Abstract
Background
Acupuncture and electroacupuncture (EA) are widely applied in the treatment of various conditions, including pain. Acupuncture stimulation is applied not only in areas close to pain sites, but also in distal regions or on the contralateral side of the body. Identifying which acupuncture paradigms produce best therapeutic effects is of clinical significance.
Material/Methods
Spared nerve injury (SNI) was applied to establish a rat model of neuropathic pain. We applied 14 sessions of EA (BL 60 and BL 40, 1–2 mA, and 2 Hz, 30 min per session) every other day from days 3 to 29 after surgery on the contralateral or ipsilateral side of pain. von Frey hair was applied to examine mechanical allodynia in the SNI model and analgesic effects of EA. All experimental procedures were approved by the Animal Care and Use Committee of our university, according to the guidelines of the International Association for the Study of Pain.
Results
SNI produced significant and long-lasting mechanical allodynia (p<0.001) in injured paws. Repeated EA on the contralateral side of the pain significantly attenuated mechanical allodynia from 14 days after surgery (p<0.05). By contrast, ipsilateral EA did not show analgesic effects (p>0.05).
Conclusions
These findings indicate that contralateral EA is superior to local EA in some types of pain disorders. Further investigations are needed for a more comprehensive understanding of the central mechanisms of acupuncture.
MeSH Keywords: Acupuncture, Analgesia, Neuropathic Pain, Rats, Spared Nerve Injury
Background
Acupuncture has a long history in China and other Asian countries and is applied in the treatment of various diseases [1–4]. Pain is one of the most intensively investigated models of acupuncture or electroacupuncture (EA). Clinical trials have confirmed acupuncture’s effects in some pain disorders such as chronic shoulder pain, knee osteoarthritis, and chronic low back pain [5,6]. Acupuncture activates/deactivates a variety of pain-relevant brain regions and promotes the release of endogenous opioid peptides to produce analgesia [7]. Acupuncture or EA protocols with different stimulating intensities and current frequencies (for electroacupuncture, EA) produce very different effects through distinct mechanisms. For example, 2 Hz EA promotes release of endorphin and shows better therapeutic effects in treating opioid craving than 100 Hz EA, whereas 100 Hz EA is more powerful for treating withdrawal symptoms [8]. The influence of stimulating sites on acupuncture effects is more controversial. Although early reports show limited differences between different acupuncture points, more recent studies do not support this conclusion [7,9–11]. One extreme example would be comparing effects of acupuncture points local to pain or disease side with contralateral acupuncture points. Our previous work has shown that contralateral acupuncture relieves chronic shoulder pain and improves shoulder mobility and quality of life [12], and that contralateral acupuncture recruits central nuclei such as anterior cingulate cortex (ACC) [9]. Kim et al. reported better therapeutic effects using acupuncture points contralateral to the paralyzed side than ipsilateral points in patients with stroke [10]. Analgesia tests in rats with chronic constriction injury of the sciatic nerve yield similar results: daily transcutaneous electric nerve stimulation reduced the development of mechanical and thermal allodynia, but only when delivered on the contralateral side [11].
However, we need to note that in some studies [10,11], peripheral nerves or the somatosensory tract that convey acupuncture signals are directly damaged by stroke or nerve injuries. By contrast, spared nerve injury (SNI) is a common animal model of neuropathic pain characterized by stable and long-lasting mechanical allodynia [13]. More importantly, the sural nerve remains intact in this model, allowing transmission of acupuncture signals. The present study aimed to compare the analgesic effects of contra- and ipsilateral EA in this model.
Material and Methods
Subjects and grouping
Adult male Sprague-Dawley rats were provided by the Department of Laboratory Animal Sciences, Peking University Health Science Center (Beijing, China). All animals were housed in standard cages with a 12-h alternating light/dark cycle and food and water were available ad libitum. All experimental procedures were approved by the Animal Care and Use Committee of our university, according to the guidelines of the International Association for the Study of Pain.
SNI model
The SNI model was established as previously described [13,14]. After anesthesia with 1% sodium pentobarbital (0.5 ml/100 g, i.p.), the left tibial nerve and common peroneal nerve were tightly ligated and sectioned distal to the ligation, leaving the sural nerve intact. Muscle and skin were closed in 2 layers. In sham-operation controls, we exposed the sciatic nerve and its branches without causing any lesions.
