Abstract
Objective:
To assess the imaging features of peritoneal mesothelioma and identify key anatomical sites that aid patient selection for complete cytoreduction.
Methods:
Pre-operative imaging of 59 (32 males, 27 females) patients who underwent cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for histologically proven peritoneal mesothelioma [36 malignant peritoneal mesothelioma, 23 cystic mesothelioma were reviewed. Imaging findings were correlated with surgical outcome. Best imaging predictors of complete cytoreduction, n = 22 and major tumour debulking, n = 12 were assessed.
Results:
Most patients (88.9%) had diffuse peritoneal disease with mean radiological peritoneal cancer index of 18 ± 12 (range 2–39). Disease in the lesser omentum (n = 10), porta hepatis (n = 8), perigastric area (n = 5), mesentery (n = 25), small bowel (n = 17), hydronephrosis (n = 1), concurrent pleural disease (n = 2), lymph nodes (n = 1) and abdominal wall disease (n = 4) was considered unfavourable. While 78.9% of patients who underwent complete cytoreduction had no disease at unfavourable sites, 75% of those who underwent MTD did have disease at these sites. There was significant difference in the radiological peritoneal cancer index, severity of upper abdominal disease, small bowel and mesenteric involvement between patients who underwent complete cytoreduction and MTD for malignant peritoneal mesothelioma. Complete cytoreduction was not achieved in the presence of a rind of soft tissue around the small bowel (p = 0.016) and was unlikely in the presence of large volume upper abdominal disease (p = 0.06).
Conclusion:
Involvement of key anatomical sites such as small bowel serosa and large volume upper abdominal disease reduced the likelihood of achieving complete cytoreduction in patients with malignant peritoneal mesothelioma.
Advances in knowledge:
Demonstration of small bowel disease and large volume upper abdominal disease on imaging in patients with malignant peritoneal mesothelioma can be used to identify patients who may not benefit from cytoreductive surgery.
Introduction
Peritoneal mesothelioma is an uncommon clinical entity with an incidence of 0.5 to 3 per million in males and 0.2 to 2 per million in females.1 The peritoneal cavity is the second most common site of mesothelioma, after the pleura.2 Over the last decade, a number of published studies from experienced peritoneal malignancy centres have supported the use of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of peritoneal mesothelioma.3–8 The strongest predictor of long-term survival after CRS and HIPEC is complete removal of all visible disease, referred to as complete cytoreduction.3–9 To achieve this, appropriate patient selection is essential. In our experience imaging findings are useful in identifying those who are unlikely to benefit from CRS and HIPEC due to the volume and the distribution of disease. We retrospectively reviewed imaging findings in patients with peritoneal mesothelioma treated with CRS and HIPEC at our institution in order to identify key imaging findings that would influence the likelihood of achieving complete cytoreduction and thus help refine patient selection in the future.
Methods and materials
Study design and patients
This is a retrospective study of 59 consecutive patients (32 males, 27 females) who underwent CRS and HIPEC for pathologically proven peritoneal mesothelioma between 1998 and 2015 in a National referral centre for peritoneal mesothelioma. 36 patients had malignant peritoneal mesothelioma and 23 had cystic mesothelioma.
Most patients were referred from other institutions and hence there was variation in imaging modalities and protocols. 58 patients had reformatted CT with slice thickness ranging from 1 to 8 mm; one patient had PET-CT and seven patients with cystic mesothelioma also had MRI of the abdomen and pelvis. 30 patients were administered iodinated oral contrast agent while the remainder had water or no oral preparation. The median duration between latest imaging and surgery was 6 weeks with a range of 1–12 weeks. Images were anonymized and reviewed by two gastrointestinal radiologists with 7 and 3 years of experience in imaging of peritoneal malignancy. They were blinded to the operative findings and the surgical outcome.
Imaging findings: (figures)
Images were studied for general findings, disease extent, severity and operability. General findings included morphological features of disease such as presence of ascites, peritoneal thickening, omental disease and solid or cystic masses. Volume of ascites was subjectively classified as mild, moderate and large. Peritoneal thickening was considered micronodular if there was perceptible non-uniform peritoneal thickening measuring <2 mm thickness; macronodular if discrete nodularity was perceived with peritoneal thickness measuring >2 mm; plaque like when there was diffuse peritoneal thickening; peritoneal masses if there were discrete measurable nodules >5 mm along the peritoneum. The greater omentum was considered to be involved if there was either omental fat stranding or omental caking. The size, enhancement characteristics and morphological features such as presence of necrosis and calcification were noted for solid masses. Masses were considered hypodense, isodense and hyperdense, if the density was less than, equal to or more than the muscle, respectively. Cystic masses were assessed for size and morphology (presence of septae and loculations; septal and mural enhancement and calcification).
The extent of disease was studied in terms of whether the disease involved the peritoneal cavity diffusely or was localized to a region of the peritoneal cavity. Radiological peritoneal cancer index (rPCI) adapted from the surgical peritoneal cancer index (PCI) described by Sugarbaker et al10 was documented (Table 1). Volume of upper abdominal disease was calculated from the sum of rPCI scores for upper abdominal sites [right upper abdomen (site 1), epigastric (site 2) and left upper abdomeninal regions (site 3)].
Table 1.
