Abstract
Objective:
To evaluate the frequency of sciatic neuromuscular variants on MR neurography and determine the interobserver variability.
Methods:
A retrospective evaluation of 137 consecutive lumbosacral plexus magnetic resonance neurography examinations was performed. All examinations were performed using nerve selective 3D imaging and independently reviewed by two readers for the presence of sciatic neuromuscular variants and piriformis muscle asymmetry. Inter- and intraobserver performance were evaluated.
Results:
There were a total of 44/268 (16.4%) extremities with sciatic neuromuscular variants. The interobserver performance in the identification of sciatic nerve variants was excellent (kappa values from 0.8–0.9). There was a total of 45/134 (33.6%) patients with piriformis muscle asymmetry. Of these, 7/134 (5.2%) had piriformis muscle atrophy and 38/134 (28.4%) had piriformis muscle hypertrophy. The interobserver performance in the identification of piriformis muscle atrophy and hypertrophy was moderate to good (kappa values from 0.39–0.61). The intraobserver performance revealed kappa values of 0.735 and 0.821 on right and left, respectively.
Conclusion:
Sciatic neuromuscular variants and piriformis muscle asymmetry are frequent on lumbosacral plexus MRN with moderate to excellent interobserver performance.
Advances in knowledge:
Sciatic neuromuscular variants and piriformis asymmetry on MR neurography are frequent and the prevalence is similar to cumulative prevalence from available scientific series. Interobserver performance for identification of sciatic neuromuscular variants is excellent, and moderate-good for piriformis muscle asymmetry.
Introduction
Sciatic neuromuscular variants have been studied in various cadaveric and surgical series (Table 1).1–18 A recent analysis emphasized the traditional Beaton and Anson classification system and summarized the results from various studies.19 These studies date since 1896, where Parsons and Keith initially described the prevalence of sciatic neuromuscular variants in a cadaveric series as 14.5%. In the imaging literature, we could find only one scientific study evaluating piriformis muscle asymmetry.20 This study used T1W images and the prevalence of piriformis muscle asymmetry was observed to range from 8 to 81%, which depended upon the threshold measurement used to define the asymmetry. The piriformis muscles ranged in size from 0.8 to 3.2 cm, with an average size of 1.9 cm. Nineteen percent of patients had greater than 3 mm of asymmetry in the size of the piriformis muscle, with a maximum asymmetry of 8 mm. The developmentally split sciatic and/or piriformis muscle variations have been shown to be associated with piriformis syndrome and sciatic nerve entrapment at the great sciatic notch leading to variable symptoms and findings of buttock pain, gluteal tenderness, sciatica and/or “wallet” anaesthesia.21 With the availability of nerve specific and vessel suppressed 3D imaging and diffusion imaging as part of 3 Tesla magnetic resonance neurography (MRN), one can confidently identify the sciatic neuromuscular anatomy and related variants in multiple planes, especially in the nerve longitudinal planes.22,23 The aims of our study were to evaluate the frequency of sciatic neuromuscular variants in a large series of consecutive patients presenting for MRN of the lumbosacral (LS) plexus and determine the interobserver variability in recognition of such findings. As a secondary aim, a simplified classification system based on the frequencies of imaging findings would be surmised for its practical use in the routine practice.
Table 1.
