Abstract
Purpose
Docosahexaenoic acid (DHA) was supplemented in a single-site, placebo-controlled, randomized clinical trial designed to slow vision loss associated with X-linked retinitis pigmentosa (XLRP); the DHAX Trial. We previously reported no significant differences between supplemented and placebo groups in intent-to-treat analysis of primary ERG outcomes. Assessed herein are hypothesis-generating measures of ancillary visual function outcomes in participants fully adhering to trial protocol.
Methods
Male participants with XLRP (range, 7–31 years) received 30 mg DHA/kg/d (n = 29) or placebo (n = 22) for 4 years. Visual outcomes were measured annually and red blood cell (RBC) DHA determined every 6 months.
Results
Oral DHA supplementation increased mean RBC-DHA levels by 4-fold (P < 0.0001) over placebo. No group differences in progression were found for visual acuity (P = 0.11), shape discrimination (P = 0.18), or fundus appearance (P = 0.70). Optical coherence tomography (OCT) became available during year 2 of the trial; no group differences were seen in ellipsoid zone constriction (P = 0.87) over 2 years. Yearly rates of progression were reduced for dark-adapted thresholds (P = 0.06) and visual field sensitivity for foveal, macular, peripheral, total, and ellipsoid zone regions by DHA supplementation (P = 0.039, P = 0.031, P < 0.0001, P < 0.0001, and P = 0.033). Rates of visual field sensitivity decline were dependent on RBC-DHA (P = 0.046 to <0.0001).
Conclusions
Supplementation of DHA significantly elevated blood DHA levels and reduced the rate of progression in final dark-adapted thresholds and visual field sensitivity. From the relationship between RBC-DHA and the rate of field sensitivity loss, we can extrapolate that an RBC-DHA level of 17% could minimize the decline in field sensitivity. (ClinicalTrials.gov number, NCT00100230.)
Keywords: hereditary retinal degeneration, omega-3 fatty acid, randomized clinical trial, lipids
In a 4-year randomized clinical trial, supplementation of docosahexaenoic acid (DHA) to patients with X-linked retinitis pigmentosa did not alter visual acuity, fundus appearance, or ellipsoid zone outcomes; however, loss of visual field sensitivity was significantly reduced by DHA intervention.
X-linked retinitis pigmentosa (XLRP) is a rare genetic retinal degenerative disease characterized by early childhood nightblindness, diminished visual acuity, appearance of retinal pigmentation, and severe progressive visual field constriction.1–3 Many patients with XLRP have lower than normal blood lipid levels of the n3 fatty acid, docosahexaenoic acid (DHA; 22:6n3) found highly enriched in the retina and brain.4,5 Using stable isotope methodology, patients with XLRP were found to have a reduction in the synthesis of DHA from shorter chain n3 fatty acids.6 Thus, oral supplementation of DHA may bypass this metabolic obstacle and possibly slow disease progression.
Although randomized trials employing n3 fatty acid-rich fish, fish oil, or DHA to slow progression have been conducted in patients with RP, a compelling demonstration of efficacy remains elusive. In a clinical trial of patients with retinitis pigmentosa (RP), 15,000 IU vitamin A plus either 1200 mg DHA/d or placebo resulted in no statistically significant group differences in peripheral visual fields, cone ERG function or visual acuity.7 However, subgroup analysis indicated that visual fields could be preserved by vitamin A plus DHA supplementation for two years.8 In addition, a subsequent analysis of dietary questionnaire data revealed that patients with RP taking vitamin A and consuming ≥200 mg DHA/d, attributable to two or more meals of fish, had a significantly reduced rate of visual acuity loss.9
In a 4-year trial in which participants with XLRP took 400 mg DHA/d,10 RBC-DHA levels were elevated to a mean of 6.9%, 2.5-fold greater than in the placebo group. Although rod and cone ERG loss was slowed in the DHA-supplemented group, statistical significance was not attained; no differences were found in progression of visual acuity or field sensitivity. A correlation was found between RBC-DHA and cone ERG function such that a target RBC-DHA level of ∼13% might eliminate cone ERG loss. Thus, a dose of 30 mg DHA/kg bodyweight/d was proposed for use in the DHAX trial. We previously reported that no significant group differences were found in the primary and secondary outcome measures of 31-Hz cone and rod ERG function.11 None of the studies with DHA supplementation have shown significant safety risks in participants with XLRP.12,13 Here, we examine ancillary outcome measures of the DHAX trial focusing on potential hypothesis-generating analysis of data from participants following the designated protocol for the entire 4-year trial.
Methods
Study Population and Trial Design
Details of recruitment, randomization, inclusion criteria, participant flow and trial design for this single-site, placebo-controlled, randomized, double-masked, clinical trial were provided previously.11 Briefly, male patients diagnosed with XLRP (aged 7–31 years) were enrolled and randomized to receive either a daily supplement enriched with DHA or placebo for 4 years. As observed in natural history studies of RP,14,15 lengthy clinical trials (i.e., 3–4 years) appear necessary to assess modification of disease progression. Participant dropout characteristics were given in our primary outcome report11 which defined a modified intent-to-treat cohort (n = 60) completing at least the first year of testing. Five participants in the placebo group and three in the DHA group failed to complete the trial and one in the DHA group was nonadherent to protocol. Thus, only participants who completed the trial with evidence of adherence to the protocol comprised the prespecified per protocol cohort (n = 51). Of 22 participants in the placebo group, 20 had RPGR mutations (91%), one had an RP2 mutation (5%), and one did not have an identified mutation (5%). Of 29 participants in the DHA-supplemented group, 25 had RPGR mutations (86%), three had RP2 mutations (10%), and one did not have an identified mutation (4%). The nine dropouts from the modified intent-to-treat cohort had RPGR mutations.