Radiological peritoneal cancer index adapted from surgical peritoneal cancer index described by Jacquet P and Sugarbaker PH10
| Region | Anatomical structures | |
|---|---|---|
| 0 | Central | Greater omentum, transverse colon |
| 1 | Right upper | Right subphrenic space |
| 2 | Epigastrium | Left lobe of liver, lesser omentum, falciform ligament |
| 3 | Left Upper | Left subphrenic space, spleen, tail of pancreas, anterior and posterior stomach surfaces |
| 4 | Left flank | Left paracolic gutter, descending colon |
| 5 | Left lower | Pelvic side wall lateral to sigmoid colon, sigmoid colon |
| 6 | Pelvis | Ovaries, tubes, uterus in female; prostate and seminal vesicles in males, bladder, pouch of Douglas, recto-sigmoid colon |
| 7 | Right lower | Right pelvic side wall, caecum, appendix |
| 8 | Right flank | Right paracolic gutter, ascending colon |
| 9 | Upper jejunum | Upper jejunum and its mesentery |
| 10 | Lower jejunum | Lower jejunum and its mesentery |
| 11 | Upper ileum | Upper ileum and its mesentery |
| 12 | Lower ileum | Lower ileum and its mesentery |
Disease severity and operability were assessed on imaging by detailed assessment of unfavourable sites. These were established after discussion with our surgeons based on their experience of cytoreductive surgery and HIPEC. Involvement of the following sites on imaging were considered unfavourable: structures around the epigastrium including the lesser omentum, lesser sac, porta hepatis, perigastric disease encasing the stomach and presence of biliary dilatation; small bowel and mesenteric disease; retroperitoneal structures (kidneys, ureters, psoas and iliacus muscles) and pelvic structures (bladder trigone, pelvic side wall and pre-sacral space). The severity of small bowel involvement was assessed using Yan’s CT classification11 which incorporates presence of ascites (class I), small bowel mural thickening and enhancement (class II); luminal distortion with kinking, nodular thickening of bowel wall or segmental bowel obstruction (class III).
Treatment and outcome
While 56 patients underwent cytoreductive surgery, 3 patients underwent diagnostic laparoscopy and biopsy. Of the 36 patients with malignant peritoneal mesothelioma, 34 underwent surgery. Completeness of cytoreduction was assessed using completeness of cytoreduction score which is based on the size of visible disease in the peritoneal cavity following surgery.12 Cytoreductive surgery was considered complete if there was no visible disease or when less than 2.5-mm sized visible disease was left behind. The term major tumour debulking was used if there was visible tumour larger than 2.5 mm. 22/34 had complete cytoreduction while 12/34 had major tumour debulking. 22 out of 23 patients with cystic mesothelioma underwent surgery; 21 of them had complete cytoreduction and one had MTD.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics software for Macintosh, Version 20.0. Armonk, NY: IBM Corp. Patients with malignant peritoneal mesothelioma and cystic mesothelioma were analysed separately as the surgical outcome and prognosis of these are different. The frequency and percentage frequency were obtained for various imaging findings. The mean with two standard deviations and the range were obtained for continuous variables such as size of the lesion and rPCI. Imaging features of different histopathological types of mesothelioma were compared and tabulated.
Only patients with malignant mesothelioma who underwent cytoreductive surgery were included when correlating imaging features with surgical outcome (n = 34). Various imaging findings were correlated with completeness of cytoreduction using Χ2 test, independent sample T test or Mann–Whitney U test. Two-by-two tables were obtained to assess the association between unfavourable sites on imaging and completeness of cytoreduction. Multivariate binary logistic regression analysis was performed to identify the key anatomical sites that reduced likelihood of complete cytoreduction if thought to be involved on CT imaging. The level of significance was set as <0.05.
Results
Malignant mesothelioma
Table 2 shows the imaging features of patients with malignant peritoneal mesothelioma. Figures 1–3 show examples of the different histological subtypes of malignant mesothelioma. Except for the presence of solid masses (p = 0.007), there were no statistically significant differences in the patient and imaging characteristics between different histological subtypes.
Table 2.
Patient and imaging characteristics in 36 patients with malignant peritoneal mesothelioma according to histological subtypes
| Variables | Epithelioid (n = 23) | Biphasic (n = 3) | Well differentiated papillary (n = 10) | p value |
|---|---|---|---|---|
| Sex | M:F = 15:8 | M:F = 2:1 | M:F = 5:5 | 0.697 |
| Age | 58.2 ± 9.8 years (40–77 years) | 68.6 ± 14.5 years (52–79 years) | 49.4 ± 15.3 years (29–78 years) | 0.057 |
| Disease extent: | ||||
| Diffuse | 21 (91.3 %) | 2 (66.6%) | 9 (90%) | 0.439 |
| Localized | 2 (8.7 %) | 1 (33.3%) | 1 (10%) | |
| Ascites | 18 (78.2%) | 1 (33.3%) | 8 (80%) | 0.219 |
| Solid masses | 4 (17.3%) | 3 (100%) | 2 (20%) | χ2 = 9.843, p = 0.007 |
| Peritoneal thickening | 21 (91.3%) | 2 (66.6%) | 7 (70%) | 0.231 |
| Micronodular | 8 (34.7%) | 1 (33.3%) | 4 (40%) | |
| Macronodular | 8 (34.7%) | 0 (0%) | 2 (20%) | |
| Plaques | 10 (43.3%) | 1 (33.3%) | 3 (30%) | |
| Papillary | 2 (8.7%) | 1 (33.3%) | 1 (10%) | |
| Omental disease | 20 (86.9%) | 3 (100%) | 9 (90%) | 0.789 |
| Omental caking | 16 (69.5%) | 1 (33.3%) | 5 (50%) | |
| Fine stranding | 4 (17.3%) | 2 (66.6%) | 4 (40%) | |
| Mesenteric involvement | 19 (82.6%) | 3 (100%) | 6 (60%) | 0.064 |
| Mesenteric fold thickening | 19 (82.6%) | 1 (33.3%) | 5 (50%) | |
| Mesenteric retraction | 1 (4.3%) | 0 (0%) | 1 (10%) | |
| Mesenteric mass | 3 (13%) | 2 (66.6%) | 0 (0%) | |
| Small bowel involvement | 13 (56.5%) | 1 (33.3%) | 3 (30%) | 0.329 |
| Mural thickening | 6 (26%) | 1 (33.3%) | 1 (10%) | |
| Distortion of bowel lumen | 11 (47.8%) | 1 (33.3%) | 2 (20%) | |
| Rind around small bowel | 7 (30.4%) | 1 (33.3%) | 1 (10%) | |
| Small bowel obstruction and compartmentalization | 2 (8.7%) | 0 (0%) | 1 (10%) | |
| Subphrenic disease | 1 (4.3%) | 1 (33.3%) | 2 (20%) | 0.186 |
| Upper abdominal disease | 14 (60.8%) | 2 (66.6%) | 6 (60%) | 0.978 |
| Radiological PCI | 21 ± 13 (3–39) | 17 ± 7 (12–25) | 15 ± 9 (2–30) | 0.824 |
Figure 1.
