Abstract
This editorial refers to ‘Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors’†, by N. El Harane et al., on page 1835.
Over the past two decades, many pre-clinical and clinical studies have been conducted to establish stem cell therapy as a potential treatment for heart failure.1 Although the original purpose was to replace the scar lesion with new cardiomyocytes generated from stem cells, there is growing evidence that therapeutic benefits may be derived mostly from cardioprotective paracrine factors released by stem cells.2 Paracrine factors from several cell sources, including bone marrow-derived cells, endothelial cells, and mesenchymal stromal cells, have been shown to stimulate growth of new blood vessels and activate the endogenous repair pathway in the myocardium. Several studies hypothesized that these paracrine effects work through extracellular vesicles (EVs) secreted from stem cells.3–6 EVs, including exosomes and microvesicles, are released by different types of cells and involved in both physiological and pathophysiological processes.3–7 EVs are believed to mediate intercellular communication by transmitting information from the cells of origin to their target cells. Thus, attempts are being made to utilize EVs as novel tools for various therapeutic approaches such as antitumour and regenerative therapies, and some antitumour EVs have already entered phase II human clinical trials.3,7
Since the discovery of human induced pluripotent stem cells (iPSCs) by Shinya Yamanaka et al.,8 they are increasingly being used in cardiovascular research for disease modelling, drug screening, personalized medicine, and regenerative medicine.9,10 Although previous studies have shown that transplantation of either human embryonic stem cell-derived cardiomyocytes (hESC-CMs)11 or iPSC-derived cardiomyocytes (iPSC-CMs)12 to non-human primates can engraft or improve cardiac function through direct cardiomyocyte replacement, there are still several hurdles to overcome, including short-term ventricular arrythmias and long-term sustainable engraftment.12–14
In this issue of the journal, El Harane et al. sought to test whether EVs secreted by iPSC-derived cardiovascular progenitors (iPSC-PGs) can recapitulate the therapeutic effects of direct transplantation of iPSC-PGs.15 They successfully isolated EVs from iPSC-PGs, and found that iPSC-CMs did not produce EVs althogh the mechanism is unclear. EVs were internalized in vitro to target cells, which improved cell survival and proliferation of cultured H9C2 cardiomyocytes, and promoted angiogenesis including scratch wound healing and tube formation. Similarly, in vivo EV injection improved cardiac function in a murine myocardial infarction model. Surprisingly, the injection of EVs outperformed transplantation of their parent progenitor cells. The EVs were enriched with 16 highly conserved microRNAs (miRNAs), which are associated with biological functions expected to ameliorate heart failure. In terms of target genes of the miRNAs, they showed that the hearts from the three groups treated with iPSC-CMs, iPSC-PGs, and EVs showed distict gene expression patterns.
The work by El Harane et al. highlights the effect of EVs secreted by iPSC-PGs on chronic heart failure following myocardial infarction. In terms of clinical application, these results have three important implications. First, the source of EVs is progenitor cells differentiated from iPSCs, which are more available than hESCs from the ethical point of view. Second, the EV therapy is considered to be relatively safe because it is a cell-free therapy. Third, EV therapy was more effective in improving cardiac function in vivo than the direct transplantation of parent stem cells.
In their previous studies, Kervadec et al. showed that the injection of EVs secreted from human embryonic stem cell-derived cardiovascular progenitor cells (hESC-PGs) could provide beneficial effects equivalent to those of cell transplantation therapy of parent hESC-PGs in the treatment of chronic heart failure in mice.16. While EV therapy for heart failure had potential for being a new cell-free therapy, there were several hurdles to put it into practice because of the limited availability and ethical issues of hESCs. To overcome this problem, they switched the cell source from hESCs to iPSCs in the current study and successfully isolated EVs from iPSC-PGs to show the therapeutic effects of the iPSC-PG-EVs. The switch is an important step for clinical application of the EV therapy, because iPSCs are more available than hESCs in terms of regulatory restrictions and ethical issues.
