Abstract
A review was undertaken of studies reporting increased DNA damage in circulating blood cells and increased organ doses, for X-ray exposures enhanced by iodinated contrast media (ICM), compared to unenhanced imaging. This effect may be due to ICM molecules acting as a source of secondary radiation (Auger/photoelectrons, fluorescence X-rays) following absorption of primary X-ray photons. It is unclear if the reported increase in DNA damage to blood cells necessarily implies an increased risk of developing cancer. Upon ICM-enhancement, the attenuation properties of blood differ substantially from surrounding tissues. Increased energy deposition is likely to occur within very close proximity to ICM molecules (within a few tens of micrometres). Consequently, in many situations, damage and dose enhancement may be restricted to the blood and vessel wall only. Increased cancer risks may be possible, in cases where ICM molecules are given sufficient time to reach the capillary network and interstitial fluid at the time of exposure. In all situations, the extrapolation of blood cell damage to other tissues requires caution where contrast media are involved. Future research is needed to determine the impact of ICM on dose to cells outside the blood itself and vessel walls, and to determine the concentration of ICM in blood vessels and interstitial fluid at the time of exposure.
Introduction
Intravenous iodinated contrast media (ICM) play a central role in X-ray imaging procedures, including CT and fluoroscopy. The chemotoxic effects of ICM, including allergic reactions, kidney damage and thyrotoxicosis, are well known, although serious adverse effects are extremely rare.1 There are also concerns, however, that contrast media may also increase the cancer risks from X-ray exposures, by increasing the DNA damage from a given radiation exposure.2,3 Since the late 1970s, studies have demonstrated increased damage, by up to several hundred percent, to blood cells following the administration of intravenous ICM during diagnostic X-ray and interventional fluoroscopy exposures, compared with unenhanced exposures.4–18 The results of other studies in which larger-than-expected levels of DNA damage were reported19,20 could, potentially, also be explained by the effect of ICM use. Other authors have reported increases in estimated organ doses of up to 71%, for ICM-enhanced, compared with unenhanced CT scans.21,22 It remains unclear what the consequences of these findings are, however. Most notably, it is unclear if the reported increase in DNA damage to blood cells necessarily implies an increased risk of cancer for ICM-enhanced exposures. This uncertainty adds to that already present regarding the risks from low dose medical radiation exposures, including risks derived from ongoing epidemiological studies.23–25
To address this issue, a review was undertaken of previously published research investigating the impact of contrast media on cell damage and radiation doses in diagnostic imaging. Relevant papers were identified through a search using PubMed. Studies investigating contrast enhanced radiotherapy and the dosimetric effects of high atomic number elements in general were also gathered. Summaries of study findings are presented in Tables 1 and 2. A meta-analysis of these data was not possible, as parameters such as iodine concentration, time-since-exposure and X-ray energy spectra varied between studies.
Table 1.
Summary of in vitro studies of DNA damage indicators for blood samples exposed with, or without iodinated contrast media (ICM)
| Study (year of publication) | Assay type | Dose (mGy) | Time since exposure | Equipment | Quoted tube potential/filtration | Iodine concentration (mg I ml–1) | Foci per cell (no ICM) | Foci per cell (with ICM) | ICM associated increase in absolute foci (%) | ICM associated increase in excess foci (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Callisen (1979) | Lymphocyte survival | Unclear | Faxitron | 70 kV/4.2 mm Al | 3.7c | 30 | ||||
| 7.4c | 70 | |||||||||
| 18.5c | 270 | |||||||||
| Hadnegy (1982) | Lymphocyte dicentrics & rings | 500 | Not stated | 250 kV/1 mm Al, 1 mm Cu | 5.6c (Urografin) | 0.064 | ||||
| 500 | 11.1c (Urografin) | 0.073 | ||||||||
| 500 | 22.2c (Urografin) | 0.119 | ||||||||
| 560 | 19.2c (Hexabrix) | 0.135 | ||||||||
| 940 | 19.2c (Hexabrix) | 0.314 | ||||||||
| 1250 | 19.2c (Hexabrix) | 0.465 | ||||||||
| 1880 | 19.2c (Hexabrix) | 1.024 | ||||||||
| Jost (2009) | Lymphocyte dicentrics | 0 | Siemens Sensation 64 (CT) | n/a | 5.0 | 0.0003 | 0 | |||
| 25 | 120 kV | 5.0 | 0.0011 | 0.0014 | 27 | |||||
| 50 | 120 kV | 5.0 | 0.0021 | 0.0025 | 19 | |||||
| 100 | 120 kV | 5.0 | 0.0042 | 0.0059 | 40 | |||||
| 1000 | 120 kV | 5.0 | 0.0920 | 0.1603 | 74 | |||||
| 0 | n/a | 50.0 | 0.0003 | 0 | ||||||