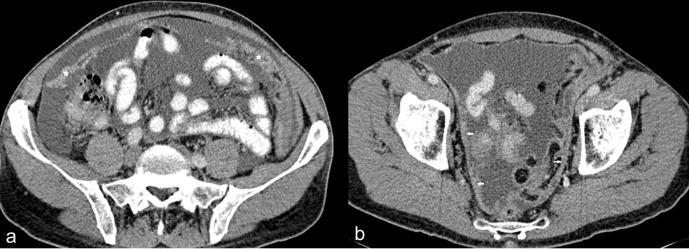
(a, b) Contrast enhanced CT of a 53-year-old male patient with epithelioid mesothelioma. (a) Axial CT section through the mid-abdomen shows caking of the greater omentum (asterisk) and nodular mesenteric fold thickening (arrows) (b) Axial CT section through the pelvis shows plaque like nodular peritoneal thickening (arrows).
Figure 3.
(a, b) Contrast enhanced CT of a 42-year-old female patient with well differentiated papillary mesothelioma showing moderate volume ascites, omental caking (asterisk) and plaque like peritoneal thickening (arrows).
Table 3 compares imaging characteristics of patients who underwent complete cytoreduction (n = 22) or major tumour debulking (MTD) (n = 12) for malignant peritoneal mesothelioma.
Table 3.
Comparison of imaging findings of malignant peritoneal mesothelioma (n = 34) between 22 patients who underwent CC and 12 patients who underwent MTD
| Variables | CC (n = 22) | MTD (n = 12) | p value |
|---|---|---|---|
| Sex | M:F = 12:10 | M:F = 8:4 | 0.377 |
| Age | 51 ± 11.2 years (29–67 years) | 65.3 ± 9.7 years (51–79 years) | 0.001 |
| Disease extent: | |||
| Diffuse | 19 | 12 | 0.257 |
| Localized | 3 | 0 | |
| Ascites | 16 | 10 | 0.402 |
| Solid masses present | 5 | 3 | 0.584 |
| Size of solid mass | 12.3 ± 7.2 cm (6.5–25 cm) | 9.4 ± 5.9 cm (4.3–16 cm) | |
| Peritoneal thickening | 17 | 11 | 0.290 |
| Micronodular | 8 | 5 | |
| Macronodular | 7 | 3 | |
| Plaques | 7 | 6 | |
| Papillary | 2 | 1 | |
| Omental disease | 19 | 12 | 0.257 |
| Omental caking | 12 | 11 | |
| Fine stranding | 7 | 1 | |
| Mesenteric involvement | 14 | 10 | 0.211 |
| Mesenteric fold thickening: | 14 | 10 | |
| Mesenteric retraction | 2 | 0 | |
| Mesenteric mass | 3 | 2 | |
| Small bowel involvement | 7 | 9 | 0.019 |
| Mural thickening | 4 | 4 | 0.279 |
| Distortion of bowel lumen | 6 | 8 | 0.031 |
| Rind around small bowel | 1 | 7 | 0.001 |
| Small bowel obstruction and compartmentalization | 1 | 1 | 0.588 |
| Yan CT grading | |||
| Class 0 | 5 | 1 | 0.694 |
| Class I | 6 | 3 | |
| Class II | 9 | 6 | |
| Class III | 2 | 2 | |
| Subphrenic disease | 2 | 1 | 0.721 |
| Upper abdominal disease | 2 ± 2 | 5 ± 4 | 0.025 |
| Radiological PCI | 16 ± 11 | 24 ± 12 | 0.048 |
| Surgical PCI | 17 ± 12 (3–39), N = 13 | 34 ± 5 (25–39), N = 8 | < 0.001 |
| Recurrence | 6 | 1 | 0.379 |
| Survival | 43.6 ± 42 months (5–89 months) | 23.5 ± 23.5 months (5–67 months) | 0.074 |
| Alive | 18 | 6 | 0.102 |
| Dead | 4 | 5 |
CC, complete cytoreduction; MTD, majortumour debulking.