Prevalence of sciatic nerve anomalies in cadaveric and surgical series
| Investigator | A | B | C | D | E | F | Total sides | Total variants | Percent variants |
|---|---|---|---|---|---|---|---|---|---|
| Parsons and Keith (1896) | 118 | 17 | 0 | 3 | 0 | 0 | 138 | 20 | 14.5 |
| Bardeen (1901) | 220 | 25 | 1 | 0 | 0 | 0 | 246 | 26 | 10.6 |
| Trotter (1932)a | 400 | 0 | 0 | 0 | 0 | 0 | 464 | 64 | 13.8 |
| Ming-Tzu (1941) | 92 | 46 | 0 | 2 | 0 | 0 | 140 | 48 | 34.3 |
| Mirsa (1954) | 262 | 18 | 12 | 8 | 0 | 0 | 300 | 38 | 12.7 |
| Anson and McVay (1971) | 1789 | 201 | 13 | 5 | 0 | 0 | 2008 | 219 | 10.9 |
| Nizankowski et al (1972) | 181 | 8 | 3 | 5 | 3 | 0 | 200 | 19 | 9.5 |
| Lee and Tsai (1974) | 118 | 8 | 3 | 5 | 3 | 0 | 168 | 50 | 29.8 |
| Pecina (1979) | 102 | 27 | 1 | 0 | 0 | 0 | 130 | 28 | 21.5 |
| Chiba (1992) | 328 | 173 | 10 | 0 | 0 | 0 | 511 | 183 | 35.8 |
| Chiba et al (1994) | 285 | 148 | 9 | 0 | 0 | 0 | 442 | 157 | 35.5 |
| Pokorny et al (1998) | 82 | 14 | 4 | 2 | 0 | 0 | 102 | 20 | 19.6 |
| Fishman et al (2002) | 65 | 0 | 0 | 0 | 0 | 0 | 76 | 11 | 14.5 |
| Benzon et al (2003) | 65 | 1 | 0 | 0 | 0 | 0 | 66 | 1 | 1.5 |
| Agur and Dailey (2005) | 557 | 79 | 3 | 0 | 0 | 0 | 639 | 82 | 12.8 |
| Ugrenovic et al (2005) | 192 | 5 | 3 | 0 | 0 | 0 | 200 | 8 | 4.0 |
| Pokorny et al (2006) | 144 | 26 | 8 | 4 | 0 | 0 | 182 | 38 | 20.9 |
| Guvencer et al (2009) | 38 | 8 | 4 | 0 | 0 | 0 | 50 | 12 | 24.0 |
| Cumulative | 5038 | 829 | 78 | 32 | 5 | 5 | 6,062 | 1,024 | 16.9 |
Individual variants not mentioned.
Methods and Materials
The study was performed following institutional review board approval in compliance with HIPPAA regulations and informed consent was waived. A consecutive series of 137 LS plexus MRN examinations over a period of 2 years were selected for retrospective evaluation.
MRN examinations
The following sequences from the LS plexus MRN were reviewed: coronal 3D nerve selective images (SHINKEI, TR-2500 ms, TE-184 ms, ETL-100, Voxel 1.5 mm isotropic), axial T1-weighted (T1W) images (TR-700 ms, TE-8 ms, ETL-7, slice-4 mm), axial T2 SPAIR images (TR-3800 ms, TE-65 ms, ETL-15, slice-4 mm, fat suppression—adiabatic inversion recovery), and axial diffusion weighted imaging (DWI) (TR-6500 ms, TE-70 ms, slice-5 mm, B-0,600) images. All the scans were performed on 3 Tesla magnet (Achieva, Philips, Best, Netherlands) using a combination of spine and torso XL coils. Among the consecutive series of 137 patients, three patients were excluded due to artefacts from regional metallic hardware. The patients presented with one or a combination of a variety of complaints of pelvic pain, numbness, genital symptoms, back pain, buttock and coccyx pain. The readers were blinded to history and clinical findings in the subjects. There was no prior sciatic or piriformis surgery in any of the cases. The remaining 134 examinations were reviewed for the presence of sciatic neuromuscular variants and piriformis muscle asymmetry.
Reading procedures
All studies were read by two radiologists with 5 and 13 years of radiology experience, respectively. The sciatic neuromuscular variants were categorized using the Beaton and Anson Classification system,24 which is summarized in Table 2. In type A, the undivided sciatic nerve exits below the piriformis muscle. In type B, the common peroneal division exits through the piriformis muscle and the tibial division exits below the piriformis muscle. In type C, the common peroneal division exits above the piriformis muscle and the tibial division exits below the piriformis muscle. In type D, the undivided sciatic nerve exits through the piriformis muscle. In type E, the common peroneal division exits above the piriformis muscle and tibial division exits through the piriformis muscle. In type F, the undivided sciatic nerve exits above the piriformis muscle. The sciatic nerves were evaluated on all sequences, including 3D coronal images. The 3D images were available on PACS (picture archiving and communication system) as uniformly reconstructed 8-mm thick slab maximum intensity projections and no other reconstructions were obtained. The piriformis muscles were classified as asymmetric or symmetric. The muscles were further classified as hypertrophic or atrophic based on the subjective assessment of increased muscle area or presence of accessory belly vs loss of volume with or without fatty infiltration relative to other regional muscles, respectively. The evaluation criteria for muscle hypertrophy and atrophy were outlined in consensus among the readers before the final review. The reader training was initially performed with approximately 10% of the cases (14/134), 2 weeks prior to the final independent review. The mean of frequencies recorded by the two readers was reported as the final number for descriptive analysis and statistical calculations. Second blinded reads were also performed by the attending radiologist. Interobserver and intraobserver performance were assessed using weighted kappa statistics and all data including frequencies were stored on a spread sheet (Excel 2010, Microsoft, Seattle, WA). Analysis of variance test was used to detect associations between the muscle size and age.