Informed consent was obtained from participants and/or parents of minors. Research adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. Study oversight was conducted by a data and safety monitoring committee.11,13
Intervention
Intervention assignment was determined using a computer-generated randomization schedule with varying block size design. All testing personnel were masked to treatment assignment. DSM Nutritional Products supplied gelatin capsules containing DHA-enriched triglyceride (DHASCO) or corn/soy oil (placebo) triglycerides. The 500-mg capsules contained 200 mg DHA or placebo oil with a sufficient amount of capsules provided to achieve a dose of 30 mg DHA/kg/d. Both placebo and DHA capsules contained small amounts of vitamins E and C as antioxidants (12.5 mg each) and a food-grade orange extract as flavoring (5.9 mg/500 mg capsule). Fatty acid content of capsules has been presented previously.13 Neither participants nor investigators were able to distinguish the placebo and active capsules based on appearance, smell, or taste. Adherence to protocol was monitored by blood fatty acid analysis as detailed previously.11,13
All participants received commercial multivitamins in order to maintain a minimum antioxidant status and provided 100% of the recommended daily amounts of vitamins A, B6, B12, C, D, and E.13
Functional and Biochemical Evaluation
Clinical, anthropometric, ERG, and biosafety assessments for the DHAX trial have been previously reported.11,13 At baseline (year 0) and annually (years 1 through 4), ancillary exploratory tests were conducted at our institution, including visual acuity, final dark-adapted threshold, shape discrimination threshold, fundus appearance progression, and ellipsoid zone (EZ) anatomic assessment from ocular coherence tomography (OCT). In addition, foveal, macular, peripheral, total, and EZ visual field sensitivity (VFS) measures were made annually.
Visual Acuity, Shape Discrimination and Dark-Adapted Thresholds
Best-corrected visual acuity was assessed by electronic Early Treatment of Diabetic Retinopathy Study for both OD and OS. Individual shape discrimination thresholds were determined and averaged between eyes as minimal detectable radial modulation amplitude on charts of varying circular D4 contour and radial deformed visual stimuli.16 Pupils were maximally dilated using 1.0% cyclopentolate hydrochloride and 2.5% phenylephrine hydrochloride. Immediately after dilation, one eye was dark-adapted for 45 minutes using a black patch (Bernell Corp., Mishawaka, IN, USA) and opaque adhesive occlusers (Coverlet Eye Occlusors; Beiersdorf, Inc., Cincinnati, OH, USA). Final dark-adapted thresholds (average of 10 determinations) were obtained with an 11° test stimulus located 7° below fixation on a Goldmann-Weekers dark-adaptometer.
Fundus Imaging
Digitized color fundus images (Escalon Medical Corp., Ardmore, PA, USA) included the ETDRS seven standard 60° fields and one additional nasal view for each eye at baseline and annual visits (Canon CF-60UD; Melville, NY, USA). After completing all visits, progression of disease was assessed using a grading technique similar to that described previously.10,17 After brightness and blue balancing, eight image pairs per eye from year 0 and year 4 were uploaded online (Viewlet Builder 7; Qarbon, Inc., San Jose, CA, USA) for side-by-side comparison by five masked judges. A group training session was followed by individual fundus image judging. In a subsequent group adjudication session, consensus scores were determined. For each set of images, two judgments were made: right or left position of 4-year images, and degree of progression grade based on changes in: (1) appearance of new retinal pigment, (2) vessel caliber, (3) retinal pigment atrophy, and (4) optic disc pallor. Progression for each image set was assessed as 0 (no change); 1 (minimal change); 2 (moderate change); and 3 (severe change). Representative fundus images for each grade are depicted in Figure 1.
Figure 1.
