Abstract
Objective:
The aim of the study was to evaluate the CT and fluorine-18-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) imaging findings of lung mucosa associated lymphoid tissue (MALT) lymphoma.
Methods:
28 patients with histologically confirmed pulmonary MALT lymphoma who underwent a chest CT and 18F-FDG PET/CT for staging were retrospectively analysed. The CT images were evaluated to determine morphological pattern of appearance, laterality, localization, number, size, presence of thoracic lymphadenopaties and secondary/combined findings. PET images were analysed visually and semi-quantitatively by measuring the maximum standardized uptake value (SUVmax), lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio. The relationship between qualitative and semi-quantitative features at 18F-FDG PET/CT and CT findings were also analysed.
Results:
A total of 57 pulmonary lesions were identified by CT: 37 areas of consolidation, 4 masses, 12 nodules and 4 ground-glass opacities. Solitary and multiple lesions were detected in 10 and 18 patients, respectively; among patients with multiple lesions, 16 were bilateral and 2 unilateral. 18F-FDG PET/CT revealed increased 18F-FDG uptake in 47/57 lesions, in 26/28 patients. 18F-FDG avidity was significantly associated only with tumour size.
Conclusions:
Pulmonary MALT lymphoma is 18F-FDG avid in most cases and 18F-FDG avidity is correlated with tumour size. Consolidation is the most frequent morphological pattern of disease presentation.
Advances in knowledge:
This study demonstrated that lung MALT lymphoma are 18F-FDG avid in most cases depending on tumour size. Single or multiple areas of consolidation are the most common pattern of presentation of lung MALT lymphoma at CT.
Introduction
Primary non-Hodgkin's lymphoma (NHL) of the lung is a very rare disease accounting for only 0.4% of all malignant lymphomas.1–4 The most common subtype of pulmonary NHL is the mucosa-associated lymphoid tissue (MALT) type.5 MALT lymphoma, also called extranodal marginal zone B cell lymphoma, involves the mucosa-associated lymphoid tissue possibly arising from any mucosal site. It originates in the marginal zone of the MALT from B cells; this B cells are post-germinal-centre cells with rearranged and mutated immunoglobulin heavy and light chain genes.6 Besides stomach, lung is one of the most frequent organs involved. Lung MALT lymphoma is a disease that arises from bronchial-associated lymphoid tissue and it is considered to be related to long-term exposure to a variety of antigenic stimuli, like smoking, inflammatory conditions or autoimmune diseases.7,8 Pulmonary MALT lymphomas have no specific morphological appearance and CT may show single or multiple areas of consolidation, masses, nodules and/or other rarer findings.9–13 This suggests that potentially any radiological abnormal parenchymal finding could be consistent with lung lymphoma.14 Fluorine-18-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) is an imaging tool used for staging, restaging and follow-up in various tumours, including Hodgkin and non-Hodgkin lymphoma15–17; so far, its real role in evaluating MALT lymphoma is still under investigation.18–20 In particular, it is still under debate if this rare type of lymphoma is 18F-FDG avid or not21–23 ; pulmonary localization of MALT lymphoma seems to be one of the most 18F-FDG avid site, together with head and neck,21 liver24 and breast localizations,25 but specific studies about its glucidic metabolic behaviour are rare in the literature and based upon a small number of patients. The purpose of this study is to describe the radiological/morphological and metabolic/functional characteristics of chest CT and of 18F-FDG PET/CT in a series of 28 patients with histological diagnosis of pulmonary MALT lymphoma.
