyto.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahlios J, Zhuang Y. Contribution of IL-13 to early exocrinopathy in Id3−/− mice. Molecular immunology. 2011;49:227–233. doi: 10.1016/j.molimm.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szczerba BM, Rybakowska PD, Dey P, Payerhin KM, Peck AB, Bagavant H, Deshmukh US. Type I interferon receptor deficiency prevents murine Sjogren’s syndrome. Journal of dental research. 2013;92:444–449. doi: 10.1177/0022034513483315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Liu G, Gong L, Zhang Y, Jiang G. Local suppression of IL-21 in submandibular glands retards the development of Sjogren’s syndrome in non-obese diabetic mice. Journal of oral pathology andmedicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2012;41:728–735. doi: 10.1111/j.1600-0714.2012.01175.x. [DOI] [PubMed] [Google Scholar]
- 58.Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Investigative ophthalmology and visual science. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.