Patients who underwent MTD were older with a mean age of 65.3 ± 9.7 years compared with 51 years for patients who underwent complete cytoreduction. Mean rPCI (24 ± 12) and surgical PCI (34 ± 5) were significantly higher in patients who had MTD (p < 0.05). Patients who underwent MTD had larger volume of disease in the upper abdomen reflected by higher mean sum of rPCI scores in the upper abdominal sites (sites 1, 2 and 3); 5 for MTD compared to 2 for complete cytoreduction, p = 0.025. Similarly, there was more frequent small bowel involvement in patients who underwent MTD, 75 vs 32% for complete cytoreduction (p = 0.019) with distortion of small bowel (66.5% among MTD vs 27.2% for complete cytoreduction, p = 0.031) and a rind of soft tissue around the small bowel being more common (58.3% among MTD vs 4% for complete cytoreduction, p = 0.001).
Table 4 lists the unfavourable sites of involvement as assessed on CT and compares patients who underwent complete cytoreduction and MTD. There was significant difference in the number of patients who had perigastric disease (33.3% among MTD vs 4.5% among complete cytoreduction, p = 0.042) and small bowel involvement (75% among MTD vs 33.3% among complete cytoreduction, p = 0.019) among those who underwent complete cytoreduction and MTD. Among patients with no CT evidence of unfavourable sites, 78.6% of patients underwent complete cytoreduction. Conversely, 75% of patients who underwent MTD had disease in one or more listed unfavourable sites on CT.
Table 4.
Table comparing the unfavourable sites of involvement among patients who underwent CC and MTD for malignant peritoneal mesothelioma
| Unfavourable sites | Site (n) | CC (n = 22) | MTD (n = 12) | p value |
|---|---|---|---|---|
| Epigastric region | Lesser omentum – 10 | 4 (18.1%) | 6 (50%) | 0.062 |
| Disease around left lobe of liver – 3 | 2 (9%) | 1 (8.2%) | 0.721 | |
| Perigastric disease – 5 | 1 (4.5%) | 4 (33.3%) | 0.042 | |
| Porta hepatis – 8 | 4 (18.1%) | 4 (33.3%) | 0.279 | |
| CBD obstruction – 0 | 0 (0) | 0 (0) | ||
| Paraduodenal disease – 0 | 0 (0) | 0 (0) | ||
| Liver involvement – 1 | 0 (0) | 1 (8.2%) | ||
| Splenic involvement – 2 | 0 (0) | 2 (16.6%) | ||
| Mesentery and small bowel | Mesentery – 24 | 14 (63.6%) | 10 (83.3%) | 0.211 |
| Root of mesentery – 0 | 0 (0) | 0 (0) | ||
| Small bowel – 16 | 7 (33.3%) | 9 (75%) | 0.019 | |
| Retroperitoneum | Hydronephrosis – 1 | 0 (0) | 1 (8.2%) | |
| Bladder trigone – 1 | 0 (0) | 1 (8.2%) | ||
| Pelvic side wall – 0 | 0 (0) | 0 (0) | ||
| Lymphnodes | Retroperitoneum and abdomen – 1 | 0 (0) | 1 (8.2%) | 0.279 |
| Epiphrenic – 8 | 4 (18.1%) | 4 (33.3%) | ||
| Concurrent intrathoracic disease | Pleural effusion and pleural nodules – 2 | 0 (0) | 2 (16.6%) | |
| Abdominal wall disease | Abdominal wall nodules – 4 | 2 (9%) | 2 (16.6%) | 0.444 |
Of all the imaging findings, multivariate binary logistic regression analysis revealed that CT evidence of a rind of soft tissue around the small bowel was the strongest predictor of inability to achieve complete cytoreduction (B = −5.395, SE = 2.23, p = 0.016). MTD was more likely when there was large volume upper abdominal disease (B = 0.795, SE = 0.433, p = 0.06).
Cystic mesothelioma
Table 5 summarizes the patient characteristics and the imaging findings of 23 patients with cystic mesothelioma. Over 90% (n = 21) of cystic mesothelioma was multiloculated and loculations were not perceptible in two patients. Figure 4 shows examples of cystic mesothelioma. There was significant gender difference in the radiological extent of disease with rPCI being 20 ± 9 in males and 11 ± 10 in females, p = 0.047. Mesenteric involvement was more commonly seen in males, Χ2 = 5.49, p = 0.026.
Table 5.