Although the exact mechanism of therapeutic benefit of EV therapy is still unclear, it is notable that the EV therapy here is cell free and is shown to be equally if not more effective than direct cell transplantation. If true, this means that EV therapy may have significant safety advantages because it is probably less likely to induce malignancy, immune reactions, and arrhythmias compared with direct transplantation of hESC-CMs or iPSC-CMs.
Surprisingly, El Harane et al. found that EV therapy not only could mimic paracrine effect of cell transplantation, but also was more effective than parent cells in improving EF in vivo. This is probably due to the concentration of cardioprotective factors such as miRNAs in the EVs, because EVs injected into mice hearts should be highly concentrated during the in vitro culture process prior to injection. This may also confer an advantage for EV therapy because the concentration of EVs can be controlled and optimized by adjusting culturing conditions, whereas, in comparison, it is much more difficult to control the behaviour of cells already transplanted into the hearts. Thus, this EV therapy potentially can even maximize the cardioprotective paracrine effects of the parent stem cells.
To translate EV therapy into an effective clinical treatment option for heart failure, it will be important to assess its possible serious side effects given that its precise mechanism is unclear at present. To that end, it will be essential to perform optimization to increase the benefits and decrease the side effects, such as by optimizing the amounts of EVs to be injected and their delivery to the heart, as well as how their parent stem cells are to be cultured. In addition, the selection of parent cell types for EVs may be important. Here, El Harane et al. used iPSC-PGs and iPSC-CMs as the source of EVs. However, other cell types or combinations of cell types may be even better at generating cardioprotective EVs, including endothelial progenitors that may be able to produce EVs with greater angiogenesis or anti-apoptotic effects. Furthermore, there are still several important unanswered points that should be investigated: (i) how consistently can iPSC-PGs produce effective EVs that contain the same set of miRNAs within the same iPSC line and also over different passage numbers; (ii) how robustly can other iPSC lines produce effective EVs because iPSCs have significant variability depending on the donors; (iii) how long is the biological half-life of EVs and how does that translate to sustained improvement in heart failure; and (iv) how can we standardize the process to make EVs with consistent quality?
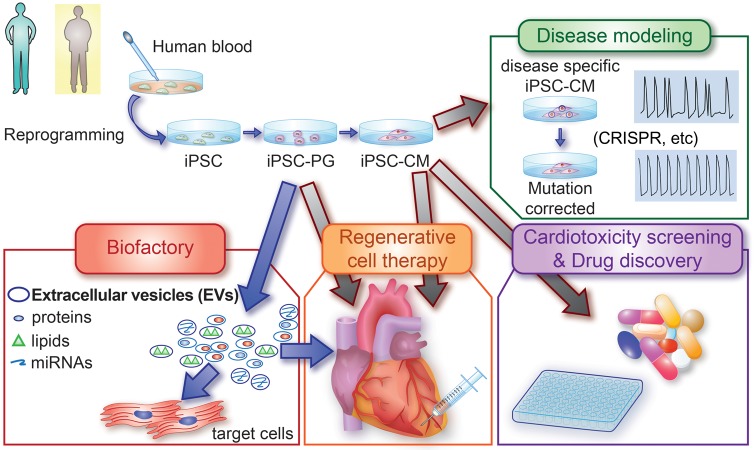
Lastly, this study also shows that EVs not only could mimic the paracrine effect of regenerative cell therapy, but also may be used as a drug delivery system.3,7 El Harane et al. notably used iPSCs as a biopharmaceutical ‘factory’ to produce EVs. Because EVs can deliver diverse substances such as proteins and nucleic acids to target tissues, and iPSCs can be differentiated into any types of cells, iPSC-derived EVs have a potential as next-generation biopharmaceutical drugs capable of targeting tissues beyond the reach of current recombinant proteins. However, additional research is necessary for these diverse applications, including ways to optimize the beneficial paracrine factors produced by iPSC-derived cells (Take home figure).17
Take home figure.