Table 2.
Comparison of Beaton and Anson and simplified classifications systems
| Beaton and anson classification system |
Simplified classification system |
||
|---|---|---|---|
| Type | Description | Type | Description |
| A | Undivided sciatic nerve exits below muscle | ||
| B | Common peroneal division exits through muscle and tibial division exits below muscle | 1 | Common peroneal division exits through muscle and tibial division exits below muscle |
| C | Common peroneal division exits above muscle and tibial division exits below muscle | 2 | Common peroneal division exits above muscle and tibial division exits below muscle |
| D | Undivided sciatic nerve exits through muscle | 3 | Undivided sciatic nerve exits through muscle |
| E | Common peroneal division exits above muscle and tibial division exits through muscle | 4 | Common peroneal and tibial divisions exit below muscle but are divided by a fibrous slip |
| F | Undivided sciatic nerve exits above muscle |
Literature search
The internet sites “pubmed” and “scopus” were searched for scientific studies related to prevalence and frequency of piriformis muscle and sciatic nerve variations. The key words included- “piriformis anatomy”, “sciatic variant”, “piriformis variant”. “piriformis syndrome”, and “sciatic anatomy”. Any study that evaluated a minimum 25 extremities was included for the cumulative analysis, resulting in a total of 18 studies (Table 1). The cumulative prevalence from 18 studies was 16.7%.
Results
A total of 134 patients (268 extremities) were evaluated. This included 35 males and 99 females.
For males, the average age was 47 years and standard deviation was 16 years. For females, the average age was 52 years and standard deviation was 15 years. The type A variant was identified in 224/268 (83.6%) of extremities. This was considered to represent the normal anatomic relationship. There were total of 44/268 (16.4%) extremities with sciatic neuromuscular variants. Figures 1–3 illustrate the appearance of sciatic neuromuscular variants on axial and coronal images. The DWI images are reconstructed in coronal planes in inverted scale corresponding to the SHINKEI images for selective depiction of the nerves. Of the sciatic neuromuscular variants identified, 32/44 (72.7%) were type B and 3/44 (6.8%) were type C. The type D, E, and F variants were not identified. In 9/44 (20.5%) extremities, a variant was identified in which the common peroneal division and tibial division exited below the piriformis muscle, but were divided by a thin fibrous slip. The sciatic neuromuscular variants were unilateral in 20/134 (14.9%) patients and bilateral in 12/134 (8.9%) patients. The interobserver performance in the identification of sciatic neuromuscular variants was excellent with kappa values measuring 0.9 and 0.8 on right and left, respectively. There were total of 45/134 (33.6%) patients with piriformis muscle asymmetry. Of these, 7/134 (5.2%) had piriformis muscle atrophy and 38/134 (28.4%) had piriformis muscle hypertrophy. Figure 4 illustrates the appearance of piriformis muscle atrophy and hypertrophy on axial images. The interobserver performance in the identification of piriformis muscle atrophy and hypertrophy was moderate to good with kappa values of 0.61 and 0.39 on right and left, respectively. The intraobserver performance revealed kappa values of 0.735 and 0.821 on right and left, respectively. Examples of inter and intraobserver disagreement are shown in Figure 5. There was no significant association between muscle size and age (p = 0.5, reader 1) and (p = 0.7, reader 2).
Figure 1.