Patient characteristics and imaging findings of 23 patients with cystic mesothelioma
| Variables | n (%) n = 23 | Male (n = 10) | Female (n = 13) | p value |
|---|---|---|---|---|
| Age | 23 | 49.9 ± 15.1 years (30–70 years) | 50.2 ± 13 years (30–67 years) | 0.956 |
| Distribution: | ||||
| Diffuse | 7 (30.4) | 2 | 5 | 0.634 |
| Localized | 16 (69.5) | 8 | 8 | |
| rPCI | 23 | 20 ± 9 | 11 ± 10 | 0.046 |
| Size of lesion | 23 | 21 ± 8.4 cm (15–43 cm) | 14.2 ± 6.7 cm (4–28 cm) | 0.06 |
| Morphology of the lesions: | ||||
| Multiloculated cystic lesion | 21 (91.3) | 10 | 11 | 0.545 |
| No perceptible loculations | 2 (8.6) | 0 | 2 | |
| Enhancing septae | 4 (17.4) | 2 | 2 | 0.632 |
| Size of the locule | 21 | 6 ± 3.6 cm | 4.5 ± 6 cm | 0.437 |
| Locations of the lesions within the peritoneal cavity: | ||||
| Sub phrenic space | 4 (17.4) | 3 | 1 | 0.200 |
| Lesser omentum/lesser sac | 5 (21.7) | 4 | 1 | 0.089 |
| Porta hepatis | 3 (13) | 2 | 1 | 0.398 |
| Greater omentum | 13 (56.6) | 8 | 5 | 0.195 |
| Small bowel mesentery | 12 (52.2) | 8 | 4 | 0.026 |
| Root of mesentry | 1 (4.3) | 0 | 1 | 0.565 |
| Small Bowel | 5 (21.7) | 4 | 1 | 0.089 |
| Pelvis | 21 91.3) | 9 | 12 | 0.189 |
| Pelvic side wall | 1 (4.3) | 0 | 1 | 0.565 |
| Hydronephrosis | 1 (4.3) | 1 | 0 | 0.435 |
| Paracolic gutters | 13 (56.5) | 6 | 7 | 0.955 |
| Other findings: | ||||
| Ascites | 6 (26) | 4 | 2 | 0.197 |
| Peritoneal thickening | 4 (17.4) | 2 | 2 | 0.596 |
| Ovarian/adnexal lesions | 4 | - | 4 | |
| Epiphrenic nodes | 2 (8.6) | 1 | 1 | 0.692 |
| Pleural plaques | 1 (4.3) | 1 | 0 | |
| Intrathoracic disease | 0 | 0 | 0 | |
| Treatment and outcome: | ||||
| Surgical PCI | 10 | 20 ± 11 | 9 ± 5 | 0.045 |
| Surgery: | ||||
| CC + HIPEC | 21 (91.3) | 9 | 12 | 0.355 |
| MTD | 1 (4.3) | 0 | 1 | |
| EPIC | 4 (17.4) | 1 | 3 | |
| Recurrence | 2 (8.6) | 2 | 0 | 0.178 |
| Time of recurrence | 2 | 5 months, 18 months | - | |
| Survival: | 23 | 49.7 ± 26.4 months | 51 ± 23 months | 0.895 |
| Alive | 22 | 10 | 12 | 0.565 |
| Dead | 1 | 0 | 1 |
CC, complete cytoreduction; EPIC, early post-operative intraperitoneal chemotherapy; HIPEC, intraoperative hyperthermic intraperitoneal chemotherapy; MTD, major tumour debulking; rPCI, radiologicalperitoneal cancer index.
Figure 4.
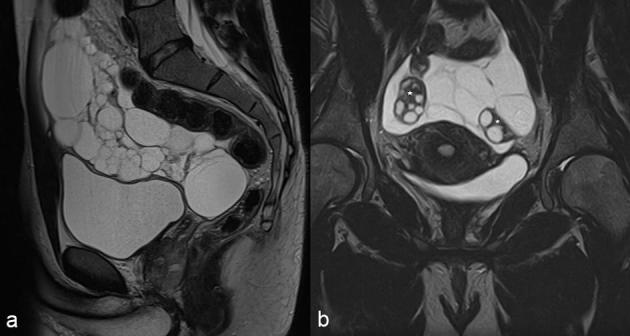
(a, b) MRI images of two different patients with cystic mesothelioma (b) Sagittal MRI of a 41-year-old male patient showing multiloculated cystic lesions in the pelvic cul-de-sac. (b) Coronal MRI of a 36-year-old female patient showing multiloculated cystic lesions in the pelvis which surrounds the normal ovaries (asterisk).
Among the female patients, 4/13 patients had concurrent adnexal lesions on imaging: two had unilateral benign ovarian cyst, one had bilateral benign ovarian cysts and one had bilateral solid ovarian masses. Ovaries were normal in seven patients and removed in two patients.
In addition to cytoreductive surgery, 21 patients received HIPEC and four patients received additional early post-operative intraperitoneal chemotherapy. All except one patient with cystic mesothelioma underwent complete cytoreduction. The mean radiological PCI of patients who underwent complete cytoreduction was 15 ± 9. One patient who underwent MTD had high volume disease with rPCI of 30 and lesions involved small bowel mesentery, surface of the left lobe of liver, lesser sac, left subphrenic space, left paracolic gutter and pelvis with unusual calcified nodules in the peritoneum and the omentum (Figure 5).
Figure 5.
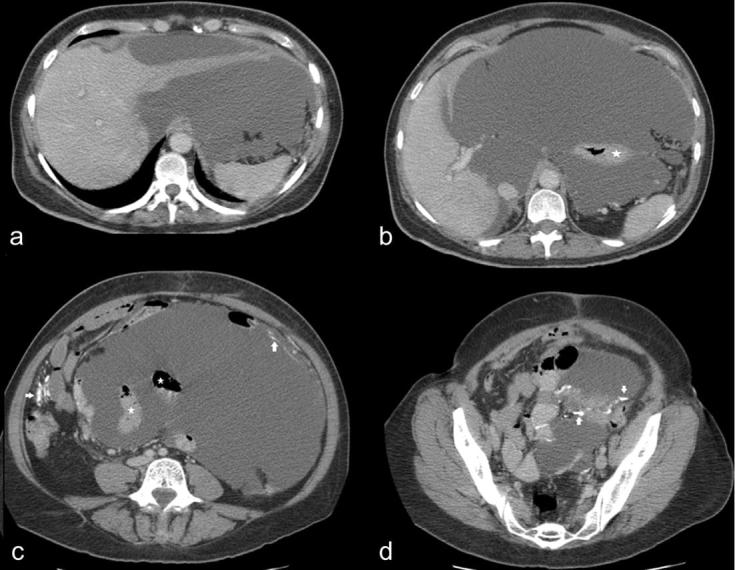
(a–d) 64-year-old female patient who underwent major tumour debulking for widespread cystic mesothelioma. CT axial sections show multiloculated cystic lesions in the peritoneal cavity encasing the left lobe of liver (a), the stomach (asterisk in b) and small bowel loops (shown as asterisk in c). This patient had unusual calcified nodules in the peritoneum and the omentum (arrows in c, d).