| 25 | 120 kV | 50.0 | 0.0011 | 0.0082 | 645 | |||||
| 50 | 120 kV | 50.0 | 0.0021 | 0.0213 | 914 | |||||
| 100 | 120 kV | 50.0 | 0.0042 | 0.0444 | 957 | |||||
| 1000 | 120 kV | 50.0 | 0.0920 | 0.9094 | 888 | |||||
| Jost (2009) | Lymphocyte γ-H2AX56 | 25 | Siemens Sensation 64 (CT) | 120 kV | 5.0 | 0.580 | 0.952 | 64 | ||
| 50 | 120 kV | 5.0 | 0.911 | 0.726 | −20 | |||||
| 100 | 120 kV | 5.0 | 1.503 | 1.547 | 3 | |||||
| 1000 | 120 kV | 5.0 | 4.697 | 6.351 | 35 | |||||
| 25 | 120 kV | 50.0 | 0.580 | 0.892 | 54 | |||||
| 50 | 120 kV | 50.0 | 0.911 | 2.232 | 145 | |||||
| 100 | 120 kV | 50.0 | 1.503 | 3.983 | 165 | |||||
| 1000 | 120 kV | 50.0 | 4.697 | 11.709 | 149 | |||||
| Grudzenski (2009) | Lymphocyte γ-H2AX | 10 | 5 min | Philips PW2184 (radiography tube) | 90 kV/1 mm Al | 33.0 (Iopromide) | 0.217a,d | 0.359a,d | 66 | |
| 30 m | 0.133a,d | 0.266a,d | 100 | |||||||
| 2.5 h | 0.080a,d | 0.190a,d | 138 | |||||||
| 5 h | 0.032a,d | 0.109ad | 241 | |||||||
| 500 | 5 min | Philips PW2184 (radiography tube) | 90 kV/1 mm Al | 33.0 (Iopromide) | 9.27a,d | 15.00a,d | 62 | |||
| 30 min | 5.51a,d | 9.90a,d | 80 | |||||||
| 2.5 h | 3.20 a,d | 6.58a,d | 106 | |||||||
| 5 h | 1.56a,d | 3.29a,d | 111 | |||||||
| 500 | 5 min | 662 keV γ source (Cs-137) | n/a | 33.0 (Iopromide) | 8.34 a,d | 7.62ad | 10 | |||
| 30 min | 5.77ad | 5.51a,d | 5 | |||||||
| 2.5 h | 4.08a,d | 4.52ad | −10 | |||||||
| 5 h | 2.00a,d | 1.92a,d | 4 | |||||||
| Grudzenski (2009) | Fibroblast γ-H2AX | 1500 | 30 min | Philips PW2184 (radiography tube) | 90 kV/1 mm Al | 33.0 (Iopromide) | 26.7a,d | 36.5a,d | 37 | |
| 2.5 h | 10.6a,d | 18.6a,d | 75 | |||||||
| 5 h | 5.9a,d | 8.4a,d | 42 | |||||||
| Fibroblast γ-H2AX | 1500 | 30 min | 90 kV/1 mm Al | 33.0 (Iomeprol) | 26.7a,d | 37.2a,d | 39 | |||
| 2.5 h | 10.6a,d | 16.9a,d | 59 | |||||||
| 5 h | 5.9a,d | 8.4a,d | 42 | |||||||
| Fibroblast 53bp1 | 1500 | 30 min | 90 kV/1 mm Al | 33.0 (Iopromide) | 26.5a,d | 34.8a,d | 31 | |||
| 2.5 h | 10.9a,d | 17.2a,d | 58 | |||||||
| 5 h | 5.9a,d | 9.5a,d | 61 | |||||||
| Fibroblast 53bp1 | 1500 | 30 min | 90 kV/1 mm Al | 33.0 (Iomeprol) | 26.5a,d | 35.9a,d | 36 | |||
| 2.5 h | 10.9a,d | 17.2a,d | 58 | |||||||
| 5 h | 5.9a,d | 10.2a,d | 73 | |||||||
| Pathe (2011) | Lymphocyte γ-H2AX | 0 | Immediate | n/a | n/a | 37.0c (Iopromide 370) | 0.18 | 0.12 | −33 | |
| 30 min | 0.05 | 0.10 | 100 | |||||||
| 1 h | 0.04 | 0.11 | 175 | |||||||
| 2 h | 0.05 | 0.10 | 100 | |||||||
| 24 h | 0.08 | 0.03 | −63 | |||||||
| 20 | Immediate | Siemens Multix M (radiography) | 102 kV | 0.55 | 1.29 | 135 | 216 | |||
| 30 min | 0.63 | 1.23 | 95 | 95 | ||||||
| 1 h | 0.51 | 0.9 | 76 | 68 | ||||||
| 2 h | 0.53 | 0.81 | 53 | 48 | ||||||
| 24 h | 0.28 | 0.3 | 7 | 35 | ||||||
| 100 | Immediate | 102 kV | 1.57 | 2.98 | 90 | 106 | ||||
| 30 min | 1.92 | 3.18 | 66 | 65 | ||||||
| 1 h | 1.50 | 2.39 | 59 | 56 | ||||||
| 2 h | 1.05 | 2.11 | 101 | 101 | ||||||
| 24 h | 0.42 | 0.36 | −14 | −3 | ||||||
| 200 | Immediate | 102 kV | 3.73 | 4.31 | 16 | 18 | ||||
| 30 min | 3.24 | 4.79 | 48 | 47 | ||||||
| 1 h | 2.38 | 3.38 | 42 | 40 | ||||||
| 2 h | 2.37 | 2.59 | 9 | 7 | ||||||
| 24 h | 0.52 | 0.36 | −31 | −25 | ||||||
| 1000 | Immediate | 102 kV | 6.10 | 9.09 | 49 | 52 | ||||
| 30 min | 6.32 | 8.49 | 34 | 34 | ||||||
| 1 h | 4.65 | 5.94 | 28 | 26 | ||||||
| 2 h | 4.56 | 5.01 | 10 | 9 | ||||||
| 24 h | 1.26 | 1.99 | 58 | 66 | ||||||
| Beels (2012) | Lymphocyte γ-H2AX | 5 | Philips MG420 generator, MCN420 tube | 100 kV/2 mm Al | 5.0 | 0.73d | 0.71d | −4d | ||
| 10.0 | 0.73d | 0.80d | 9d | |||||||
| 20.0 | 0.73d | 0.70d | −5d | |||||||
| 10 | 5.0 | 0.96d | 1.13d | 16d | ||||||
| 10.0 | 0.96d | 0.89d | −8d | |||||||
| 20.0 | 0.96d | 0.89d | −8d | |||||||
| 50 | 5.0 | 1.41d | 1.51d | 6d | ||||||
| 10.0 | 1.41d | 1.55d | 9d | |||||||
| 20.0 | 1.41d | 1.51d | 6d | |||||||
| Deinzer (2014) | Lymphocyte γ-H2AX | 20 | Siemens Axiom Multix MT | 102 kV | 7.5 | 0.240 | 0.313b | 30 | ||
| 15.0 | 0.240 | 0.379b | 58 | |||||||
| 500 | 7.5 | 3.581 | 3.956b | 10 | ||||||
| 15.0 | 3.581 | 4.926b | 38 | |||||||
| Gould (2015) | Lymphocyte γ-H2AX | 70 | Carestream DRX-Evolution (radiography) | 120 kV | 15.0 | 44 | ||||
| 17.5 | 82 | |||||||||
| 30.0 | 89 | |||||||||
| 35.0 | 111 | |||||||||
| 45.0 | 89 | |||||||||
| 52.5 | 99 | |||||||||
| Gould (2015) | Lymphocyte γ-H2AX | 140 | Carestream DRX-Evolution (radiography) | 120 kV | 15.0 | 42 | ||||
| 17.5 | 50 | |||||||||
| 30.0 | 75 | |||||||||
| 35.0 | 64 | |||||||||
| 45.0 | 86 | |||||||||
| 52.5 | 76 | |||||||||
| 250 | 120 kV | 15.0 | 14 | |||||||
| 17.5 | 31 | |||||||||
| 30.0 | 35 | |||||||||
| 35.0 | 35 | |||||||||
| 45.0 | 49 | |||||||||
| 52.5 | 49 | |||||||||
| 450 | 120 kV | 15.0 | 11 | |||||||
| 17.5 | 36 | |||||||||
| 30.0 | 25 | |||||||||
| 35.0 | 48 | |||||||||
| 45.0 | 33 | |||||||||
| 52.5 | 59 |
Foci numbers in excess of baseline levels.