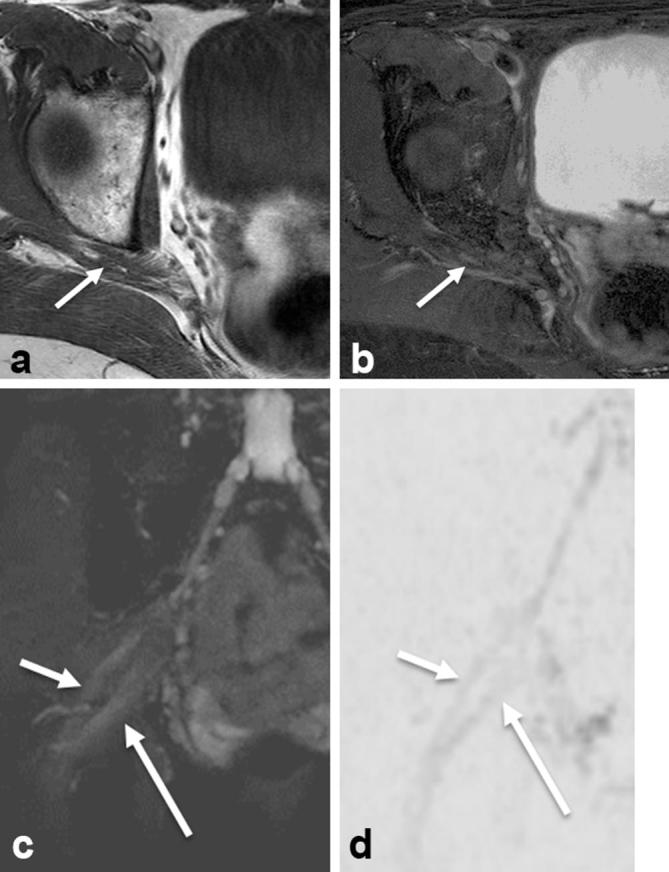
Axial T1W (a), axial T2 SPAIR (b), coronal SHINKEI (c) and coronal reconstructed DWI (inverted grey scale, (d) show that the common peroneal division (small arrows) exits through and the tibial division (large arrows) exits below the piriformis muscle, respectively. DWI, diffusion weighted imaging; T1W, T1 weighted.
Figure 3.
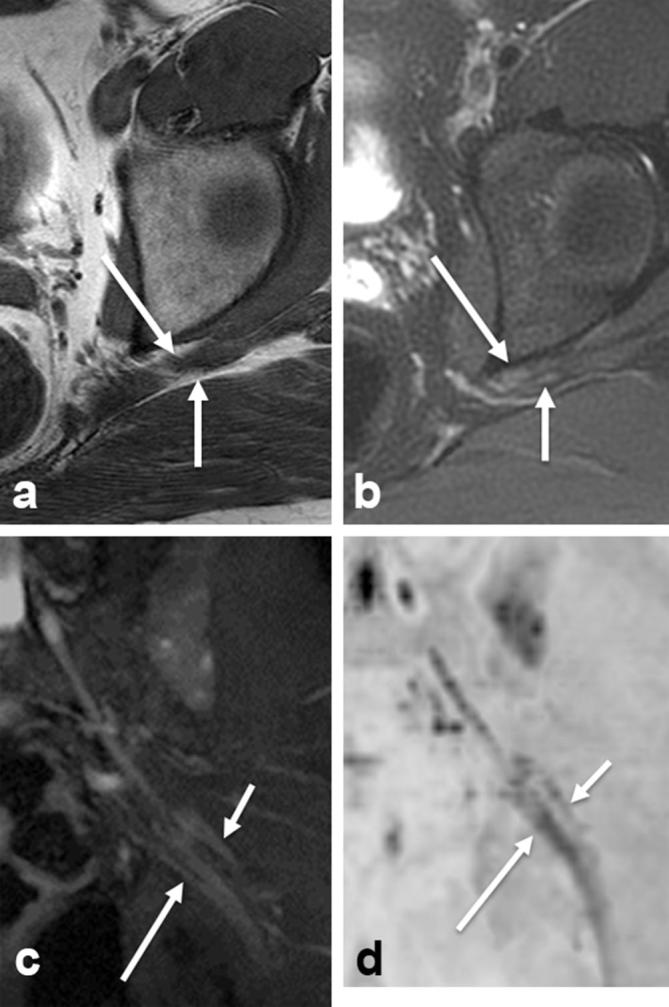
Type 4 variant. Axial T1W (a), axial T2 SPAIR (b), coronal SHINKEI (c) and coronal reconstructed DWI (inverted grey scale, (d) show that common peroneal (small arrows) and tibial (large arrows) divisions exit below muscle but are divided by a fibrous slip. DWI, diffusion weighted imaging; T1W, T1 weighted.