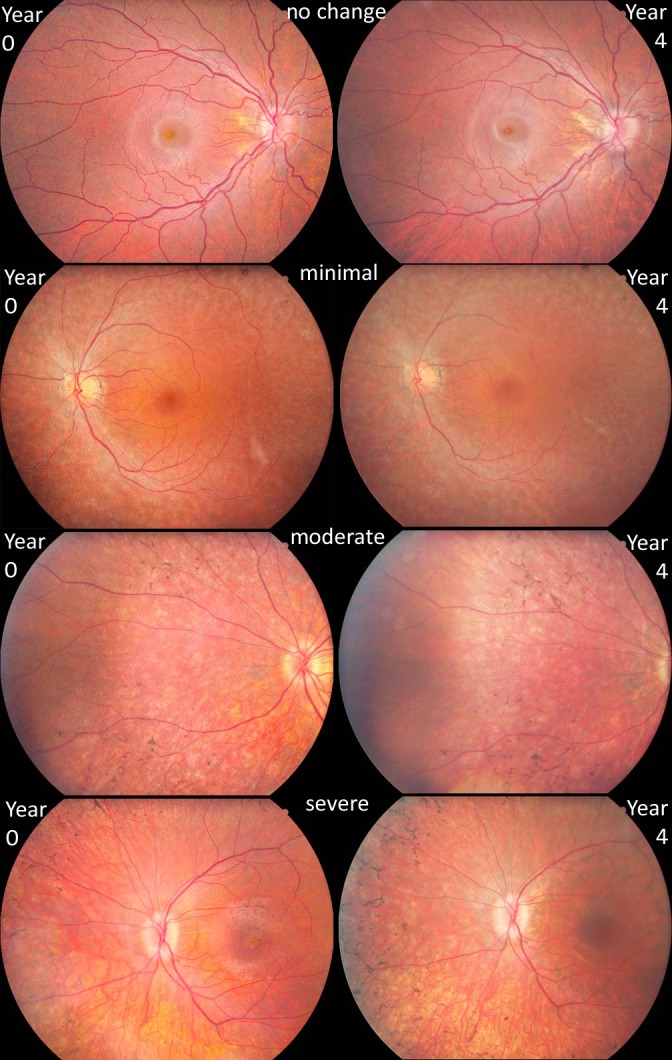
Fundus image scoring. Representative images of scoring grades comparing fundus appearance progression from trial year 0 to year 4. A score of 0 = no identifiable change; 1 = minimal change; 2 = moderate change including new pigmentation and/or further vessel attenuation; and 3 = severe progression with multiple new deposits of pigmentation.
Humphrey Visual Fields
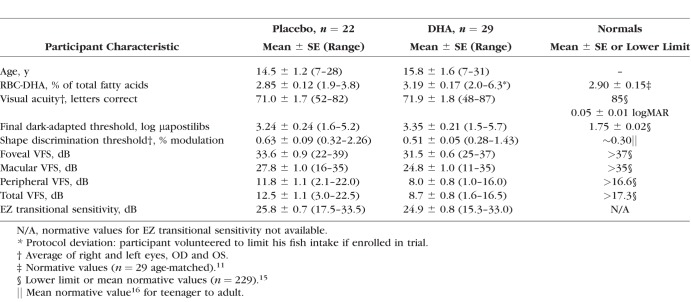
Visual fields were obtained from the participant's eye with poorer visual acuity on a model 640 Humphrey Field Analyzer (Zeiss-Humphrey Systems, Dublin, CA, USA). To minimize practice effects, two fields per eye were obtained before year 0 (baseline). Program 30-2, spot size V was used to assess static perimetric thresholds at 75 locations within the central 30°. For participants with fields >30°, program 30/60-2 was used to test 60 locations between 30° and 60°. Foveal VFS was determined from a single stimulus location (<3°). Macular VFS was the summed sensitivity of four locations within a macular annulus extending from 3.4 to 10° and peripheral VFS is the sum of 130 locations out to 60° excluding foveal and macular sensitivities. Post hoc exploratory analysis included summation of a priori independent measures of foveal, macular, and peripheral VFS to define a total VFS outcome (tVFS; 135 locations). Results are presented as decibels (dB) sensitivity per point; lower limits of normal VFS values are given in Table 1.
Table 1.
Baseline Demographics and Ocular Characteristics of Per Protocol Cohort of the 4-Year DHAX Trial
Ellipsoid Zone (EZ) Anatomic and EZ Transitional Sensitivity Assessment
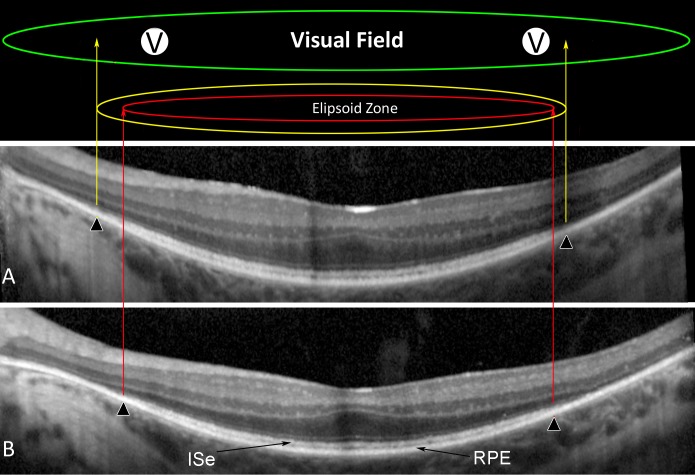
As described previously,18 beginning with the third trial visit, horizontal midline frequency-domain OCT scans were obtained yearly with an OCT device (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany). Automatic real-time registration was used with an average of 100 scans. All scans had a quality index of greater than 25 dB. Repeat scans were captured with the aid of automatic registration to ensure the same scan placement on each test. Semiautomated segmentation was aided by previously published routines.19 The dense EZ outer photoreceptor segment band lies between the hyperreflective inner segment band (ISe) and the hyperreflective RPE band (Fig. 2). The anatomic EZ width was defined by the nasal and temporal points where the thickness of the outer segment layer declined to zero. Based on the segmented scans, two visual field locations at the nasal and temporal transition zones were selected for each patient for evaluation of EZ transitional sensitivity. The rate of change in EZ transitional VFS at these locations from baseline to year 4 was calculated for each participant.