Mean of 7 contrast media products.
Calculated by current authors based on data quoted in original paper.
Data not quoted numerically. Read from figure(s).
Table 2.
Summary of in vivo studies assessing DNA damage foci after clinical X-ray exposures enhanced or unenhanced by iodinated contrast media
| Study (year of publication) | Sample size—enhanced/unenhanced | Assay type | Doseenhanced/unenhanced | Dose type | Time since exposure | Equipment | Tube potential | Iodine administered | Foci per cell (no ICM) | Foci per cell (with ICM) | ICM associated increase in absolute foci (%) | ICM associated increase in excess foci (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grudzenski (2009) | 14/13 | Lymphocyte γ-H2AX | 480/470 mGy*cm | DLP | Philips MX8000 (CT) | 120 kV | 34.9 g | ≈30b | |||||
| 5/5 | 371/287 mGy*cm | DLP | Siemens Somatom Sensation 10 or 64 (CT) | 120 kV | 28.8 g | ≈30 | |||||||
| Pathe (2011) | 15/15 | Lymphocyte γ-H2AX | 392/336 mGy*cm | DLP | Immediate | Siemens Sensation 64 (CT) | 120 kV | 37–44.4 g Iopromid 370 | 0.12a | 0.19a | 58 | ||
| 1 h | 0.07a | 0.11a | 57 | ||||||||||
| 2 h | 0.04a | 0.06a | 50 | ||||||||||
| 24 h | 0.01a | 0.01a | 0 | ||||||||||
| Gould (2015) | 57/12 | Lymphocyte γ-H2AX | 116 mGy | AK | Philips Allura Xper (fluoroscopy) | 19 mg I ml–1 (Iomeron 350) | 0.48 | 0.9 | 88d | ||||
| 0.7 | 0.86 | 23d,e | |||||||||||
| Piechowiak (2015) | 179/66 | Lymphocyte γ-H2AX | 301/342 mGy*cm | DLP | Siemens Sensation 64 (CT) | 120 kV | 18.6 g Ultravist 300 | 0.100 | 0.128 | 28 | 107 | ||
| 0.100 | 0.128 | 60 | 267c | ||||||||||
| Wang | 48/22 | Lymphocyte γ-H2AX | 294/276 | DLP | Siemens Sensation 64 (CT) | 100–120 kV | 33 g (Ultravist 370) | 0.700 | 0.945 | 35 | 38 |
AK, air kerma; DLP, dose length product.
Foci numbers in excess of baseline levels.
Normalized using in vitro response.
Foci numbers normalized by dose.
With ANCOVA test to adjust for dose.
Calculated by current authors based on data quoted in original paper.
Impact of ICM on radiation doses
Most dosimetric evaluations of ICM in diagnostic imaging have focused on absorbed dose to the blood. Blood doses, where estimated,14,26 appear to be in the range 1–10 mGy, although could potentially be much higher for interventional fluoroscopy procedures. The blood dose enhancement factor (DEF) following contrast administration, at a particular photon energy (E), can be calculated from the ratio of the mass energy absorption coefficients of iodine (μen/ρ)I and blood (μen/ρ)blood:6
The variable r is the mass fraction of iodine in the blood.6 The overall DEF for a spectrum of photon energies, each with a relative intensity N(E), is given by11
The upper and lower limits of integration in the above equation are principally defined by the tube potential and filtration, respectively.
Callisen et al6 used the above methodology to estimate the DEF to blood for eight paediatric cardiac catheterization procedures, utilizing a Siemens biplane fluoroscopy unit (half value layer, HVL, ~2.8 mm Al). These enhancement factors ranged from 1.7 to 3.0 (mean = 2.3) depending on exposure factors. DEF estimates were also compared to lymphocyte survival following in vitro exposures of blood samples combined with varying concentrations of Renografin® contrast media. Good agreement was reported between the two methodologies for Renografin® concentrations of 1% and 2%, while at 5%, the “empirical” DEF (i.e. based on lymphocyte survival) was higher, at 3.7, than the equivalent “physical” DEF based on the methodology outlined above, of 2.9. A similar approach was taken by Hadnegy et al9 who calculated physical DEFs of 1.25, 1.50 and 2.00 for Urografin® concentrations of 1%, 3% and 6%, respectively, based on a mono-energetic 100 keV X-ray beam. More recently, Jost et al11 calculated the DEF for two concentrations of ICM, using the beam spectrum of a Siemens Sensation 64 CT scanner (120 kV, HVL = 9.1 mm Al equivalent).27 These were then compared to observed levels of ICM-related damage determined from dicentrics and γ-H2AX foci. The agreement between the physical DEF (1.56), dicentric yield (increased by a factor of 1.74) and γH2AX yield (increased by a factor of 1.35) was reasonable for 5 mg ml−1 iodine concentrations, at 1 Gy. At 50 mg I ml–1, the physical DEF of 6.3 was much higher than the increase in γH2AX foci (2.3) but 50% lower than the increase in dicentrics (9.5).
Little research investigating the overall impact of ICM on patient tissues other than the blood has been published. Amato et al28 developed a methodology for estimating the increase in organ dose based on differences in mean Hounsfield unit (HU) between enhanced and non-enhanced CT scans. Using this methodology, they reported an increase in dose to the liver, kidneys, spleen/pancreas and thyroid of 19%, 71%, 33% and 41%, respectively.21 In a further study, He et al29 constructed a simple computational phantom model consisting of a 28 cm diameter sphere of water within which were placed several "contrast spheres" of various diameters (0.5, 4 and 16 cm) containing varying concentrations of iodine (1, 10 and 100 mg ml−1). X-ray exposures were simulated using the Monte Carlo code MCNP530 for mono-energetic beams. While absorbed doses to the contrast spheres were increased by a factor of 1.63 to 2.38 (60 keV photon energy, 10 mg ml−1 iodine concentration) depending on sphere size, the overall impact on mean dose to the whole phantom was less than 1%. This appears to be due to the increase in dose to the contrast spheres being balanced out by a corresponding reduction in energy imparted to unenhanced structures located downstream. The increase in dose to the contrast spheres was reasonably similar to the DEFs for blood dose described above. In a more recent study, Sahbaee et al22,31 used a patient-specific pharmacokinetic modelling approach to estimate iodine concentrations within abdominal organs as a function of time since ICM injection. These figures were incorporated into Monte Carlo simulations to derive estimates of ICM-associated increases in organ doses of 53, 30, 35, 54, 27, 18, 17 and 24%, respectively, for the heart, spleen, liver, kidneys, stomach, colon, small intestine and pancreas.