Behavioral test for mechanical allodynia
Mechanical allodynia of bilateral hind paws was tested at 9: 00–12: 00 a.m. before and after surgery at different time points (Figure 1). The 50% paw withdrawal threshold (PWT) in response to a series of von Frey hair (Stoelting, Wood Dale, IL, USA) was examined by the “up and down” method as described previously [14]. The experimenter was blind to animal grouping.
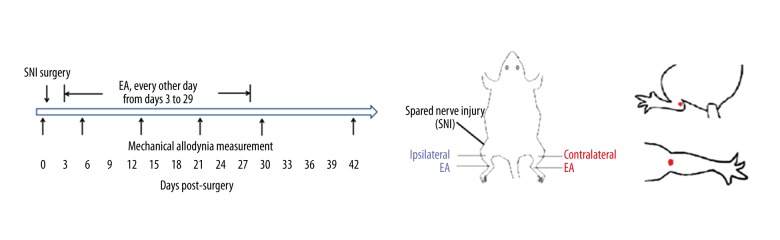
Figure 1.
Flowchart of the study. (A) EA and sham EA were performed at 14: 00–17: 00 p.m. every other day from days 3 to 29 after surgery. Mechanical allodynia of bilateral hind paws was tested at 9: 00–12: 00 a.m. before and after surgery at different time points. (B) Ipsilateral or contralateral EA was applied in BL 40 and BL 60. (C) Localization of acupoints used (red dots). “Kunlun” (BL 60, top) localized in the posterolateral aspect of the ankle, and in the depression between the tip of the external malleolus and Achilles’ tendon. “Weizhong” (BL 40, bottom) localized on the posterior aspect of the knee, and in the midpoint of the transverse crease of the popliteal fossa.
Electroacupuncture
Animals were randomly divided into 3 groups: SNI + contralateral EA (n=10), SNI + ipsilateral EA (n=9), and SNI + sham EA (n=10). EA and sham EA were performed bat 14: 00–17: 00 p.m. every other day from days 3 to 29 after surgery (Figure 1A, 1B).
For EA application, animals were placed into a plastic tube with their legs protruding out. Two commonly used acupoints – “Kunlun” (BL 60, on the posterolateral aspect of the ankle, in the depression between the tip of the external malleolus and Achilles’ tendon) and “Weizhong” (BL 40, on the posterior aspect of the knee, midpoint of the transverse crease of the popliteal fossa) (Figure 1C) – were stimulated with square waves of 0.2 ms in pulse width and 2 Hz in frequency from a Han’s Acupoint Nerve Stimulator (HANS, LH series, manufactured in our university) [9,15]. Their intensities were increased in a stepwise manner at 1.0–1.5–2.0 mA, each lasting 10 min.
For sham EA, animals entered the tube and needles were attached to the skin but not inserted or electrically stimulated. Since we did not observe differences between ipsilateral or contralateral sham EA, they were pooled into the sham EA group.
Statistics
Data are presented as means ±SEM. One- and two-way ANOVA with Bonferroni post hoc tests were used for the comparison between groups. The differences were calculated with SPSS and statistical significance was defined as p<0.05.
Results
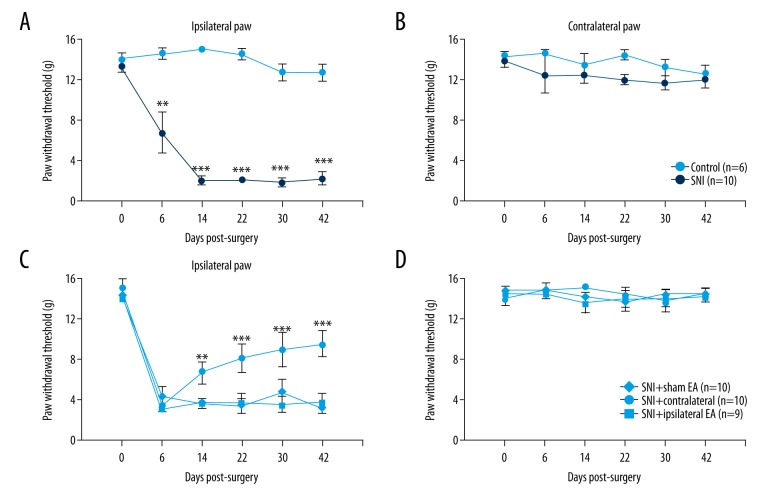
SNI rats exhibited mechanical allodynia after nerve injury (group: F1,96=293.22, p<0.001; time point: F5,96=13.89, p<0.001; interaction: F5,96=11.71, p<0.001. Figure 2A). Mechanical thresholds of contralateral hind paws remained unchanged throughout the procedure (group: F1,96=1.35, p>0.05; time point: F5,96=1.07, p>0.05; interaction: F5,96=0.57, p>0.05, two-way ANOVA with Bonferroni post hoc test. Figure 2B).