Methods And Materials
Patients
We have retrospectively screened about 30,000 patients studied with 18F-FDG PET/CT in our Nuclear Medicine centre from January 2006 to January 2017 using our institutional radiology information system (RIS). Among these patients, pulmonary MALT lymphoma was identified in 32 patients. The histopathological diagnosis was based on the World Health Organization criteria.26 In all patients, immunologic phenotyping on paraffin sections was done to demonstrate heavy and light chain restriction and phenotype CD20 + CD5 − CD10 − cyclinD1 which were microscopically consistent with lung MALT lymphoma. We excluded patients who did not have an histological diagnosis available and those without a whole body 18F-FDG PET/CT: two patients were excluded because of lack of histological data and two patients because of the absence of a 18F-FDG PET/CT study. We reviewed the medical records and pathology reports of these 28 patients: epidemiological features (age at diagnosis, gender), tumour stage according to Ann Arbor classification, morphological/radiological pattern by thoracic CT, metabolic features by 18F-FDG PET/CT, treatment modality and follow-up data were collected and analysed in all patients. 20 patients underwent chest CT and whole body 18F-FDG PET/CT scans before any treatment while 8 patients underwent only 18F-FDG PET/CT; in these patients the low-dose CT of the PET study was considered; the maximum interval between chest CT and PET/CT was 3 weeks. Baseline features of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of 28 patients
| n (%) | |
|---|---|
| Age average (range) | 60.5 (33–76) |
| Sex male | 18 (64%) |
| female | 10 (36%) |
| Tumour stage at diagnosis | |
| I | 16 (57%) |
| II | 6 (21%) |
| III | 1 (4%) |
| IV | 5 (18%) |
| Tumour size (mm) average (range) | 35 (5–114) |
| Location | |
| Unilateral | 12 (43%) |
| Bilateral | 16 (57%) |
| Single lesion | 10 (36%) |
| Multiple lesions | 18 (64%) |
| Pleural effusion | 2 (7%) |
| Mosaic pattern | 1 (3%) |
| Lung cysts | 4 (145) |
| Thoracic lymph nodes | 8 (29%) |
| 18F-FDG PET/CT pos | 26 (93%) |
| SUVmax average (range) | 5.3 (1.1–13) |
| lesion-to-liver SUVmax ratio average (range) | 2.3 (0.4–6) |
| lesion-to-blood pool SUVmax ratio average (range) | 2.8 (0.5–8.2) |
CMT, chemotherapy; FDG, fluorodesoxyglucose; Mm, millimeter; Pos, positive; RTT, radiotherapy; SUV, standardized uptake value.
Chest CT imaging and interpretation
20 patients were scanned using a 128-detector CT scanner (Somatom Definition Flash; Siemens, Erlangen, Germany) with the following parameters: collimation, 128 × 0.6 mm; beam pitch, 1.2; rotation time, 0.5 s; tube voltage, 120 kV; and tube current, 110 mA. The acquisition, extending from lung apex to lung base, was performed in inspiratory apnoea without spirometric control of lung volume. The volume was reconstructed as 1-mm thick sections, applying a sharp reconstruction algorithm and a lung window setting (window width, 1600 HU; window level, −600 HU), and as 2-mm thick sections, applying a smooth reconstruction algorithm and mediastinal window setting (window width, 400 HU; window level, 40 HU).
As aforementioned, in those eight patients who did not performed chest CT the radiological analysis was made evaluating the low dose CT of PET/CT scan (tube voltage 120 kV; tube current 80 mA, rotation time 0.5–0.8 s). The volume was reconstructed as 3.8-mm thick slices; lung window setting: window width 1600 HU; window level −600 HU; mediastinal window setting: window width 400 HU; window level, 40 HU).
All chest CT scans were retrospectively reviewed by two experienced radiologists with more than 10 years of experience, to describe the morphological features of MALT lymphomas: laterality, localization, number, size, presence of enlarged thoracic lymph nodes, pleural effusion, mosaic pattern and pulmonary cyst. Based on the predominant radiologic abnormalities the lung MALT lymphomas were categorized into four major patterns: (1) consolidation; (2) mass; (3) nodule and (4) ground glass opacity (GGO). Area of consolidation was defined as homogeneous increase in pulmonary parenchymal attenuation obscuring the vessels margins and airways walls27; mass was defined as rounded well-defined or moderately well-defined opacity greater than 3 cm in maximum diameter27; nodule was defined as rounded well-defined or moderately well-defined opacity equal to or less than 3 cm in maximum diameter27; GGO was defined as a hazy increased attenuation of lung with preservation of bronchial and vascular margins.27 For every lung lesion was described the presence of secondary associated features as air bronchogram, bronchiectasis, calcification and cavitation. Other CT findings, including thoracic lymphadenopathy, pleural effusion, pulmonary cysts and mosaic pattern, were also recorded. Mosaic pattern was a condition in which there was a patchwork of regions of different lung attenuation on thin-section CT. For every lung lesion the presence of secondary associated features like air bronchogram, bronchiectasis, calcification and cavitation was described.