The above dose estimations are essentially macroscopic in nature, i.e. calculating the mean doses to whole organs or phantoms. The microscopic pattern of absorbed dose distribution within organs was not explicitly assessed. Yet this is a critical issue in determining the potential for increased damage to cells outside of blood vessels, and thus the potential for increased cancer risks.32 Due to the inhomogeneous distribution of cells prone to malignant transformation on a microscopic scale, an increase in the mean dose to an organ does not necessarily imply an increased number of traversals, nor dose to, these vulnerable cells. In an attempt to address this issue, the above mentioned study by Sahbaee et al22 utilized a simplified microdosimetric model to estimate the proportion of dose deposited outside of blood vessels. This figure, calculated to be 51%, was used to estimate a “biologically relevant” ICM-associated dose increase of 0–18% for the liver and 27% for the kidneys.
Impact on exposures
As contrast media increase beam attenuation, the possibility of compensatory increases in X-ray output must also be considered. For CT, in which exposure is typically set from the unenhanced “scout” image prior to the scan, there is mixed evidence of variation in dose length product (DLP) or CT dose index (CTDI) between ICM-enhanced and unenhanced scans. Piechowiak et al17 reported a significantly lower mean DLP and volumetric CTDI figures for 179 ICM-enhanced CT chest scans (301 mGy*cm and 8.1 mGy, respectively) compared to 66 unenhanced equivalent scans (342 mGy*cm and 9.4 mGy) (p = 0.02). Pathe et al reported slightly higher mean DLP for 15 enhanced (392 mGy*cm) vs 15 non-enhanced (336 mGy*cm) abdominal scans (p > 0.05). Grudzenski et al12 reported a mean DLP of 480 mGy*cm for 12 ICM-enhanced chest scans and one chest/abdomen scan, vs 470 mGy*cm for 14 unenhanced chest scans. The same authors reported a larger difference using a different scanner, of 371 mGy*cm vs 287 mGy*cm for 5 enhanced and 5 unenhanced scans, respectively, despite patients in each group being matched for age. The small sample sizes of these studies (particularly the latter two) prevent the drawing of firm conclusions.
As real-time automatic exposure control is always used in fluoroscopic imaging, an increase in patient attenuation due to contrast media would be expected to result in a compensatory increase in output. No studies investigating this were found in our search, however.
Impact of ICM on cell damage
Findings of increased cell damage following X-ray exposures in the presence of iodine, compared to unenhanced exposures, began to appear in the late 1970s and early 1980s.4–10 There has been a resurgence in interest in recent years,11–18 partly owing to improved techniques for detecting cell damage, such as γH2AX assays. Readers are directed to previous reviews33 for a description of these techniques. Both in vitro and in vivo methodologies have been used. In the former, blood samples from one or more volunteers are mixed with various concentrations of contrast media and exposed to an estimated dose of radiation. These concentrations are typically in the range 0–50 mg of iodine per ml of blood and designed to represent “clinically relevant” concentrations. While it is possible that the upper limit of this range may be reachable in trans-catheter bolus injections, in most cases clinical concentrations are less than 10 mg I ml–1.22 This issue is addressed further in the Discussion section of this paper. In vivo studies involve obtaining blood samples from patients, before, and at one or more times after contrast-enhanced clinical examinations (CT or fluoroscopy). Cell damage indicators (including micronuclei, dicentrics, and γH2AX foci) are then compared with those of patients undergoing non-enhanced exposures, or expected levels based on dose estimates.
In vitro studies
The majority of in vitro studies show increased damage to cells irradiated in the presence of ICM, compared to those irradiated without ICM or in the presence of Mannitol only (Table 1). The methodology for calculating sample dose was rarely described in detail. In all reviewed studies, the “dose” to the sample appeared to refer to the estimated dose in the absence of ICM based on X-ray output or ionization chamber measurements, i.e. no DEF adjustment was made. Thus "higher-than-expected" refers to increased DNA damage for a given X-ray output, i.e. the number of X-rays the sample is exposed to. It was also noted that some studies reported the absolute number of damage foci, while others reported excess foci, based on comparison between pre- and post-exposure levels. These are shown as separate columns in Tables 1 and 2.
All studies, except one,12 assayed blood lymphocytes only. Grudzenski et al12 assessed both lymphocytes and fibroblasts, finding a slightly higher level of ICM-related damage increase in the former. Most recent studies were based on γH2AX assays (an indicator of DNA double strand breaks) only. Increased damage in fibroblasts was reasonably similar for γH2AX and 53BP1 foci in the above mentioned study by Grudzenski et al.12 Another study reported a greater ICM-related increase in lymphocyte dicentric yields, compared to γH2AX, at 50 mg of iodine per ml of blood, while the effect was more comparable between assay types at 5 mg ml−1.11 Increased damage does not appear to occur when ICM are added in the absence of irradiation,9,13 added after irradiation,12 or if ICM are pre-irradiated before mixing with blood.12,16
In two studies, DNA damage was assessed at various time intervals following in vitro exposure, for the same cell population.12,13 In both cases, excess γH2AX foci were observed for ICM-enhanced exposures at the earliest time interval, compared to unenhanced exposures. Both groups also reported decreasing γH2AX yields with increasing time since exposure, for both enhanced and unenhanced exposures. This suggests that contrast media increase the initial yield of DNA damage, without affecting damage repair,12 although measures to assess cell viability throughout the experiment in the above studies are unclear.