Discussion
Peritoneal mesothelioma is an uncommon tumour arising from the mesothelial cells lining the peritoneal cavity. The peritoneal cavity is the second commonest site of disease accounting for a third to fifth of all mesothelioma.2 Unlike the more common pleural counterpart, only half of patients with peritoneal mesothelioma give a history of asbestos exposure.12 Cystic mesothelioma is considered borderline malignant and amenable to cure with favourable long-term outcome after cytoreductive surgery and HIPEC. Prior to the era of cytoreductive surgery and HIPEC, malignant peritoneal mesothelioma was considered to be a tumour with bad prognosis and was uniformly fatal. As with other peritoneal malignancies, the survival benefits after cytoreductive surgery and HIPEC come at the cost of high morbidity and potential mortality and are mainly dependant on completeness of cytoreduction.3–9,13–20 As complete cytoreduction is the strongest predictor of long-term survival in patients with malignant peritoneal mesothelioma it is essential to identify radiological features that might prevent this. In this study, we aimed to identify key imaging findings which reduced the chance of complete cytoreduction being achieved.
Malignant mesothelioma
The histological subtypes of malignant mesothelioma include epithelioid, biphasic or sarcomatoid and well-differentiated papillary mesothelioma with epithelioid being the most common. Although there are imaging features which are commonly seen in a particular histological subtype of malignant mesothelioma, imaging cannot reliably differentiate one subtype from the other. For example, the majority (91.3%) of the patients with epithelioid mesothelioma had diffuse peritoneal disease with ascites, peritoneal thickening, caking of the omentum and mesenteric fold thickening (Figure 1). Solid masses were less common (17%) in epithelioid mesothelioma but a common feature in biphasic mesothelioma. These findings are similar to those previously described.21,22 One patient with biphasic mesothelioma had unusual imaging appearance with intensely enhancing right perihepatic masses (Figure 2) secondarily involving the liver. Su et al23 have described five patients with similar perihepatic involvement. Most (90%) patients with well differentiated papillary mesothelioma had diffuse peritoneal disease with moderate to large volume ascites (80%) and omental caking (90%) (Figure 3).
Figure 2.
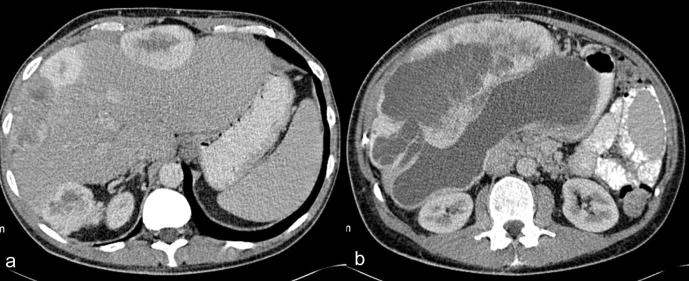
(a, b) Contrast enhanced CT of a 57-year-old male patient with biphasic mesothelioma showing (a) multiple intensely enhancing perihepatic masses with central necrosis and (b) a large subhepatic mass with intensely enhancing solid component and non-enhancing necrotic areas.
We compiled a list of unfavourable sites based on the experience of our surgeons who see involvement at these sites lead to increased surgical complexity or inability to achieve complete cytoreduction. This was similar to radiological criteria proposed by Tan et al24 for resectability of peritoneal malignancy in general, the exception being the omental cake, which we did not include in our list as an unfavourable finding, since the greater omentum can be easily removed at surgery. We also quantified the volume of upper abdominal disease by adding the rPCI scores of the upper abdominal sites (site1 + site2 + site3). Univariate analysis showed more common occurrence of incomplete cytoreduction in older patients; those with high radiological PCI; large volume upper abdominal disease and in those with small bowel involvement, p < 0.05.
We found a significant difference in the unfavourable sites of involvement and the volume of upper abdominal disease among patients who underwent MTD compared to complete cytoreduction. 75% of patients who underwent MTD had involvement of one or more unfavourable sites on imaging while no unfavourable sites were involved in 79% of patients who had a complete cytoreduction.
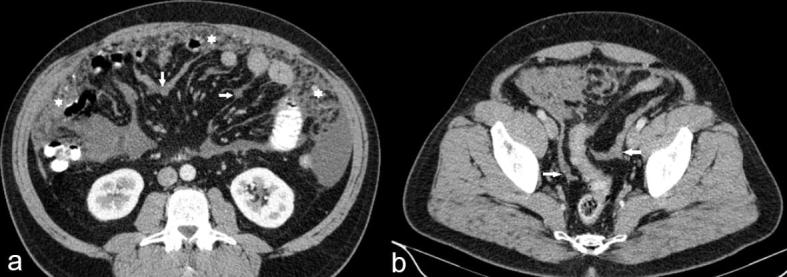
Multivariate analysis supported the findings of univariate analysis. Two imaging findings which best predicted the outcome of cytoreductive surgery in patients with malignant peritoneal mesothelioma were the presence of a rind of soft tissue around the small bowel (Figure 6) and large volume upper abdominal disease.