Figure 2.
Repeated contralateral but not ipsilateral EA attenuated mechanical allodynia in SNI. SNI produced significant mechanical allodynia in the injured side (A) but not in the contralateral (B) side. (C) This analgesic effect was not transient, in that even 12 days after the last EA treatment, the relief of mechanical allodynia was still maintained. (D) Contralateral paw withdrawal threshold was not affected. ** p<0.01, *** p<0.001, compared with sham EA and ipsilateral EA groups, two-way ANOVA with Bonferroni post hoc test.
From 3 days after model establishment, EA in the contralateral side of SNI significantly attenuated mechanical allodynia (group: F2,162=19.04, p<0.001; testing day: F5,162=46.84, p<0.001; interaction: F10,162=2.17, p<0.05. Figure 2C). This effect lasted even 12 days after EA had terminated. By contrast, ipsilateral EA did not show obvious effects. This effect was also confirmed by calculating the area under curves (AUC) of the 3 groups (F2,28=8.03, p<0.01). Contralateral paw withdrawal threshold was not affected (Figure 2D).
Discussion
Neuropathic pain is characterized by spontaneous pain and sensory allodynia, and has complex mechanisms, including both peripheral and central sensitization. Chronic neuropathic pain is difficult to treat, at least partially due to the structural and functional reorganization of the central nervous system [16]. Somatosensory stimulation, including acupuncture, could thus act as an additional input to re-arrange the neural loop. A prerequisite of this mechanism would be integration of nociceptive and acupuncture signals at spinal and supraspinal levels. Obviously, this could not be achieved in animals receiving acupuncture in the same side with extensive lesions of peripheral nerves such as chronic constriction injury (CCI) or spinal nerve ligation (SNL). The SNI model, by contrast, involves an intact sural nerve and overcomes this issue. The sural nerve innervates the lateral side of the hindlimb, where significant and persistent mechanical allodynia occur after SNI surgery [13,14]. The present study shows that in this model, contralateral but not ipsilateral acupuncture produces clear analgesia. We need to note, however, that the sural nerve is indeed not completely “intact” in SNI. The uninjured sural nerve re-innervates the epidermis of injured fibers, and evoked activities in these uninjured afferents may induce evoked pain through sensitized spinal neurons [17]. This could explain the ineffectiveness of ipsilateral acupuncture in the present study. Previous work has shown that repeated acupuncture normalizes connectivity in brain regions dysregulated in chronic pain [18]. Obviously, such “reorganization” is slow and accumulative, but is stable and more clinically significant. Unlike other animal pain models such as inflammatory pain, CCI, or SNL, whose pain behaviors gradually recover over a period of weeks, mechanical allodynia in the SNI model stably persists for more than 6 months [13]. Consistently, we found that apparent EA effects are not achieved until 2 weeks after model establishment (after 6 sessions of EA treatment) but are long-lasting even after treatment termination. We need to note that this persistent but late-onset pain-relieving effect does not exclude an immediate analgesic effect, which is commonly observed in EA research. In the present study, we examined mechanical allodynia in a relatively sparse frequency to avoid sensitization to repeated noxious stimulation.
In Chinese medicine, the choice of appropriate acupuncture protocols is crucial in acupuncture treatment. Intensities, frequencies, duration, and sites of acupuncture or EA all affect analgesic effects [7–11]. Differential neurochemical responses of low- versus high-frequency EA have been well established [7,8]. In the present study, the choice of 2 Hz EA was based on previous work showing its superior effect in relieving mechanical allodynia in neuropathic pain compared to 100 Hz [19,20]. The intensity applied in the present study (1.0–1.5–2.0 mA) is slightly lower than that necessary to induce analgesia in normal animals (3.0 mA) [15] but is sufficient to relieve pain in rats with neuropathic pain. The highest level of EA intensity without causing discomfort is approximately 3-fold higher in humans than in rats [15,21], consistent with the fact that we did not observe stress-like behaviors in animals in the present study.