There is insufficient in vitro evidence to determine the impact of X-ray energy on the impact of ICM on DNA damage. The majority of samples were irradiated using general radiography equipment with aluminium filtration, at around 100 kV. Grudzenski et al12 found a substantial ICM-related damage increase following exposure of lymphocytes to X-rays generated at 90 kV, but almost no effect for caesium-137 gamma rays (661.7 keV). Studies of CERT suggest photon energies exceeding the iodine k-shell electron binding energy (33.4 keV) by 0 to 30 keV yield the greatest enhancement effect.34,35
In several studies, DNA damage was found to increase with increasing iodine concentration, at a given radiation dose, ranging from 5.0 to 52.5 mg ml−1.11,15,16 There appears to be little variation between different contrast media products (i.e.manufacturers, brands), providing the concentration of iodine is taken into account.15,36 No pattern of ICM-related increase in γH2AX foci was found by Beels et al14 for iodine concentrations of 5, 10 and 20 mg ml−1, each exposed to doses of 5, 10 and 50 mGy. No clear pattern of γH2AX yield enhancement was found by Jost et al11 for lymphocytes at 5 mg ml−1 iodine concentrations, compared to 0 mg ml−1. Yields were significantly higher for the former at 25 mGy (64%) but significantly lower at 50 mGy (−20%). At iodine concentrations of 50 mg ml−1, yields of γH2AX were raised at all doses (25, 50, 100, 1000 mGy), by an average factor of 2.3, compared to 0 mg ml−1 concentrations. These findings, along with those of Beels et al14 were interpreted as suggesting the impact of contrast media, at “clinically relevant” concentrations, is limited. The lack of an ICM-associated increase in DNA damage foci in spite of the increased dose to blood is unusual, although could be explained by uncertainties in γH2AX assays.33
In vivo studies
A number of in vivo studies have also suggested increased DNA damage following ICM-enhanced clinical X-ray exposures, compared to unenhanced imaging. The largest effect was reported by Piechowiak et al17 who reported a 107% greater excess of γH2AX foci in lymphocytes following contrast enhanced adult chest CT scans (0.056 per cell), compared to non-enhanced equivalent scans (0.027). Surprisingly, given the large sample size (179 and 66 in respective groups), this effect was not significant (p = 0.44). When yields were normalized by dose length product, the enhancement effect was increased to 267% (p = 0.001). Despite using the same Siemens Sensation 64 CT scanner model, more modest ICM-associated increases in excess lymphocyte γH2AX foci of 58% (p = 0.04) and 38% (p = < 0.01) were found by Pathe et al13 and Wang et al18 respectively, for abdominal CT scans. An even smaller dose-adjusted damage enhancement of 19%, based on absolute, rather than excess foci numbers, was reported by Gould et al16 for paediatric cardiac catheterizations (we calculated a figure of 23% using the same data). Grudzenski et al12 determined inter-individual damage response (in terms of foci per mGy, based on linear regression analysis) using in vitro exposures for 27 patients, then used these findings to normalize each patient’s lymphocyte γH2AX foci yield obtained following enhanced (mean iodine administration of 34.9 g) or unenhanced CT scans. Following this normalization, excess numbers of γH2AX foci were approximately 30% higher in the patients undergoing ICM-enhanced scans (without the normalization, there was no difference). A second phase of the same study in which DNA damage for patients matched for age and health status also suggested a 30% higher yield of excess γH2AX foci among individuals undergoing ICM-enhanced imaging. These in vivo findings suggest significant ICM-enhanced DNA damage does occur at clinically relevant iodine concentrations, despite some contrary in vitro findings.
With the exception of the one in vitro study in which damage to fibroblasts was also assessed,12 all findings have been based on assays of peripheral lymphocytes in the blood itself. Although lymphocytes are not thought to be prone to radiation-induced malignant transformation themselves, they are considered a good surrogate for overall DNA damage throughout the exposed region, along with associated cancer risk.2,37 While this may be true under non-ICM-enhanced conditions, in which the attenuation properties of blood may reasonably approximate those of surrounding tissues, contrast media, by design, modify the attenuation properties of blood. Consequently, DNA damage to blood cells in the presence of ICM may no longer adequately represent damage to cells outside of blood vessels. As such, it is unclear if ICM administration increases damage to cells prone to malignant transformation, for example, bone marrow stem cells, breast glandular cells, or thyroid follicular cells, thus increasing the risk of cancer.
Discussion
The implication of the studies described above; that both the radiation doses and, potentially, the cancer risks from contrast-enhanced X-ray exposures are underestimated, needs to be interpreted with considerable caution. The main point of contrast-enhanced imaging is to increase the attenuation properties of blood, relative to surrounding tissues. Consequently, the absorbed dose to blood and damage to blood cells, should not be considered representative of dose and damage to surrounding tissues under contrast-enhanced conditions.
The genoclastic effect of ICM is almost certainly dosimetric in nature, rather than chemical.36 This is suggested by the lack of excess DNA damage foci where ICM are added in the absence of irradiation or if ICM are pre-irradiated before mixing with blood samples.9,12,13,16 Furthermore, the increase in DNA damage is approximately similar to the estimated increase in blood dose, albeit using the crude methodology based on attenuation coefficients.
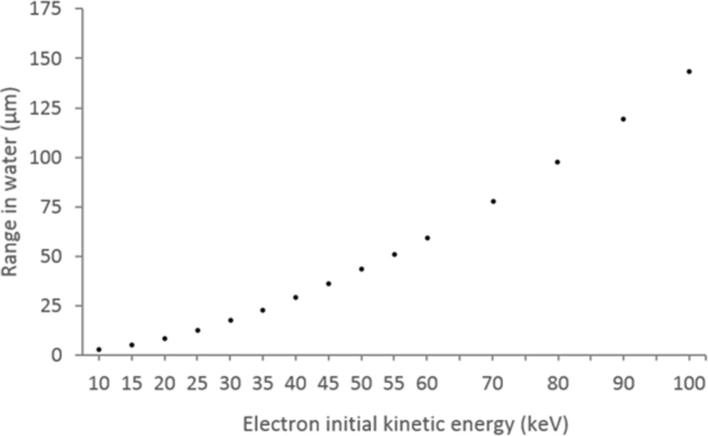
ICM molecules have a high X-ray absorption efficiency because the binding energy of inner (K-shell) electrons of iodine (33.4 keV) is close to the peak photon energies in diagnostic medical imaging (40–50 keV). Upon absorption, the interacting electron is ejected (ionized) with a kinetic energy equal to the incident photon energy (), minus the electron’s binding energy. These so-called photoelectrons typically have sufficient energy to cause hundreds or thousands of further ionizations and excitations by direct Coulombic interaction. The vacancy left by the ejected electron is filled by an electron from another shell (typically L or M). This transition results in the emission of either a photon or Auger electron. For iodine, L→K Auger electrons have kinetic energies of ∼28 keV and also capable of many ionizations. Thus, ICM molecules can be considered a source of secondary radiation, i.e. photoelectrons, Auger electrons and fluorescence photons. Any cells within range of these particles may be subject to increased damage. Theoretically, Auger/photoelectrons may escape blood vessels and reach the cell nuclei of surrounding tissues (Figure 1). However, at diagnostic X-ray energy levels, these electrons have relatively low kinetic energies ranging from just over zero to around 85 keV, with the majority being below 50 keV. The range of these electrons in soft tissue is correspondingly low, i.e. less than 100 µm (Figure 2) - meaning dose enhancement is highly localized.