Figure 4.
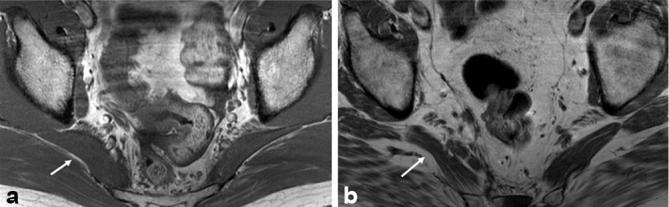
Piriformis muscle asymmetry. Axial T1W (a,b) images from different patients showing the right sided piriformis muscle hypertrophy (arrow in a) and atrophy with mild fatty infiltration (arrow in b). T1W, T1 weighted.
Figure 5.
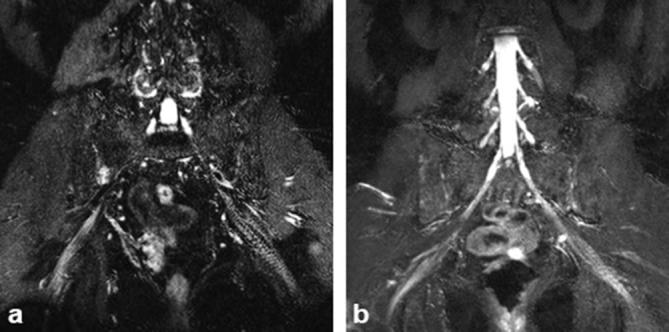
(a) Interobserver disagreement—left sciatic nerve: catagorized as Type 1 by Observer 1 (attending) and type 4 by Observer 2 (fellow). (b) Intraobserver disagreement—right sciatic: catagorized as Type 4 by first read and not split by second read.
Figure 2.
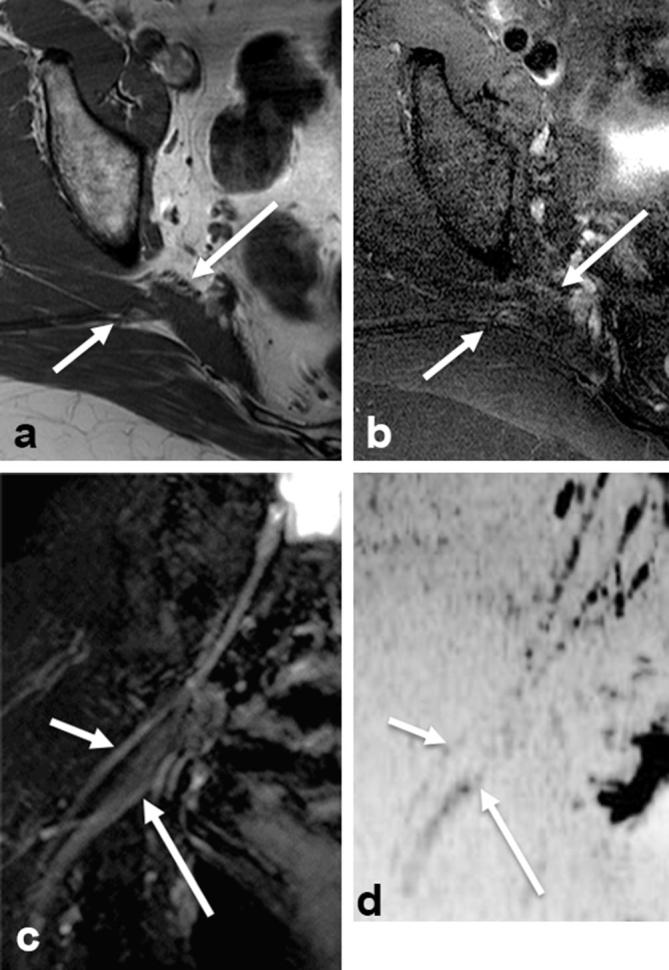
Type 2 variant. Axial T1W (a), axial T2 SPAIR (b), coronal SHINKEI (c) and coronal reconstructed DWI (inverted grey scale, (d) show that the common peroneal division (small arrows) exits above and the tibial division (large arrows) exits below the piriformis muscle, respectively. DWI, diffusion weighted imaging; T1W, T1 weighted.