Figure 2.
Ellipsoid zone anatomic and EZ transitional visual field sensitivity. Identified by semiautomated segmentation of retinal frequency-domain OCT from participant G101 (segmentation lines not shown) is the dense layer of outer photoreceptor segments termed the EZ which lies between the hyperreflective ISe and the hyperreflective RPE band. The anatomic EZ width was defined by the nasal and temporal points where the outer segment layer has declined to zero (▴) at trial year 2 (A) and year 4 (B). By superimposing the visual fields over the segmented scans, two nasal visual field locations just inside the EZ edge were selected for each patient for evaluation of EZ transitional visual field sensitivity (V).
Statistical Analysis
This report focuses on analysis of data from the per protocol compliant cohort who completed the trial (n = 51). The statistical significance of variations in each ancillary outcome measure was determined using a repeated measures mixed model regression analysis with restricted maximum likelihood estimation. Additional statistical analyses were performed using two-tailed t-tests to compare group differences in overall trial changes. For visual acuity and shape discrimination, data for both eyes was averaged as XLRP is a bilateral disease although interclass correlation analysis was conducted.20 We used χ2 and Kendall's tau-B tests for comparison of fundus imaging results. Regression analysis was used to assess the association between visual function outcomes and RBC-DHA levels. Statistical analyses were performed with commercial software (SPSS ver. 22; IBM Corp., Armonk, NY, USA, and Statistica ver.12; StatSoft, Inc., Tulsa, OK, USA).
Results
Baseline Characteristics
For the per protocol cohort, anthropometric, blood chemistry, ERG,11 and ancillary ocular characteristics at baseline were balanced between randomized groups with the exception of poorer visual field sensitivities in the DHA group (Table 1). Mean RBC-DHA levels in the XLRP cohort were comparable to normally-sighted controls although other visual function measures were characteristically abnormal.
RBC-DHA Levels
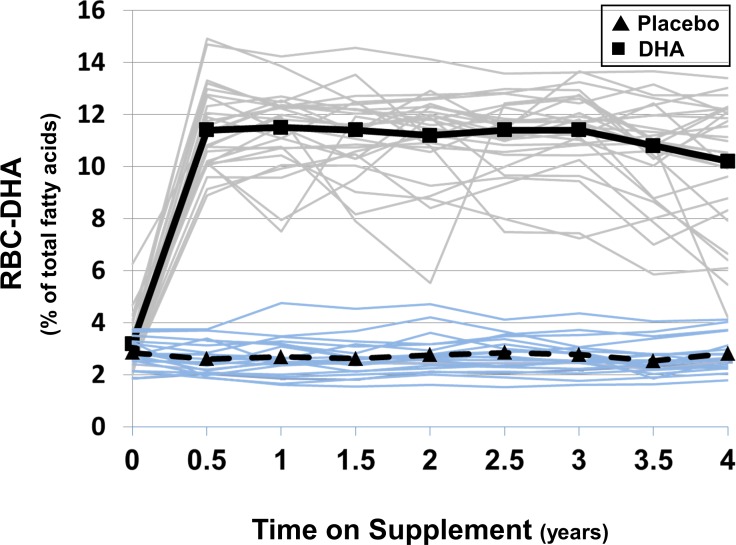
At each trial visit beyond baseline, RBC-DHA in the DHA-supplemented group of the per protocol cohort was significantly elevated above baseline and above the placebo group (P < 0.0001 for both). The mean RBC-DHA level from 0.5 to 4 years in the DHA group was 10.9% ± 0.3% with individuals ranging from 8.9% to 14.0%. In contrast, RBC-DHA in the placebo group changed very little over the course of the trial reflecting good compliance with protocol and abstaining from over-the-counter supplementation with individuals ranging from 1.7% to 4.3% with a mean of 2.7 ± 0.1% (Fig. 3).
Figure 3.
Docosahexaenoic acid in red blood cells (RBCs) of per protocol participants as a function of time. Values for %RBC-DHA at each 6-month time point are depicted for placebo (blue lines) and DHA-supplemented individuals (gray lines). Mean values for the placebo (▴···▴) and DHA (▪ — ▪) groups were significantly different at each time point between 0.5 and 4 years; P < 0.0001.
Ancillary Ocular Functions
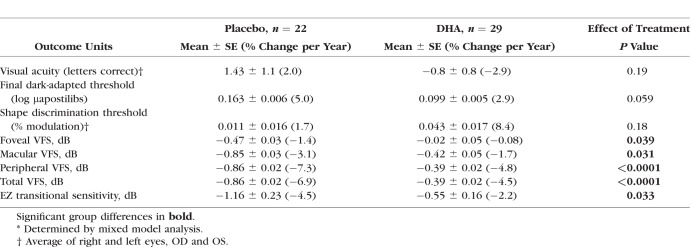
For the per protocol cohort, no significant differences between placebo and DHA groups were evident using mixed model analysis of visual acuity as measured by averaging across both eyes (P = 0.19; Table 2) or by interclass correlation analysis (P = 0.63). Similarly, no statistically significant group differences were found for shape discrimination threshold. There was a trend toward a reduced annual rate in progression of final dark-adapted thresholds in the DHA-supplemented group compared to placebo (P = 0.059; Table 2).