Figure 1.
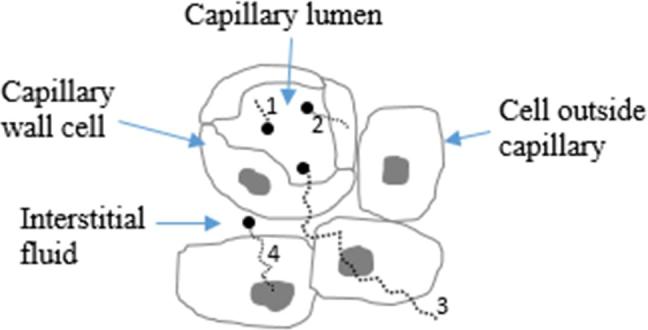
Contrast media molecules (black circles) absorb X-rays (not shown) resulting in release of secondary electrons (dotted lines). Four scenarios are shown: (1) electron restricted to blood only, (2) electron reaches vessel wall, (3) electron reaches nucleus of cell outside blood vessel, (4) electron released from contrast agent molecule in interstitial medium reaches nucleus of cell outside blood vessel.
Figure 2.
Mean linear range of electrons in water as a function of initial kinetic energy. Data obtained from National Institute of Standards and Technology ESTAR database, available from http://physics.nist.gov/PhysRefData/Star/Text/ESTAR.html.
The ability of contrast media to increase the risk of developing cancer may therefore depend upon the ability of ICM molecules to get within a few tens of micrometres to cells prone to malignant transformation at the time of X-ray exposure. This is only likely to occur if they have reached the capillary network of an organ, or the interstitial fluid outside these vessels. While such situations can undoubtedly occur in clinical practice, determining the concentration of iodine in capillaries and interstitial fluid is challenging. The upper limit is the concentration of the contrast media solution itself (i.e. the patient’s blood is entirely replaced by contrast media), typically around 270–400 mg I ml–1. A further estimate can be obtained by assuming the ICM is distributed uniformly throughout the patient’s blood volume. Based on the above solution concentrations, quoted injected volumes of 60–120 ml and average adult blood volume of 5000 ml, this gives iodine concentrations of 3.2 to 9.6 mg I ml–1.
The mean difference in HU between unenhanced and venous phase ICM-enhanced CT scans calculated by Amato et al21 was 87 (range: 46–205) for the thyroid, 49 (28-80) for the liver, and 71 (39-135) for the spleen. Using the conversion factor of Bae38 of 26.18 HU per ml I ml–1 of blood at 120 kV, this suggests iodine concentrations of 3.3 mg ml−1 (range: 1.8–7.8) for the thyroid, 1.9 mg ml−1(1.1–3.1) for the liver and 2.7 mg ml−1(1.5–5.2) for the spleen. The pharmacokinetic modelling approach developed by Sahbaee et al31 suggests peak iodine concentrations of 2–6 mg ml−1 for most organs and up to 10 mg ml−1 for the heart. These estimates are for the average concentration across the whole organ, however. The concentration is likely to be higher in blood vessels and lower outside blood vessels. Furthermore, venous phase scans are acquired with a relatively long delay between ICM injection and scanning, meaning ICM has more time to reach capillaries and interstitial fluids than would be the case for arterial phase exposures. More research is required investigating the concentration of ICM in the blood and interstitial medium of organs, for different methods of delivery (i.e. catheter, peripheral vein) and different phases (i.e. venous or arterial).
If contrast media are restricted to large/medium blood vessels (wall thicknesses > 50 µm) at the time of exposure, released electrons are unlikely to reach cells other than those of the vessel wall and the blood itself. Assays of circulating blood cells in such situations may give the impression of substantial "damage increase", despite little or no increase in traversals of cells known to be prone to malignant transformation. Likewise, due to the potentially highly uneven energy deposition in contrast enhanced tissues, it is possible that macroscopic organ doses (i.e. calculated from average HU of the organ) would be increased with minimal increase in energy imparted to cells outside of blood vessels. There may indeed be situations in which contrast media do increase cancer risks, compared to non-enhanced exposures. However, this is only likely to be for certain organs and particular timings between ICM administration and X-ray exposure. It appears unlikely that contrast media would increase overall cancer risks by a similar extent to that suggested by assays of circulating blood cells.36
Aside from dosimetric considerations, the uncertainties inherent in radiobiological assays must be taken into account, and could explain some of the effect variation between studies, including the negative findings of Beels et al14 or the lack of a clear relationship between damage foci and ICM concentration reported by Jost et al.11 While the γ-H2AX assay has been shown to give good dosimetric estimates in well characterized exposure scenarios,39 a very large number of associated uncertainties are recognized, including those due to the delay between exposure and foci appearance, possible coalescence of foci33 and different scoring methods. As such, additional effort on standardization and regular performance testing will be required to fully establish DNA damage foci assays as routine biodosimetric tools.33
Epidemiological analysis of the potential modification of radiation-induced cancer risks by contrast media is (theoretically) possible, given that ICM administration is often recorded in radiology information system (RIS) records of CT scans. Such a study is likely to be exceptionally challenging, due to the different clinical indications for enhanced and unenhanced imaging. An alternative possibility, suggested by Gould et al16 is to conduct assays of other cell types, such as bone marrow, following ICM-enhanced exposures, yielding findings perhaps more applicable to the final endpoint of cancer.
As contrast media may increase absorbed dose to the blood vessel wall, the potential for increased risk of non-malignant circulatory disease should also be considered. The target cells for radiation-induced circulatory disease and risks at diagnostic dose levels are poorly understood,40 although it is likely that the endothelium of the vessel wall is a critical tissue.41 Despite attempts to assess the potential risk of radiation-induced cardiovascular disease from cardiac catheterizations,42,43 dose estimates did not account for the impact of contrast media (the Monte Carlo (STUK, Helsinki, Finland) software used, PCXMC, SKUK, Helinski, Finland,44 does not allow for this). Such risks may, therefore, be underestimated.
Conclusion
Caution is urged in extrapolating the apparent increase in DNA damage to blood cells following contrast-enhanced X-ray exposures, to the risk of developing cancer. Blood cells may be unsuitable biomarkers for DNA damage and cancer risk under contrast-enhanced conditions, unless the increased dose to blood is accounted for. A microdosimetric evaluation, using Monte Carlo simulations (MCNP 6.1), of the impact of contrast media on tissue doses at the scale of capillaries is currently underway. Future research investigating the concentration of contrast media within blood vessels and interstitial media, along with an evaluation of the impact of contrast enhancement on automatic X-ray output control, is required. This will allow a more thorough evaluation of the impact of contrast media on the cancer risks from X-ray procedures.
