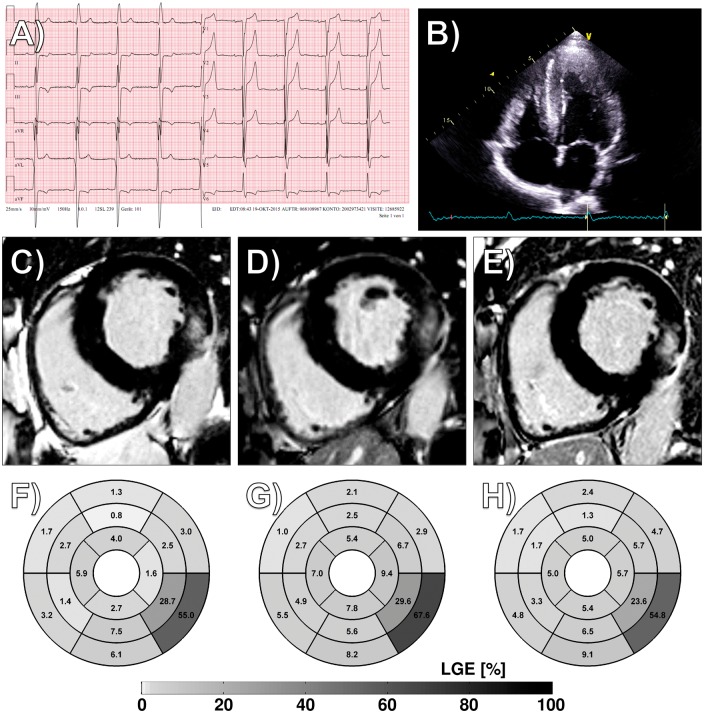
A 52-year-old male patient diagnosed for hypertrophic cardiomyopathy was admitted to our clinic in October 2015 for unusual rapid worsening of heart failure symptoms. The electrocardiogram showed negative T-waves in inferior leads and a positive Sokolow index for left ventricular (LV) hypertrophy (Panel A). Echocardiography confirmed non-obstructive LV hypertrophy (Panel B). Cardiac serum biomarkers showed troponin and N-terminal pro-brain natriuretic peptide (NT-ProBNP) levels of 49.1 and 365 pg/mL. Cardiac magnetic resonance imaging revealed diffuse late gadolinium enhancement (LGE) in the posterolateral wall (Panels C and F). Genetic analysis excluded sarcomeric hypertrophic cardiomyopathy but revealed the mutation N215S in the alpha-galactosidase A gene, one of the most prevalent Fabry mutations in Europe and recently described for a specific cardiac form of the disease. Heart failure medication was started, but enzyme replacement therapy refused by the patient. Due to further disease progression at 10 months follow-up regarding symptoms, further increase of ventricular mass (+21 g/+7%, Panel D/Supplementary material online, Video S1) and LGE in the Fabry-typical Segment 5 (+13%, Panel G), as well as troponin and NT-ProBNP levels (70.9 and 729 pg/mL), Fabry-specific therapy with Migalastat was initiated July 2016. After 12 months of chaperone therapy, LV hypertrophy decreased (−18 g/−6%, Panel E/Supplementary material online, Video S2), LGE fell by 13% (Panel H), and cardiac serum biomarkers dropped (62.5 and 327 pg/mL).
Just recently, several specific mutations have been reported responsible for cardiac variants of Fabry disease, most prominently N215S and IVS4. For the Chinese hotspot mutation IVS4, a Taiwanese screening study revealed a prevalence of >1:1000 individuals, implying a disease frequency comparable to sarcomeric hypertrophic cardiomyopathy in related populations. While treatment for classic Fabry disease in form of enzyme replacement therapy has been introduced more than 15 years ago, both short- and long-term data question substantial positive cardiac effects specifically in advanced stages of the disease. The chaperone Migalastat, approved for therapy of patients with amenable mutations since mid-2016, stabilizes the altered enzyme and thus depletes accumulation of pathogenic cellular substances, representing a novel therapy mechanism that might prove superior especially when treating cardiac manifestations of Fabry disease.
Supplementary material is available at European Heart Journal online.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.