18F-FDG PET/CT imaging and interpretation
All patients underwent disease staging 18F-FDG PET/CT before treatment (local surgery, chemotherapy, radiotherapy and/or combination); it was performed after at least 6 h fasting with glucose level lower than 150 mg dl−1. An activity of 3.5–4.5 MBq Kg−1 of 18F-FDG was administered intravenously; images were acquired 60 min after injection from the skull basis to the mid-thigh on a Discovery 690 tomograph or Discovery ST PET/CT tomograph (General Electric Company—GE®—Milwaukee, WI) with standard parameters (CT: 80 mA, 120 Kv without contrast; 2.5–4 min per bed-PET-step of 15 cm) and the reconstruction was performed in a 128 × 128 or 256 × 256 matrix and 60 cm field of view. Patients were instructed to void before imaging scan, no oral or intravenous contrast agents were administrated or bowel preparation used for any patient. All 18F-FDG PET/CT scans were retrospectively reviewed by two experienced nuclear medicine physicians with more than 10 years of experience. PET images were analysed both visually and semiquantitatively. Readers had knowledge of clinical history, and every focal tracer uptake deviating from physiological distribution and background was regarded as suggestive of MALT; it was defined as discrete 18F-FDG activity higher than the surrounding tissue on visual analysis. Semiquantitative analysis was made by measuring the maximum standardized uptake value (SUVmax), lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio for each pulmonary lesion. We measured the SUV of detectable lesions by drawing a region of interest (ROI) over the area of maximum activity and the SUVmax was calculated as the highest SUV of the pixels within the ROI. SUVmax of the blood-pool was calculated at the aortic arch by use of transaxial PET images with a round-shape 10 mm ROI not involving the wall of the vessel; SUVmax of the liver was calculated at the VIII hepatic segment of transaxial PET images using a round-shape 10 mm ROI.
Statistical analysis
All statistical analysis was carried out using Statistical Package for Social Science (SPSS) version 23.0 for Windows (IBM, Chicago, IL) and MedCalc Software version 17.1 for Windows (Ostend, Belgium). The descriptive analysis of categorical variables comprised the calculation of simple and relative frequencies. The numeric variables were described as mean, standard deviation, minimum and maximum.
Univariate logistic regression analysis was performed to assess the relationship between qualitative 18F-FDG PET/CT (FDG avidity) and the other clinical, pathological and radiological parameters. Variables with p < 0.05 in the univariate analysis were further analysed using multivariate logistic regression analysis to determinate which variables were independently associated.
The relationship between semi-quantitatively features (SUVmax, lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio) and the morphological parameters were calculated using Anova test and independent t test.
The Pearson correlation coefficient was applied to find correlation between the semiquantitative features (SUVmax, lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio) and the tumour size. We applied these test because the SUVmax, lesion-to-liver SUVmax ratio, lesion-to-blood pool SUVmax ratio and the tumour size were normally distributed. A p-value < 0.05 was considered statistically significant.
Duration of follow-up was calculated from the date of diagnosis to the last control or date of death.
Results
Patient’s features
We evaluated 28 patients with lung MALT lymphoma histological proven, 18 (64%) were male and 10 (36%) female; mean age was 60.5 ± 12.5 years (range 33–76 years). Patients were staged according to the Ann Arbor system as follows: stage I (n = 16), stage II (n = 6), stage III (n = 1) and stage IV (n = 5). Among patients with stage IV MALT lymphoma, all had extrapulmonary disease histological proven: three in stomach, one in kidney and one in liver (Table 1). All patients had no history or concomitant oncological disease different from MALT lymphoma. Four patients had associated conditions as follows: two patients systemic lupus erythematosus and two patients Sjogren syndrome. Patients were treated according to the stage and location of the disease: 5 patients underwent surgical resection (in three cases single lobectomy, in the other two cases wedge resection); 17 received chemotherapy (CMT), 2 underwent surgery plus CMT, 1 patient underwent radiotherapy and 3 patients didn’t undergo any therapy (1 patient was lost at follow-up before starting treatment and the other 2 patients did not received therapy due to the early stage of disease and asymptomatic condition). CMT regimen consisted of R-CVP (rituximab-cyclophosphamide, vincristine and prednisolone) or R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine and prednisolone). The average duration of follow-up was 62 months (9–125 months).