Disclaimer
The authors are affiliated with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Chemical and Radiation Threats and Hazards at Newcastle University in partnership with Public Health England (PHE). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
Contributor Information
Richard Harbron, Email: richard.harbron@ncl.ac.uk.
Elizabeth A Ainsbury, Email: Liz.Ainsbury@phe.gov.uk.
Simon D Bouffler, Email: simon.bouffler@phe.gov.uk.
Rick J Tanner, Email: Rick.Tanner@phe.gov.uk.
Jonathan S Eakins, Email: jonathan.eakins@phe.gov.uk.
Mark S Pearce, Email: mark.pearce@newcastle.ac.uk.
References
- 1.Thomsen H, Reimer P. Intravascular contrast media for radiography, CT, MRI and ultrasound : Schaefer-Prokop C, Dixon A, Grainger & Allison’s Diagnostic Radiology. Vol 1 Edinburgh: Churchill Livingstone; 2015. 26–51. [Google Scholar]
- 2.Berrington de Gonzalez A, Kleinerman RA. CT scanning: is the contrast material enhancing the radiation dose and cancer risk as well as the image? Radiology 2015; 275: 627–9.https://doi.org/10.1148/radiol.2015150605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley P. Does iodinated contrast medium amplify DNA damage during exposure to radiation. Br J Radiol 2015; 88: 20150474https://doi.org/10.1259/bjr.20150474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams FH, Norman A, Mello RS, Bass D. Effect of radiation and contrast media on chromosomes. Preliminary report. Radiology 1977; 124: 823–6.https://doi.org/10.1148/124.3.823 [DOI] [PubMed] [Google Scholar]
- 5.Adams FH, Norman A, Bass D, Oku G. Chromosome damage in infants and children after cardiac catheterization and angiocardiography. Pediatrics 1978; 62: 312–6. [PubMed] [Google Scholar]
- 6.Callisen HH, Norman A, Adams FH. Absorbed dose in the presence of contrast agents during pediatric cardiac catheterization. Med Phys 1979; 6: 504–9.https://doi.org/10.1118/1.594613 [DOI] [PubMed] [Google Scholar]
- 7.Norman A, Adams FH, Riley RF. Cytogenetic effects of contrast media and triiodobenzoic acid derivatives in human lymphocytes. Radiology 1978; 129: 199–203.https://doi.org/10.1148/129.1.199 [DOI] [PubMed] [Google Scholar]
- 8.Cochran ST, Khodadoust A, Norman A. Cytogenetic effects of contrast material in patients undergoing excretory urography. Radiology 1980; 136: 43–6.https://doi.org/10.1148/radiology.136.1.7384520 [DOI] [PubMed] [Google Scholar]
- 9.Hadnagy W, Stephan G, Kossel F. Enhanced yield of chromosomal aberrations in human peripheral lymphocytes in vitro using contrast media in X-irradiation. Mutat Res 1982; 104: 249–54.https://doi.org/10.1016/0165-7992(82)90152-X [DOI] [PubMed] [Google Scholar]
- 10.Stephan G, Hadnagy W. Chromosomal aberrations in patients exposed to X-rays and contrast medium. Eur J Radiol 1981; 1: 335–7. [PubMed] [Google Scholar]
- 11.Jost G, Golfier S, Pietsch H, Lengsfeld P, Voth M, Schmid TE, et al. The influence of x-ray contrast agents in computed tomography on the induction of dicentrics and gamma-H2AX foci in lymphocytes of human blood samples. Phys Med Biol 2009; 54: 6029–39.https://doi.org/10.1088/0031-9155/54/20/001 [DOI] [PubMed] [Google Scholar]
- 12.Grudzenski S, Kuefner MA, Heckmann MB, Uder M, Löbrich M. Contrast medium-enhanced radiation damage caused by CT examinations. Radiology 2009; 253: 706–14.https://doi.org/10.1148/radiol.2533090468 [DOI] [PubMed] [Google Scholar]
- 13.Pathe C, Eble K, Schmitz-Beuting D, Keil B, Kaestner B, Voelker M, et al. The presence of iodinated contrast agents amplifies DNA radiation damage in computed tomography. Contrast Media Mol Imaging 2011; 6: 507–13.https://doi.org/10.1002/cmmi.453 [DOI] [PubMed] [Google Scholar]
- 14.Beels L, Bacher K, Smeets P, Verstraete K, Vral A, Thierens H. Dose-length product of scanners correlates with DNA damage in patients undergoing contrast CT. Eur J Radiol 2012; 81: 1495–9.https://doi.org/10.1016/j.ejrad.2011.04.063 [DOI] [PubMed] [Google Scholar]
- 15.Deinzer CK, Danova D, Kleb B, Klose KJ, Heverhagen JT. Influence of different iodinated contrast media on the induction of DNA double-strand breaks after in vitro X-ray irradiation. Contrast Media Mol Imaging 2014; 9: 259–67.https://doi.org/10.1002/cmmi.1567 [DOI] [PubMed] [Google Scholar]
- 16.Gould R, McFadden SL, Horn S, Prise KM, Doyle P, Hughes CM. Assessment of DNA double-strand breaks induced by intravascular iodinated contrast media following in vitro irradiation and in vivo, during paediatric cardiac catheterization. Contrast Media Mol Imaging 2016; 11: 122–9.https://doi.org/10.1002/cmmi.1671 [DOI] [PubMed] [Google Scholar]
- 17.Piechowiak EI, Peter JF, Kleb B, Klose KJ, Heverhagen JT. Intravenous Iodinated Contrast Agents Amplify DNA Radiation Damage at CT. Radiology 2015; 275: 692–7.https://doi.org/10.1148/radiol.14132478 [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Li Q, Wang XM, Hao GY, Jie-Bao , Hu S, et al. Enhanced radiation damage caused by iodinated contrast agents during CT examination. Eur J Radiol 2017; 92: 72–7.https://doi.org/10.1016/j.ejrad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 19.Beels L, Bacher K, De Wolf D, Werbrouck J, Thierens H. gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: are we underestimating radiation risks? Circulation 2009; 120: 1903–9.https://doi.org/10.1161/CIRCULATIONAHA.109.880385 [DOI] [PubMed] [Google Scholar]
- 20.Geisel D, Zimmermann E, Rief M, Greupner J, Laule M, Knebel F, et al. DNA double-strand breaks as potential indicators for the biological effects of ionising radiation exposure from cardiac CT and conventional coronary angiography: a randomised, controlled study. Eur Radiol 2012; 22: 1641–50.https://doi.org/10.1007/s00330-012-2426-1 [DOI] [PubMed] [Google Scholar]