Table 2.
Yearly Rates of Change* in Functional Outcome Measures for per Protocol Cohort
Fundus Appearance
In side-by-side comparison of 50 image sets from the per protocol cohort (one set was inadvertently omitted), the percentages of “no change,” “minimal,” “moderate,” and “severe” in the placebo group were 5%, 27%, 27%, and 41% compared to 11%, 25%, 36%, and 28% in the DHA group. Disease progression of four sets were judged to have “no change” between year 0 and year 4 and were excluded from further analysis (3 from the DHA group and one from placebo). No significant differences were found in the degree of disease progression between placebo and DHA-supplemented groups (χ2; P = 0.67; Kendall's tau-B: P = 0.65).
Visual Field Sensitivity (VFS)
Foveal VFS in the placebo and DHA groups of the per protocol cohort were not significantly different at trial year 1 (P = 0.057), year 2 (P = 0.26), year 3 (P = 0.33), or year 4 (P = 0.55). However, in the DHA group, the change from baseline to year 4 in foveal VFS was significantly less than for placebo (P = 0.039; Table 2). Similarly, the yearly group differences in macular VFS were not significant (P > 0.12), but the overall annual rate of loss of macular VFS was significantly reduced in DHA-supplemented participants (P = 0.031).
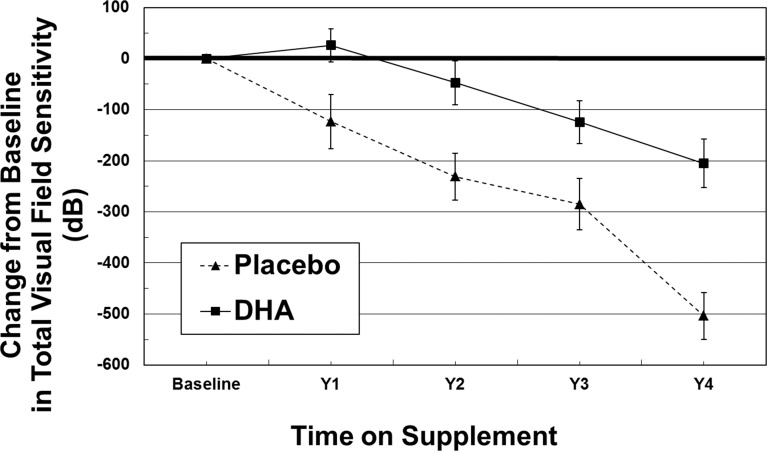
Peripheral VFS measures were not statistically different between groups at year 1 (P = 0.19), year 2 (P = 0.24), year 3 (P = 0.18), or year 4 (P = 0.55). However, yearly changes in peripheral VFS were significant: year 1 (P = 0.017), year 2 (P = 0.006), year 3 (P = 0.019), and year 4 (P < 0.0001). The annual rate of peripheral VFS progression for the overall reduction of loss in the placebo group of 113 dB/y compared with 50 dB/y in the DHA group was highly significant (P < 0.0001; Table 2). In a post hoc summation of foveal + macular + peripheral VFS, total VFS showed a similar functional profile. DHA-related change in total VFS was significant at each yearly time point (year 1 [P = 0.016]; year 2 [P = 0.005], year 3 [P = 0.018], and year 4 [P < 0.0001]). By mixed-model analysis, the overall 56% reduction in the annual rate of loss was highly significant (P < 0.0001; Table 2, Fig. 4). In a modified intent-to-treat cohort (n = 60), similar significant group differences in VFS were observed (macular VFS, P = 0.03; peripheral VFS, P < 0.0001; total VFS, P < 0.0001). The visual field sensitivity results among the per protocol DHA-supplemented group were not significant according to genotype for limited numbers of participants with RP2 versus RPGR mutations nor for null, protein variants, or splicing RPGR mutations.
Figure 4.
Yearly change in tVFS as a function of time on intervention in the per protocol cohort. Values are the mean ±SE decibels (dB) for each trial year of participants receiving placebo (▴···▴) or DHA (▪ — ▪) supplement. Placebo and DHA group means were significantly different at year 1 (P = 0.016); year 2 (P = 0.005); year 3 (P = 0.018); and year 4 (P < 0.0001).