Figure 6.
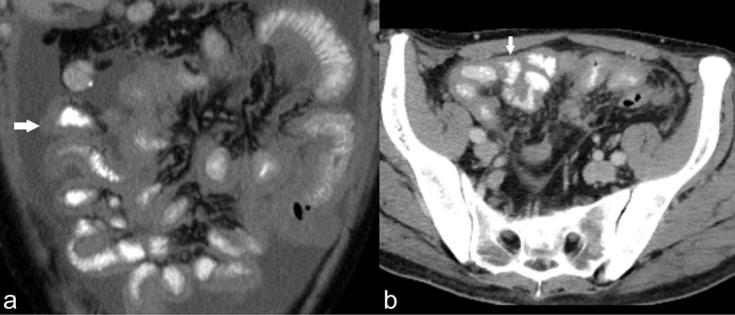
(a, b) (a) Coronal and (b) axial CT sections of two different patients with epithelioid malignant mesothelioma showing a rind of soft tissue around the small bowel (arrows).
Systematically reviewing the unfavourable sites on imaging in patients with malignant peritoneal mesothelioma may prove useful in selecting patients in whom complete cytoreduction is unlikely. Yan et al11 studied the utility of CT in selecting patients with malignant mesothelioma for cytoreductive surgery. They described a significant association between incomplete cytoreduction and two imaging findings such as epigastric disease measuring larger than 5 cm and Yan’s class III involvement of small bowel and mesentery.11 Although our results were in principle similar to that of Yan et al,11 we did not find significant association between Yan’s CT grading of small bowel and mesenteric involvement, and the surgical outcome. Although large volume upper abdominal disease was associated with incomplete cytoreduction, we did not find an association between the size of epigastric disease and the outcome. This may be because patients with small bowel obstruction on imaging (Yan’s class III) and large volume disease measuring >5 cm in the epigastric region would not have been selected for cytoreductive surgery at our centre.
Cystic mesothelioma
Cystic mesothelioma is a rare condition and most of the information available in the literature is in the form of case reports and case series. Cystic mesothelioma has always been described as a condition which is more common in females, but we did not find similar findings in our series.21,25,26 The majority (91%) presented with multiloculated cystic lesions in the peritoneal cavity with the pelvis and the paracolic gutters being the most common sites of involvement as also described in literature.21,25–27
More than half of our patients (56%) had cystic lesions in the small bowel mesentery and greater omentum. Nearly a quarter (22%) of the patients had lesions in the lesser omentum. Abdominal disease is generally considered to be an extension of pelvic disease in cystic mesothelioma.27 Thus, radiologists must consider cystic mesothelioma in the differential diagnosis of multiloculated cystic lesion in the peritoneal cavity, especially when the epicentre of the lesion is in the pelvis and the paracolic gutters. Other conditions which closely mimic the appearance of cystic mesothelioma include cystic lymphangioma and pseudomyxoma peritonei. Cystic lymphangioma has no pelvic predilection and is more commonly seen in the retroperitoneum. Identification of a mucinous primary neoplasm would help immensely in the diagnosis of pseudomyxoma peritonei. Other cystic conditions like endometriosis and peritoneal hydatidosis in endemic locations may mimic cystic mesothelioma.21,28
There was a gender difference in the size and radiological extent of cystic mesothelioma. Male patients had larger lesions reflected by higher rPCI and the mesentery was more commonly involved. These findings have not been described so far and need to be validated in a larger number of patients.
Limitations of the study
Since this study was conducted in a tertiary referral centre, image quality and protocol was heterogeneous and this is a limitation of the study. In view of the retrospective nature of the study, data on surgical PCI were incomplete and available only for 36 patients. Thus, correlation between surgical and radiological PCI was not possible in this study. Lastly, imaging features of peritoneal mesothelioma described in this study are limited to those who underwent a surgical procedure in our centre and thus may not represent the entire spectrum of imaging findings in this entity.
Conclusion
Presence of small bowel involvement in the form of a rind of soft tissue around the small bowel and large volume upper abdominal disease on imaging were identified as key findings that reduced the likelihood of achieving complete cytoreduction in patients with malignant peritoneal mesothelioma. There may be gender differences in cystic mesothelioma with males presenting with larger lesions and more frequent mesenteric involvement. However, these findings must be confirmed in a multicentre study.
Contributor Information
Anuradha Chandramohan, Email: anuradhachandramohan@gmail.com.
Andrew Thrower, Email: Andrew.Thrower@hhft.nhs.uk.
Nehal Shah, Email: Nehal.Shah@hhft.nhs.uk.
Faheez Mohamed, Email: Faheez.Mohamed@hhft.nhs.uk.