- 21.Amato E, Salamone I, Naso S, Bottari A, Gaeta M, Blandino A. Can contrast media increase organ doses in CT examinations? A clinical study. AJR Am J Roentgenol 2013; 200: 1288–93.https://doi.org/10.2214/AJR.12.8958 [DOI] [PubMed] [Google Scholar]
- 22.Sahbaee P, Abadi E, Segars WP, Marin D, Nelson RC, Samei E. The effect of contrast material on radiation dose at CT: part II. a systematic evaluation across 58 patient models. Radiology 2017; 283: 749–57.https://doi.org/10.1148/radiol.2017152852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosch de Basea M, Pearce MS, Kesminiene A, Bernier MO, Dabin J, Engels H, et al. EPI-CT: design, challenges and epidemiological methods of an international study on cancer risk after paediatric and young adult CT. J Radiol Prot 2015; 35: 611–28.https://doi.org/10.1088/0952-4746/35/3/611 [DOI] [PubMed] [Google Scholar]
- 24.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346: f2360https://doi.org/10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baysson H, Réhel JL, Boudjemline Y, Petit J, Girodon B, Aubert B, et al. Risk of cancer associated with cardiac catheterization procedures during childhood: a cohort study in France. BMC Public Health 2013; : 266https://doi.org/10.1186/1471-2458-13-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandevoorde C, Franck C, Bacher K, Breysem L, Smet MH, Ernst C, et al. γ-H2AX foci as in vivo effect biomarker in children emphasize the importance to minimize x-ray doses in paediatric CT imaging. Eur Radiol 2015; 25: 800–11.https://doi.org/10.1007/s00330-014-3463-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The ImPACT Group. Comparative specifications: 64 slice CT scanners. Report No.CEP 08027 London: The ImPACT Group; 2009. [Google Scholar]
- 28.Amato E, Lizio D, Settineri N, Di Pasquale A, Salamone I, Pandolfo I. A method to evaluate the dose increase in CT with iodinated contrast medium. Med Phys 2010; 37: 4249–56.https://doi.org/10.1118/1.3460797 [DOI] [PubMed] [Google Scholar]
- 29.He W, Huda W, Mah E, Yao H. Does administering iodine in radiological procedures increase patient doses? Med Phys 2014; 41: 113901: 113901https://doi.org/10.1118/1.4898594 [DOI] [PubMed] [Google Scholar]
- 30.Sweezy JE, Booth TE, Hughes HG, Zukaitis A, Brown FB, Mosteller RD, et al. MCNP - A general Monte Carlo N-Particle transport code. Report No: Version 5. LANL, Report LA-UR-03-1987. Los Alamos National Laboratory; 2005. [Google Scholar]
- 31.Sahbaee P, Segars WP, Marin D, Nelson RC, Samei E. The effect of contrast material on radiation dose at CT: part I. incorporation of contrast material dynamics in anthropomorphic phantoms. Radiology 2017; 283: 739–48.https://doi.org/10.1148/radiol.2016152851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boone JM, Hernandez AM. The effect of iodine-based contrast material on radiation dose at CT: It’s complicated. Radiology 2017; 283: 624–7.https://doi.org/10.1148/radiol.2017170611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, Burdak-Rothkamm S. DNA damage foci: Meaning and significance. Environ Mol Mutagen 2015; 56: 491–504.https://doi.org/10.1002/em.21944 [DOI] [PubMed] [Google Scholar]
- 34.Verhaegen F, Reniers B, Deblois F, Devic S, Seuntjens J, Hristov D. Dosimetric and microdosimetric study of contrast-enhanced radiotherapy with kilovolt x-rays. Phys Med Biol 2005; 50: 3555–69.https://doi.org/10.1088/0031-9155/50/15/005 [DOI] [PubMed] [Google Scholar]
- 35.Corde S, Joubert A, Adam JF, Charvet AM, Le Bas JF, Estève F, et al. Synchrotron radiation-based experimental determination of the optimal energy for cell radiotoxicity enhancement following photoelectric effect on stable iodinated compounds. Br J Cancer 2004; 91: 544–51.https://doi.org/10.1038/sj.bjc.6601951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman A, Cochran ST, Sayre JW. Meta-analysis of increases in micronuclei in peripheral blood lymphocytes after angiography or excretory urography. Radiat Res 2001; 155: 740–3.https://doi.org/10.1667/0033-7587(2001)155[0740:MAOIIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 37.Bonassi S, Norppa H, Ceppi M, Strömberg U, Vermeulen R, Znaor A, et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis 2008; 29: 1178–83.https://doi.org/10.1093/carcin/bgn075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010; 256: 32–61.https://doi.org/10.1148/radiol.10090908 [DOI] [PubMed] [Google Scholar]
- 39.Moquet J, Barnard S, Staynova A, Lindholm C, Monteiro Gil O, Martins V, et al. The second gamma-H2AX assay inter-comparison exercise carried out in the framework of the European biodosimetry network (RENEB. Int J Radiat Biol 2017; 93: 58–64.https://doi.org/10.1080/09553002.2016.1207822 [DOI] [PubMed] [Google Scholar]
- 40.Authors on behalf of ICRP, Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs--threshold doses for tissue reactions in a radiation protection context. Ann ICRP 2012; 41: 1–322.https://doi.org/10.1016/j.icrp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 41.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys 2007; 67: 10–18.https://doi.org/10.1016/j.ijrobp.2006.08.071 [DOI] [PubMed] [Google Scholar]
- 42.Keiller DA, Martin CJ. Radiation dose to the heart in paediatric interventional cardiology. J Radiol Prot 2015; 35: 257–64.https://doi.org/10.1088/0952-4746/35/2/257 [DOI] [PubMed] [Google Scholar]
- 43.Vañó E, Miller DL, Dauer L. Implications in medical imaging of the new ICRP thresholds for tissue reactions. Ann ICRP 2015; 44(1 Suppl): 118–28.https://doi.org/10.1177/0146645314562322 [DOI] [PubMed] [Google Scholar]
- 44.Tapiovaara M, Siiskonen T. PCXMC: A Monte Carlo program for calculating patient doses in medical x-ray examinations : STUK - Radiation and Nuclear Safety Authority. Helsinki, Finland; 2008. [Google Scholar]