How different stimulating sites affect acupuncture effects is controversial. Although early reports of limited differences between different acupuncture points are consistent with the opioid-based neurochemical basis of acupuncture analgesia [4], these studies were mostly performed in normal animals and stimulation of different acupuncture points definitely recruits distinct neural circuits. Neuroimaging studies reveal distinct brain responses to acupuncture stimulation at different points [21–24]. Clinical trials of acupuncture frequently choose local acupuncture points with or without the aid of distal points. Goldman et al. showed that local acupuncture produces analgesic effects through inhibiting nociceptors by activation of adenosine A1 receptors [25]. Obviously, this mechanism could not account for the effect of contralateral acupuncture, where pain and acupuncture sites are far away from each other.
How does contralateral acupuncture produce analgesia? We propose several mechanisms. The well-established neurochemical changes upon EA (e.g., release of endorphin by 2 Hz EA) [7] remains a possible mechanism of contralateral EA. In addition, Zhu et al. [26] showed that spinal transection blocks analgesic effects of contralateral but not ipsilateral acupuncture, indicating the involvement of brain mechanisms in contralateral acupuncture. It is accepted that EA significantly promotes neurogenesis in the hippocampus [27], which promotes the recovery from chronic pain [28]. More specifically, somatosensory information from 2 sides of the body integrate at various spinal and supraspinal levels. The anterior cingulate cortex (ACC) is one of the candidate regions. ACC neurons have large bilateral receptive fields for somatosensory stimulation that often include the whole body [29], which implies that contralateral acupuncture may competitively inhibit nociceptive signals. In addition, as a core region affected by pain, ACC manipulations such as meditation, placebo, and cognitive training relieve pain, especially chronic pain [30]. Finally, ACC innervates the descending inhibitory system, whose lesions abolish acupuncture analgesia [31]. The first evidence supporting a role of ACC in contralateral acupuncture comes from our unexpected finding that contralateral EA is more effective than ipsilateral EA in relieving formalin-induced paw-licking behaviors [9], which are mediated by forebrain mechanisms [32]. In this study, we applied EA on the ipsilateral or contralateral side of rats with inflammatory pain and observed distinct anti-nociceptive effects and mechanisms between them [9]. Both ipsilateral and contralateral EA relieve pain through spinal mechanisms, whereas contralateral EA has an additional supraspinal mechanism. Lesioning ACC completely abolished analgesic effects of contralateral but not ipsilateral EA [9].
Conclusions
In summary, our findings indicate that contralateral EA is superior to local EA in some types of pain disorders. Further investigations are needed for a more comprehensive understanding of the central mechanisms of acupuncture.
Footnotes
Conflict of interests
None.
Source of support: This work was funded by the National Natural Science Foundation of China (81603446), the Beijing Municipal Natural Science Foundation (7174363), and the Beijing Municipal Science and Technology Project (Z141107002514022)
References
- 1.NIH Consensus Conference: Acupuncture. JAMA. 1998;280(17):1518–24. [PubMed] [Google Scholar]
- 2.Schnyer R, Lao L, Hammerschlag R, et al. Society for Acupuncture Research: 2007 conference report: “The status and future of acupuncture research: 10 years post-NIH Consensus Conference”. J Altern Complement Med. 2008;14(7):859–60. doi: 10.1089/acm.2008.SAR-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han JS, Ho YS. Global trends and performances of acupuncture research. Neurosci Biobehav Rev. 2011;35(3):680–87. doi: 10.1016/j.neubiorev.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Han JS. Acupuncture analgesia: Areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41–48. doi: 10.1016/j.pain.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Molsberger AF, Schneider T, Gotthardt H, et al. German Randomized Acupuncture Trial for chronic shoulder pain (GRASP) – a pragmatic, controlled, patient-blinded, multi-centre trial in an outpatient care environment. Pain. 2010;151(1):146–54. doi: 10.1016/j.pain.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Witt C, Brinkhaus B, Jena S, et al. Acupuncture in patients with osteoarthritis of the knee: A randomised trial. Lancet. 2005;366(9480):136–43. doi: 10.1016/S0140-6736(05)66871-7. [DOI] [PubMed] [Google Scholar]
- 7.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 8.Cui CL, Wu LZ, Luo F. Acupuncture for the treatment of drug addiction. Neurochem Res. 2008;33(10):2013–22. doi: 10.1007/s11064-008-9784-8. [DOI] [PubMed] [Google Scholar]