New application of iPSCs as a ‘biofactory’. The blood cells from patients or healthy individuals can be reprogrammed into iPSCs and differentiated to cardiovascular progenitor cells (iPSC-PGs) and cardiomyocytes (iPSC-CMs). These cells have been used for cardiac disease modelling, high-throughput drug screening, and regenerative medicine. Notably, this study also presented a new way to use iPSCs as a ‘biofactory’ of extracellular vesicles (EVs). EVs released from iPSC-PGs were shown to be potentially effective for treating chronic heart failure in a murine model.
Acknowledgement
This publication was supported in part by research grants from the National Institutes of Health (NIH) R01 HL113006 and NIH R01 HL133272, American Heart Association (AHA) 17MERIT3361009, and the California Institute of Regenerative Medicine (CIRM) DR2A-05394 and RT3-07798 (J.C.W.).
Conflict of interest: none declared.
References
- 1. Nguyen PK, Neofytou E, Rhee JW, Wu JC.. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol 2016;1:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cahill TJ, Choudhury RP, Riley PR.. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discov 2017;16:699–717. [DOI] [PubMed] [Google Scholar]
- 3. Ibrahim A, Marban E.. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 2016;78:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karantalis V, Hare JM.. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong SG, Huber BC, Lee WH, Kodo K, Ebert AD, Ma Y, Nguyen PK, Diecke S, Chen WY, Wu JC.. Microfluidic single-cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes after acute myocardial infarction. Circulation 2015;132:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee WH, Chen WY, Shao NY, Xiao D, Qin X, Baker N, Bae HR, Wei TT, Wang Y, Shukla P, Wu H, Kodo K, Ong SG, Wu JC.. Comparison of non-coding RNAs in exosomes and functional efficacy of human embryonic stem cell- versus induced pluripotent stem cell-derived cardiomyocytes. Stem Cells 2017;35:2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B.. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles 2015;4:30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 9. Chen IY, Matsa E, Wu JC.. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol 2016;13:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sayed N, Liu C, Wu JC.. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 2016;67:2161–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U.. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–391. [DOI] [PubMed] [Google Scholar]
- 13. Anderson ME, Goldhaber J, Houser SR, Puceat M, Sussman MA.. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ Res 2014;115:335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu K, Wu Q, Ni C, Zhang P, Zhong Z, Wu Y, Wang Y, Xu Y, Kong M, Cheng H, Tao Z, Yang Q, Liang H, Jiang Y, Li Q, Zhao J, Huang J, Zhang F, Chen Q, Li Y, Chen J, Zhu W, Yu H, Zhang J, Yang HT, Hu X, Wang J. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ Res 2018; doi: 10.1161/CIRCRESAHA.117.311578. [DOI] [PubMed] [Google Scholar]
- 15. El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier MC, Lima Correa B, Thiébault L, Cagnard N, Duché A, Brunaud C, Lemitre M, Gauthier J, Bourdillon AT, Renault MP, Hovhannisyan Y, Paiva S, Colas AR, Agbulut O, Hagège A, Silvestre JS, Menasché P, Renault NKE.. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 2018;39:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kervadec A, Bellamy V, El Harane N, Arakelian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Perier MC, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagege A, Ruel M, Boulanger CM, Silvestre JS, Menasche P, Renault NK.. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 2016;35:795–807. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Aviles F, Sanz-Ruiz R, Climent AM, Badimon L, Bolli R, Charron D, Fuster V, Janssens S, Kastrup J, Kim HS, Luscher TF, Martin JF, Menasche P, Simari RD, Stone GW, Terzic A, Willerson JT, Wu JC; TACTICS (Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes) Writing Group; Authors/Task Force Members. Chairpersons; Basic Research Subcommittee; Translational Research Subcommittee; Challenges of Cardiovascular Regenerative Medicine Subcommittee; Tissue Engineering Subcommittee; Delivery, Navigation, Tracking and Assessment Subcommittee; Clinical Trials Subcommittee; Regulatory and funding strategies subcommittee; Delivery, Navigation, Tracking and Assessment Subcommittee. Global position paper on cardiovascular regenerative medicine. Eur Heart J 2017;38:2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]