RBC-DHA and VFS
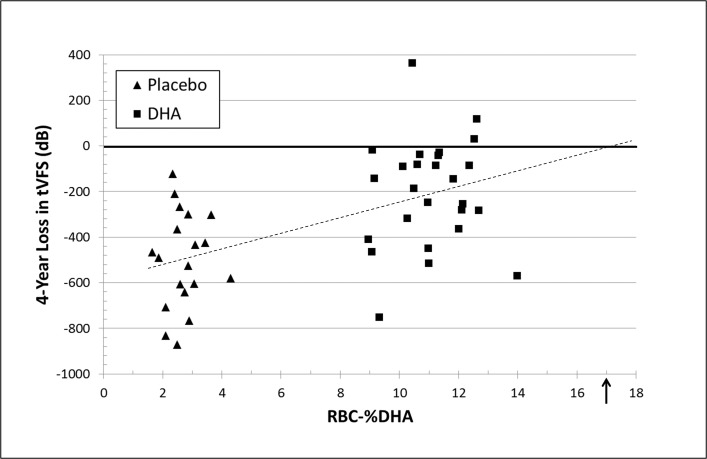
Intervention-related changes in foveal, macular, and peripheral VFS over the 4-year trial were inversely correlated with RBC-DHA (r = −0.29, P = 0.046; r = −0.45, P = 0.001; and r = −0.55, P < 0.0001, respectively) such that participants with higher RBC-DHA levels had less change in all visual fields. Similarly, the correlation of RBC-DHA content to change in total VFS was significant (n = 51; r = −0.55, P < 0.0001; Fig. 5). To assess if participants with early stage XLRP might be differentially influenced by DHA compared with those with more advanced RP, total VFS in subgroups of younger than 14 years of age (placebo, n = 12; DHA, n = 17) and older participants (placebo, n = 10; DHA, n = 12) were analyzed. The Pearson correlation between RBC-DHA and total VFS for older participants was less (n = 22; r = −0.46; P = 0.031) than in young participants (n = 29; r = −0.63; P = 0.0002). Extrapolation of the trendline for all participants to the zero intercept (Fig. 5) yields a level of RBC-DHA in which the loss of total VFS by year 4 could be eliminated (17% RBC-DHA). The level of RBC-DHA estimated to maximally reduce loss of total VFS in the older subgroup was 21% compared to 15% in those with earlier stage XLRP.
Figure 5.
Association of RBC-DHA and 4-year loss of tVFS. Values are mean RBC-DHA (in total percent of fatty acids) for each per protocol participant between year 0.5 and year 4 compared with loss of tVFS (in dB) from year 0 to year 4 for placebo (▴) and DHA (▪) groups. The dashed line represents a Pearson regression (r = −0.55, P < 0.0001) with an intercept = 17% RBC-DHA with (↑) indicating absence of tVFS loss.
EZ Anatomic Width and EZ Transitional VFS
Over a 2-year span of the trial, the mean annual loss of the retinal outer segment layer, depicted by width of the EZ, for the placebo group was 0.83 (±0.15 SE) mm/y (8.6% loss/y) and did not differ significantly from the DHA group (0.75 [±0.10 SE] mm/y; 6.9% loss/y; P = 0.61]).
The visual field sensitivity of points in the transitional zone (just inside the EZ edge) were tracked for each participant from baseline to Year 4. The annual rate of loss of the EZ transitional VFS in the placebo group was twice that of the DHA group (P = 0.033; Table 2).
Discussion
A daily supplementation of DHA at 30 mg/kg bodyweight for 4 years elevated RBC-DHA to a mean of 10.9% compared with 2.7% of total fatty acids in participants receiving placebo. Despite a 4-fold difference in RBC-DHA, visual acuity, shape discrimination threshold, fundus appearance and EZ anatomical width were not significantly different between groups. These results are consistent with our previous report that no significant group differences were found for the primary and secondary outcomes of cone and rod ERG function.11 In contrast, psychophysical measures of final dark-adapted thresholds in the DHA-supplemented group showed less progression over 4 years compared with the placebo group (Table 2). In addition, DHA-supplementation resulted in significant reductions in the annual rates of disease progression for measures of foveal, macular, peripheral, total, and EZ transitional VFS. Since unbalanced VFS values were evident at baseline, different stages of disease progression may have existed between groups prior to treatment. To minimize quasi-floor and -ceiling effects, data were sorted into quintiles based on total VFS at baseline. Total VFS data from the balanced, middle three quintiles (placebo, n = 14; DHA, n = 19) showed that DHA supplementation significantly reduced visual function loss by 57% (P = 0.0005).
An inconsistency evident among the various outcome measures of the DHAX trial are the differences in response to DHA supplementation in ERG11 and VFS. One possible explanation is that the full-field ERG, which shows no benefit from DHA, represents the summed response from primarily diseased photoreceptors. VFS, on the other hand, reflects the small percentage of healthier photoreceptors which detect visual stimuli at threshold. The neuroprotection afforded by DHA may primarily preserve the healthiest photoreceptors resulting in slowing of field sensitivity decline.
By optimizing the lipid microenvironment of retinal cell membranes, various mechanisms may account for the neuroprotective actions of DHA-supplementation. In general, the chain length and the high degree of unsaturation of DHA suggest a role in maintaining a membrane with fluidity and permeability properties required for the dynamic behavior of components of the visual cascade.21 Rod outer segment membranes rich in DHA (22:6n3)22 and DHA-enriched phosphatidylcholine bilayers23 both exhibited 2- to 6-fold enhancement of rhodopsin formation, cGMP phosphodiesterase activity and metarhodopsin-transducin complex formation compared to membranes and bilayers containing primarily the n6 long-chain fatty acid, docosapentaenoic acid (22:5n6). A reduction in apoptotic cell death as indexed by DNA fragmentation and caspase-3 was found in mouse neurons exposed to DHA.24 The neuroprotective derivative of DHA, neuroprotectin, NPD1, has been shown to promote survival, inhibit cell death and scavenge free radicals in photoreceptors and brain neural cells.25 In addition, we described the influence of DHA on expression of genes associated with neurogenesis and both pro- and antiapoptosis in cultured human fetal retina.26 Thus, several viable mechanisms may explain why optimizing the DHA content of retinal membranes may be beneficial in slowing progression in retinal degenerative diseases.