- 9.Yi M, Zhang H, Lao L, et al. Anterior cingulate cortex is crucial for contra- but not ipsi-lateral electro-acupuncture in the formalin-induced inflammatory pain model of rats. Mol Pain. 2011;7:6. doi: 10.1186/1744-8069-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MK, Choi TY, Lee MS, et al. Contralateral acupuncture versus ipsilateral acupuncture in the rehabilitation of post-stroke hemiplegic patients: A systematic review. BMC Complement Altern Med. 2010;10:41. doi: 10.1186/1472-6882-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: The effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Phys Ther. 2006;86(5):698–709. [PubMed] [Google Scholar]
- 12.Zhang H, Sun J, Wang C, et al. Randomised controlled trial of contralateral manual acupuncture for the relief of chronic shoulder pain. Acupunct Med. 2016;34(3):164–70. doi: 10.1136/acupmed-2015-010947. [DOI] [PubMed] [Google Scholar]
- 13.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87(2):149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Liu J, Zhou MM, et al. Anxiolytic effects of hippocampal neurosteroids in normal and neuropathic rats with spared nerve injury. J Neurochem. 2017;141(1):137–50. doi: 10.1111/jnc.13965. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Chen X, Zhang C, et al. Comparison of electroacupuncture in restrained and unrestrained rat models. Evid Based Complement Alternat Med. 2013;2013:404956. doi: 10.1155/2013/404956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuo M. Neuronal mechanism for neuropathic pain. Mol Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringkamp M, Meyer RA. Injured versus uninjured afferents: Who is to blame for neuropathic pain? Anesthesiology. 2005;103(2):221–23. doi: 10.1097/00000542-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin. 2015;9:430–35. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208(2):323–32. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Li HT, Shi YS, et al. Ketamine potentiates the effect of electroacupuncture on mechanical allodynia in a rat model ofneuropathic pain. Neurosci Lett. 2004;368(3):327–31. doi: 10.1016/j.neulet.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WT, Jin Z, Luo F, et al. Evidence from brain imaging with fMRI supporting functional specificity of acupoints in humans. Neurosci Lett. 2004;354(1):50–53. doi: 10.1016/j.neulet.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Bai L, Zhang W, et al. Investigation of acupoint specificity by whole brain functional connectivity analysis from fMRI data. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2784–87. doi: 10.1109/IEMBS.2011.6090762. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Bai L, Zhang W, et al. Investigation of acupoint specificity by multivariate granger causality analysis from functional MRI data. J Magn Reson Imaging. 2011;34(1):31–42. doi: 10.1002/jmri.22585. [DOI] [PubMed] [Google Scholar]
- 24.Claunch JD, Chan ST, Nixon EE, et al. Commonality and specificity of acupuncture action at three acupoints as evidenced by FMRI. Am J Chin Med. 2012;40(4):695–712. doi: 10.1142/S0192415X12500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman N, Chen M, Fujita T, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13(7):883–88. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu B, Xu WD, Rong PJ, et al. A C-fiber reflex inhibition induced by electroacupuncture with differenIntensis appied at homotopic and heterotopic acupoints in rats selectively destructive effects on myelinated and unmyelinated afferent fibers. Brain Res. 2004;1011(5):228–37. doi: 10.1016/j.brainres.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Fan XW, Liu HH, Wang HB, et al. Electroacupuncture improves cognitive function and hippocampal neurogenesis after brain irradiation. Radiat Res. 2017;187(6):672–81. doi: 10.1667/RR14561.1. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J, Jiang YY, Xu LC, et al. Adult hippocampal neurogenesis along the dorsoventral axis contributes differentially to environmental enrichment combined with voluntary exercise in alleviating chronic inflammatory pain in mice. J Neurosci. 2017;37(15):4145–57. doi: 10.1523/JNEUROSCI.3333-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68(5):1720–32. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- 30.Bingel U, Lorenz J, Schoell E, et al. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1–2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Pertovaara A. Plasticity in descending pain modulatory systems. Prog Brain Res. 2000;129:231–42. doi: 10.1016/S0079-6123(00)29017-1. [DOI] [PubMed] [Google Scholar]
- 32.Porro CA, Cavazzuti M, Lui F, et al. Independent time courses of supraspinal nociceptive activity and spinally mediated behavior during tonic pain. Pain. 2003;104(1–2):291–301. doi: 10.1016/s0304-3959(03)00015-0. [DOI] [PubMed] [Google Scholar]