Discussion
The frequency of sciatic neuromuscular variants on MRN is 16.4%, which is similar to the cumulative prevalence from various available cadaveric and surgical series (16.7%). Variants D, E and F were not identified in our study. This is similar to our experience in reading these scans over the years, e.g. the senior authors have not encountered complete intramuscular course of the sciatic nerve or complete sciatic nerve exiting above the piriformis muscle. Although limiting the sample size and not including many years of data may have overlooked these variants. On review of the available series (Table 1), it is also apparent that none of the studies found variant F while 2/18 studies reported variant E and 8/18 reported variant D.1,7,8 Piriformis asymmetry on MRN was found in almost 1/3 of the patients, with hypertrophy seen much more commonly than atrophy. This is within the range previously published in the imaging literature (8–81%). It should be noted that our study used a subjective assessment to categorize piriformis asymmetry with pre-defined qualitative criteria as opposed to a threshold measurement to mirror the routine practice. The interobserver performance on MRN was excellent for identification of sciatic nerve variants, which is in part related to excellent MR neurographic images. The sciatic nerves were conspicuous on high resolution T1W, T2 SPAIR, DWI and 3D images. The interobserver performnce was inferior (moderate to good) for the identification of piriformis muscle asymmetry, which is partly explained by the fact that muscle was only evaluated to axial T1W images. It is difficult to evaluate the muscle on fat suppressed T2 weighted (T2W) coronal images and axial DWI. The coronal T1W or Dixon images were not part of this LS plexus protocol. The overall interobserver reliability is encouraging as one can employ this less time consuming qualitative method of evaluation in the routine practice. The intraobserver performance was good to excellent for the attending radiologist.
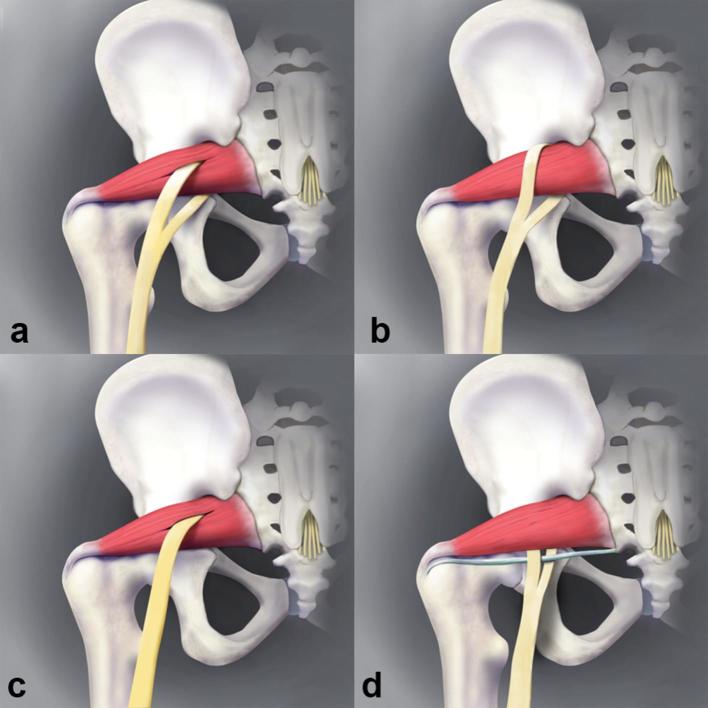
Some of the sciatic neuromuscular variants described in the Beaton and Anson classification were not identified in our study. Although a larger series might find these variants, their existence is questionable. Also, an additional variant was identified in our study, in which the tibial and common peroneal components are divided by a thin fibrous slip, possibly from the piriformis or gemellus muscles. This variant is different from the Beaton and Anson classification system and encompassed 20.5% of all the variants. Diligent interrogation and high-resolution imaging are essential to find this variant. Based on these results, we suggest a simplified classification system encompassing 4 variant types, which is summarized in Table 2 and illustrated in Figure 6. The original type D variant (new Type 3 variant) has been kept in the classification. Admittedly, in our many years of neuromuscular practice, we haven’t encountered it. However, since it has been reported in the literature, if present, it would represent a very significant finding with the whole sciatic nerve traversing through the muscle due to implications with regards to dynamic nerve compression during everyday muscle action.