By extrapolation of results comparing mean RBC-DHA and loss of total VFS, a 17% level of RBC-DHA may fully attenuate visual field loss. To attain this level of RBC-DHA, it is estimated that a dose of 45 mg DHA/kg bodyweight/d would be needed. This high DHA dose is within the range of natural nutriture as breastmilk compositions throughout the world provide between 3 and 84 mg/kg/d of DHA dependent upon maternal dietary intake.27–29 In another “at risk” population, intervention studies with preterm infants routinely use DHA levels ranging from 42 to 59 mg/kg/d to improve functional outcome measures.29 Improvements in select outcomes were found in other studies where patients with the neural degenerative disease, Rett syndrome,30 receiving 72.9 mg DHA/kg/d and children with cystic fibrosis receiving 50 mg DHA/kg/d had RBC-DHA elevated from 2.8% to 11%.31 Patients with hypertriglyceridemia are routinely prescribed four 1-g capsules containing 3.36 g eicosapentaenoic acid (EPA) plus DHA/d32 (Omacor/Lovaza), which for average men (88 kg) and women (75 kg)33 equates to 38 and 45 mg n3/kg/d, respectively. Children with necrotizing enterocolitis for whom an intravenous fish oil emulsion was the exclusive source of lipid over many months attained a mean whole blood level of 23.8% EPA+DHA (Brenna JT, unpublished observations, 2015). Thus, it remains uncertain if a maximum ceiling for blood DHA (and/or EPA) has been attained; however, the safety profile of high dose DHA is favorable.13,34 Dietary n3 fatty acids are necessary for optimal structure and function of cell membranes; however, these have been systematically diminished in Western diets.35 The obsession with increasing shelf life for preserved foods has led to unhealthy imbalances in n6-to-n3 ratios; thus marked elevation of n3 fatty acids in the diet by fish consumption or fish oil/DHA supplementation should not be considered pharmaceutical but is nutritionally sound.
Strengths of the DHAX trial include genetic homogeneity, use of a single study site, a long intervention period, RBC-DHA monitoring of compliance, and close adherence to protocol. Limitations include small cohort sizes, variability in measures of field sensitivity, and inconsistent results of outcome measures. Assessment of a modified intent-to-treat cohort showed similar functional results to the Per Protocol cohort, thus drop-outs did not impose an untoward bias.
Supplementation of DHA to trial participants significantly elevated RBC-DHA levels but did not alter disease progression of visual acuity, EZ anatomy, shape discrimination, or fundus appearance associated with XLRP. Supplementation of DHA reduced the elevation in final dark-adapted thresholds and significantly slowed visual field sensitivity loss. From the relationship between RBC-DHA and the rate of field sensitivity loss, we can extrapolate that a level of 17% could minimize the decline in field sensitivity.
Acknowledgments
The authors thank the trial participants and their families for their long-term dedication to completion of the DHAX trial. We thank Joost Felius, PhD (Dallas, TX, USA) for calculation of the interclass correlations. DHASCO and placebo capsules were donated by DSM Nutritional Products (Columbia, MD, USA).
Supported by grants from the Orphan Products Development program of the U.S. Food and Drug Administration (5R01FD002543; DRH) and the Foundation Fighting Blindness (Columbia, MD, USA, C-TX02-0704-0274; DGB). The authors alone are responsible for the content and writing of the paper.