References:
- 1.Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007; 18: 985–90.10.1093/annonc/mdl345 [DOI] [PubMed] [Google Scholar]
- 2.Mohamed F, Sugarbaker PH. Peritoneal mesothelioma. Curr Treat Options Oncol 2002; 3: 375–86.10.1007/s11864-002-0003-6 [DOI] [PubMed] [Google Scholar]
- 3.Baratti D, Kusamura S, Iusco D, Bonomi S, Grassi A, Virzì S, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014; 57: 858–68.10.1097/DCR.0000000000000149 [DOI] [PubMed] [Google Scholar]
- 4.Alexander HR Jr, Bartlett DL, Pingpank JF, Libutti SK, Royal R, Hughes MS, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013; 153: 779–86.10.1016/j.surg.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magge D, Zenati MS, Austin F, Mavanur A, Sathaiah M, Ramalingam L, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014; 21: 1159–65.10.1245/s10434-013-3358-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robella M, Vaira M, Mellano A, Marsanic P, Cinquegrana A, Borsano A, et al. Treatment of diffuse malignant peritoneal mesothelioma (DMPM) by cytoreductive surgery and HIPEC. Minerva Chir 2014; 69: 9–15. [PubMed] [Google Scholar]
- 7.Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015; 22: 1686–93.10.1245/s10434-014-3978-x [DOI] [PubMed] [Google Scholar]
- 8.Desantis M, Bernard JL, Casanova V, Cegarra-Escolano M, Benizri E, Rahili AM, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg 2015; 400: 37–48.10.1007/s00423-014-1253-z [DOI] [PubMed] [Google Scholar]
- 9.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009; 27: 6237–42.10.1200/JCO.2009.23.9640 [DOI] [PubMed] [Google Scholar]
- 10.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996; 82: 359–74. [DOI] [PubMed] [Google Scholar]
- 11.Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 2005; 103: 839–49.10.1002/cncr.20836 [DOI] [PubMed] [Google Scholar]
- 12.Antman KH, Pomfret EA, Aisner J, MacIntyre J, Osteen RT, Greenberger JS. Peritoneal mesothelioma: natural history and response to chemotherapy. J Clin Oncol 1983; 1: 386–91.10.1200/JCO.1983.1.6.386 [DOI] [PubMed] [Google Scholar]
- 13.Alexander HR Jr, Burke AP. Diagnosis and management of patients with malignant peritoneal mesothelioma. J Gastrointest Oncol 2016; 7: 79–86.10.3978/j.issn.2078-6891.2015.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baratti D, Kusamura S, Deraco M. Diffuse malignant peritoneal mesothelioma: systematic review of clinical management and biological research. J Surg Oncol 2011; 103: 822–31.10.1002/jso.21787 [DOI] [PubMed] [Google Scholar]
- 15.Chiesa JC. Malignant peritoneal mesothelioma: advances in diagnosis and management. J Am Osteopath Assoc 1986; 86: 31–3. [PubMed] [Google Scholar]
- 16.Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol 2006; 32: 676–81.10.1016/j.ejso.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 17.Miura JT, Johnston FM, Gamblin TC, Turaga KK. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol 2014; 21: 3947–53.10.1245/s10434-014-3803-6 [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker PH, Turaga KK, Alexander HR, Deraco M, Hesdorffer M. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. J Oncol Pract 2016; 12: 928–35.10.1200/JOP.2016.011908 [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker PH, Yan TD, Stuart OA, Yoo D. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2006; 32: 686–91.10.1016/j.ejso.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 20.Yan TD, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Morbidity and mortality assessment of cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma—a prospective study of 70 consecutive cases. Ann Surg Oncol 2007; 14: 515–25.10.1245/s10434-006-9187-5 [DOI] [PubMed] [Google Scholar]
- 21.Park JY, Kim KW, Kwon HJ, Park MS, Kwon GY, Jun SY, et al. Peritoneal mesotheliomas: clinicopathologic features, CT findings, and differential diagnosis. AJR Am J Roentgenol 2008; 191: 814–25.10.2214/AJR.07.3628 [DOI] [PubMed] [Google Scholar]
- 22.Levy AD, Arnáiz J, Shaw JC, Sobin LH. From the archives of the AFIP: primary peritoneal tumors: imaging features with pathologic correlation. Radiographics 2008; 28: 583–607.10.1148/rg.282075175 [DOI] [PubMed] [Google Scholar]
- 23.Su SS, Zheng GQ, Liu YG, Chen YF, Song ZW, Yu SJ, et al. Malignant peritoneum mesothelioma with hepatic involvement: a single institution experience in 5 patients and review of the literature. Gastroenterol Res Pract 2016; 2016: 6242149.10.1155/2016/6242149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan GH, Kwek JW, Hosseini R, Chanyaputhipong J, Tham CK, Soo KC, et al. Proposed radiological criteria for pre-operative determination of resectability in peritoneal-based malignancies. J Med Imaging Radiat Oncol 2016; 60: 337–43.10.1111/1754-9485.12456 [DOI] [PubMed] [Google Scholar]
- 25.Wong WL, Johns TA, Herlihy WG, Martin HL. Best cases from the AFIP: multicystic mesothelioma. Radiographics 2004; 24: 247–50.10.1148/rg.241035068 [DOI] [PubMed] [Google Scholar]
- 26.Katsube Y, Mukai K, Silverberg SG. Cystic mesothelioma of the peritoneum: a report of five cases and review of the literature. Cancer 1982; 50: 1615–22.10.1002/1097-0142(19821015)50:8<1615::AID-CNCR2820500826>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 27.Weiss SW, Tavassoli FA. Multicystic mesothelioma. an analysis of pathologic findings and biologic behavior in 37 cases. Am J Surg Pathol 1988; 12: 737–46. [PubMed] [Google Scholar]
- 28.Acharya AN, Gupta S. Peritoneal hydatidosis: a review of seven cases. Trop Gastroenterol 2009; 30: 32–4. [PubMed] [Google Scholar]