Figure 6.
Simplified classification system: Type 1 (a), Type 2 (b), Type 3 (c) and Type 4 (d).
A limitation of our study is the lack of surgical correlation, but that would be impossible to obtain on a larger series of clinical subjects, since most of these patients are currently treated with image-guided injections. Additionally, about 8.9% of these variants were bilateral. We did not attempt to correlate the imaging findings with the clinical symptoms as this study was purely focused on finding the variants. Although the cumulative frequency of variants in the literature is similar to our study, this study was performed on patients referred for MR neurography, many of which may have clinical symptoms. Thus, the results may not be completely generalizable and a comparison of patients with groin pain vs gluteal pain or sciatica may help in finding true frequency of incidental vs symptom related neuromuscular variations. Evaluation of the sciatic or muscle signal alterations on T2W images, their clinical correlation and injection outcomes are also subjects of future studies.
To summarize, the sciatic neuromuscular variants and piriformis muscle asymmetry are frequent and are well evaluated on MR neurography with moderate to excellent interobserver performance. A simplified classification system with 4 variation types can be used for their imaging description.
Disclosures
Avneesh Chhabra has previously received research grants from GE-AUR (GERRAF), Siemens Medical Solutions, Gatewood Fellowship Award and Integra Life Sciences unrelated to this work. He also served as a research consultant with Siemens CAD group and ICON Medical. Dr Chhabra receives royalties from Jaypee and Wolters.
Contributor Information
Jason Eastlack, Email: jason.eastlack@utsouthwestern.edu.
Vibhor Wadhwa, Email: vibhorwadhwa90@gmail.com.
Adam Starr, Email: adam.starr@utsouthwestern.edu.
Avneesh Chhabra, Email: avneesh.chhabra@utsouthwestern.edu.
References
- 1.Parsons FG, Keith A. Sixth Annual Report of the Committee of Collective Investigation of the Anatomical Society of Great Britain and Ireland, 1895-96. J Anat Physiol 1896; 31(Pt 1): 31–44. [PMC free article] [PubMed] [Google Scholar]
- 2.Bardeen CR, Elting AW. A statistical study of the variations in the formation and position of the lumbosacral plexus in man. Anatomical Record 1901; 19: 209–32. [Google Scholar]
- 3.Trotter M. The relation of the sciatic nerve to the piriformis muscle in American whites and negroes. Anat Rec . 1932; 52: 321–3. DOI: https://doi.org/10.1002/ar.1090520404 [Google Scholar]
- 4.Ming-Tzu P'an. The relation of the sciatic nerve to the piriformis muscle in the Chinese. Am J Phys Anthropol 1941; 28: 375–80. DOI: https://doi.org/10.1002/ajpa.1330280403 [Google Scholar]
- 5.Misra B. The relations of the sciatic nerve to the piriformis in Indian cadavers. J Anat Soc India 1954; 3: 28–33. [Google Scholar]
- 6.Surgical Anatomy Surgical Anatomy. Vols. 1 and 2 Fifth edition. by B. J. Anson, M.A., Ph.D. (Med. Sc.), Chicago, and C. B. McVay, Ph.D., M.D., F.A.C.S., South Dakota. 10⅕ × 7⅕ in. Pp. 1282 +xl. Illlustrated. 1971. London: W. B. Saunders Company Ltd. £19.15. Br J Surg 1972; 59: 82–3. DOI: https://doi.org/10.1002/bjs.1800590134 [Google Scholar]
- 7.Nizankowski C, Slociak J, Szybejko J. Variations in the anatomy of the sciatic nerve in man. Folia Morphol 1972; 31: 507–13. [PubMed] [Google Scholar]
- 8.Lee CS, Tsai TL. The relation of the sciatic nerve to the piriformis muscle. Taiwan Yi Xue Hui Za Zhi 1974; 73: 75–80. [PubMed] [Google Scholar]