Disclosure: D.R. Hoffman, DSM Nutritional Products (F); D.K. Hughbanks-Wheaton, None; R. Spencer, None; G.E. Fish, None; N.S. Pearson, None; Y.-Z. Wang, None; M. Klein, None; A. Takacs, None; K.G. Locke, None; D.G. Birch, None
References
- 1. Bird AC. . X-linked retinitis pigmentosa. . 1975; 59: 177– 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fishman GA, Farber MD, Derlacki DJ. . X-linked retinitis pigmentosa. Profile of clinical findings. . 1988; 106: 369– 375. [DOI] [PubMed] [Google Scholar]
- 3. Birch DG, Anderson JL, Birch EE. . Early abnormalities of rod function in children with X-linked retinitis pigmentosa. . 1993; 8: 329– 335. [Google Scholar]
- 4. Hoffman DR. . Fatty acids and visual dysfunction. : Chow CK, . New York: Marcel Dekker; 2000: 817– 842. [Google Scholar]
- 5. Hoffman DR, Birch DG. . Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. . 1995; 36: 1009– 1018. [PubMed] [Google Scholar]
- 6. Hoffman DR, DeMar JC, Heird WC, Birch DG, Anderson RE. . Impaired synthesis of DHA in patients with X-linked retinitis pigmentosa. . 2001; 42: 1395– 1401. [PubMed] [Google Scholar]
- 7. Berson EL, Rosner B, Sandberg MA,et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. . 2004; 122: 1297– 1305. [DOI] [PubMed] [Google Scholar]
- 8. Berson EL, Rosner B, Sandberg MA,et al. Further evaluation of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. . 2004; 122: 1306– 1314. [DOI] [PubMed] [Google Scholar]
- 9. Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Willett WC. . ω-3 Intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. . 2012; 130: 707– 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffman DR, Locke KG, Wheaton DH, Fish GE, Spencer R, Birch DG. . A randomized, placebo-controlled clinical trial of docosahexaenoic acid supplementation for X-linked retinitis pigmentosa. . 2004; 137: 704– 718. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman DR, Hughbanks-Wheaton DK, Pearson NS,et al. Four-year placebo-controlled trial of docosahexaenoic acid in X-linked retinitis pigmentosa (DHAX trial): A randomized clinical trial. . 2014; 132: 866– 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wheaton DH, Hoffman DR, Locke KG, Watkins RB, Birch DG. . Biological safety assessment of docosahexaenoic acid supplementation in a randomized clinical trial for X-linked retinitis pigmentosa. . 2003; 121: 1269– 1278. [DOI] [PubMed] [Google Scholar]
- 13. Hughbanks-Wheaton DK, Birch DG, Fish GE,et al. Safety assessment of docosahexaenoic acid in X-linked retinitis pigmentosa: the 4-year DHAX trial. . 2014; 55: 4958– 4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. . Natural course of retinitis pigmentosa over a three year interval. . 1985; 99: 240– 251. [DOI] [PubMed] [Google Scholar]
- 15. Birch DG, Anderson JL, Fish GE. . Yearly rates of rod and cone functional loss in retinitis pigmentosa and cone-rod dystrophy. . 1999; 106: 258– 268. [DOI] [PubMed] [Google Scholar]
- 16. Wang YZ, Morale SE, Cousins R, Birch EE. . Course of development of global hyperacuity over lifespan. . 2009; 86: 695– 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. . Natural course of retinitis pigmentosa over a three-year interval. . 1985; 99: 240– 251. [DOI] [PubMed] [Google Scholar]
- 18. Birch D, Locke KG, Felius J,et al. Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. . 2015; 122: 833– 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. . Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. . 2009; 50: 2328– 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Q, Teo Y-Y, Saw S-M. . Application of advanced statistics in ophthalmology. . 2011; 52: 6059– 6065. [DOI] [PubMed] [Google Scholar]
- 21. Dratz EA, Deese AJ. . The role of docosahexaenoic acid (22:6 ω-3) in biological membranes: examples from photoreceptors and model membrane bilayers. : Simopoulos AP, Kifer RR, Martin RE, . New York: Academy Press; 1986: 319– 351. [Google Scholar]
- 22. Niu SL, Mitchell DC, Lim SY,et al. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. . 2004; 279: 31098– 31104. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell DC, Niu SL, Litman BJ. . Quantifying the differential effects of DHA and DPA on the early events in visual signal transduction. . 2012; 165: 393– 400. [DOI] [PubMed] [Google Scholar]
- 24. Kim H-Y. . Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). . 2000; 275: 35215– 35223. [DOI] [PubMed] [Google Scholar]
- 25. Bazan NG, Molina MF, Gordon WC. . Docosahexaenoic acid signalolipidomics in nutrition: significance in aging neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. . 2011; 31: 321– 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rojas CV, Martinez JI, Hoffman DR, Uauy R. . Gene expression analysis in human fetal retinal explants treated with docosahexaenoic acid. . 2003; 44: 3170– 3177. [DOI] [PubMed] [Google Scholar]
- 27. Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. . Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. . 2007; 85: 1457– 1464. [DOI] [PubMed] [Google Scholar]
- 28. Hoffman DR, Theuer RC, Castañeda YS,et al. Maturation of visual acuity is accelerated in breast-fed term infants fed baby food containing DHA-enriched egg yolk. . 2004; 134: 2307– 2313. [DOI] [PubMed] [Google Scholar]
- 29. Lipillonne A, Groh-Wargo S, Gonzalez CHL, Uauy R. . Lipid needs of preterm infants:updated recommendations. . 2013; 162: S37– S47. [DOI] [PubMed] [Google Scholar]
- 30. DeFelice C, Signorini C, Durand T,et al. Partial rescue of Rett syndrome by ω-3 polyunsaturated fatty acids (PUFAs) oil. . 2012; 7: 447– 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lloyd-Still JD, Powers CA, Hoffman DR,et al. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients; a randomized controlled study. . 2006; 22: 36– 46. [DOI] [PubMed] [Google Scholar]
- 32. Bays H. . Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. . 2006; 98: 71i– 76i. [DOI] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. Body measurements. Available at: http://www.cdc.gov/nchs/fastats/body-measurements.htm. Accessed June 23, 2015.
- 34. Harris WS. . Expert opinion: omega-3 fatty acids and bleeding—cause for concern? . 2007; 99: 44c-. [DOI] [PubMed] [Google Scholar]
- 35. Meyer BJ. . Are we consuming enough long chain omega-3 polyunsaturated fatty acids for optimal health? . 2011; 85: 275– 280. [DOI] [PubMed] [Google Scholar]