- 9.Pećina M. Contribution to the etiological explanation of the piriformis syndrome. Acta Anat 1979; 105: 181–7. [PubMed] [Google Scholar]
- 10.Chiba S. Multiple positional relationships of nerves arising from the sacral plexus to the piriformis muscle in humans. Kaibogaku Zasshi 1992; 67: 691–724. [PubMed] [Google Scholar]
- 11.Chiba S, Ishibashi Y, Kasai T. Perforation of dorsal branches of the sacral nerve plexus through the piriformis muscle and its relation to changes of segmental arrangements of the vertebral column and others. Kaibogaku Zasshi 1994; 69: 281–305. [PubMed] [Google Scholar]
- 12.Pokorný D, Sosna A, Veigl P, Jahoda D. Anatomic variability of the relation of pelvitrochanteric muscles and sciatic nerve. Acta Chir Orthop Traumatol Cech 1998; 65: 336–9. [PubMed] [Google Scholar]
- 13.Fishman LM, Dombi GW, Michaelsen C, Ringel S, Rozbruch J, Rosner B, et al. Piriformis syndrome: diagnosis, treatment, and outcome--a 10-year study. Arch Phys Med Rehabil 2002; 83: 295–301. DOI: https://doi.org/10.1053/apmr.2002.30622 [DOI] [PubMed] [Google Scholar]
- 14.Benzon HT, Katz JA, Benzon HA, Iqbal MS. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology 2003; 98: 1442–8. [DOI] [PubMed] [Google Scholar]
- 15.Agur AMR, Dalley AF. Grant’s atlas of anatomy. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 16.Ugrenovic S, Jovanovic I, Krstic V, Stojanovic V, Vasovic L, Antic S, et al. The level of the sciatic nerve division and its relations to the piriform muscle. Vojnosanit Pregl 2005; 62: 45–9. DOI: https://doi.org/10.2298/VSP0501045U [DOI] [PubMed] [Google Scholar]
- 17.Pokorný D, Jahoda D, Veigl D, Pinskerová V, Sosna A. Topographic variations of the relationship of the sciatic nerve and the piriformis muscle and its relevance to palsy after total hip arthroplasty. Surg Radiol Anat 2006; 28: 88–91. DOI: https://doi.org/10.1007/s00276-005-0056-x [DOI] [PubMed] [Google Scholar]
- 18.Guvencer M, Iyem C, Akyer P, Tetik S, Naderi S. Variations in the high division of the sciatic nerve and relationship between the sciatic nerve and the piriformis. Turk Neurosurg 2009; 19: 139–44. [PubMed] [Google Scholar]
- 19.Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequence: a review. Clin Anat 2010; 23: 8–17. DOI: https://doi.org/10.1002/ca.20893 [DOI] [PubMed] [Google Scholar]
- 20.Russell JM, Kransdorf MJ, Bancroft LW, Peterson JJ, Berquist TH, Bridges MD. Magnetic resonance imaging of the sacral plexus and piriformis muscles. Skeletal Radiol 2008; 37: 709–13. DOI: https://doi.org/10.1007/s00256-008-0486-8 [DOI] [PubMed] [Google Scholar]
- 21.Siddiq MAB, Hossain MS, Uddin MM, Jahan I, Khasru MR, Haider NM, et al. Piriformis syndrome: a case series of 31 Bangladeshi people with literature review. Eur J Orthop Surg Traumatol 2017; 27: 193–203. DOI: https://doi.org/10.1007/s00590-016-1853-0 [DOI] [PubMed] [Google Scholar]
- 22.Kasper JM, Wadhwa V, Scott KM, Rozen S, Xi Y, Chhabra A. SHINKEI-a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol 2015; 25: 1672–7. DOI: https://doi.org/10.1007/s00330-014-3552-8 [DOI] [PubMed] [Google Scholar]
- 23.Carpenter EL, Bencardino JT. Focus on advanced magnetic resonance techniques in clinical practice: magnetic resonance neurography. Radiol Clin North Am 2015; 53: 513–29. [DOI] [PubMed] [Google Scholar]
- 24.Beaton LE, Anson BJ. The relation of the sciatic nerve and of its subdivisions to the piriformis muscle. Anat Rec 1937; 70: 1–5. DOI: https://doi.org/10.1002/ar.1090700102 [Google Scholar]