CT findings
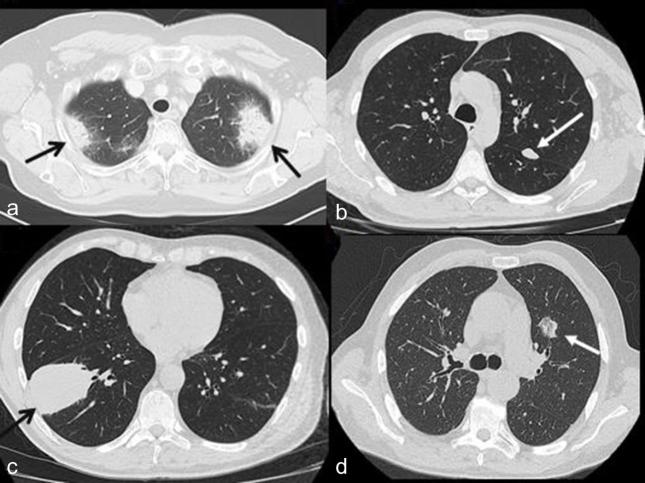
57 pulmonary lesions were identified in 28 patients: 37 (65%) presenting as areas of consolidation, 12 (21%) as nodules, 4 (7%) as masses and 4 (7%) as GGOs (Figure 1).
Figure 1.
Example of the main four morphological pattern of presentation of lung lesion at chest CT. (a) Axial CT scan revealing two areas of consolidation in both upper lobes (black arrows) in a 71-year-old female. (b) Axial CT scan showing a nodule of 18 mm of diameter in the upper lobe of left lung (white arrow) in a 58-year-old male. (c) Axial CT scan revealing a big mass of 74 mm of diameter in the lower lobe of right lung (black arrow) in a 58-year-old male (d) Axial CT scan showing a part solid GGO in the left lung (white arrow) in a 72-year-old male.
Solitary lesion was observed in 10 of 28 patients (36%), multiple lesions in the remaining 18 (64%) patients. Among 18 patients with multiple lesions, 2 demonstrated unilateral pulmonary MALT lymphoma and the remaining 16 had bilateral disease. 32 (56%) lesions were in the right lung: 15 in the upper lobe, 9 in the middle lobe and 8 in the lower lobe. 25 (44%) lesions were distributed in the left lung: 18 in the upper lobe and 7 in the lower lobe (Table 2).
Table 2.
CT features of 57 lung lesions
| Radiologic features | n (%) |
|---|---|
| Localization | |
| Right lung | 32 (56%) |
| RUL:RML:RLL | 15:9:8 (26%:16%:14%) |
| Left lung | 25 (44%) |
| LUL:LLL | 18:7 (32%:12%) |
| Morphological pattern | |
| Consolidation | 37 (65%) |
| Nodule | 12 (21%) |
| Mass | 4 (7%) |
| GGO | 4 (7%) |
| Secondary associated features | |
| Air broncogram | 33 (58%) |
| Bronchiectasis | 17 (30%) |
| Cavitation | 1 (2%) |
| Calcification | 1 (2%) |
GGO, ground glass opacity; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right medium lobe; RUL, right upper lobe.
Lesion size, considering diameter maximum of the tumour, was 35 mm ± 26 mm (range 5–114 mm).
In consolidation areas average diameter maximum was 38.7 ± 21 mm (range 5–114 mm), in masses 65 ± 38 mm (range 35–89 mm), in nodules 13.6 ± 6 mm (range 6–28 mm) and in GGO 29 ± 13 mm (range 20–45 mm). The commonest secondary associated features of the pulmonary lesions were the presence of an air bronchogram: a total of 33 air bronchogram findings were detected in 31 consolidation areas and in 2 GGOs. Bronchiectasis was present in 17 lesions, all presenting as areas of consolidation. Whereas we recorded only one case of cavitation and one case of calcification in a consolidation lesion (Table 2). Evaluating other CT findings, 2 patients had pleural effusion, 4 presences of pulmonary cystic lesions and 1 mosaic pattern presentation. Thoracic lymph nodes were identified in 8 of 28 patients (29%) (Table 1).
18F-FDG PET/CT findings
Among 28 patients, 26 (93%) were 18F-FDG-avid showing the presence of a hypermetabolic lesion corresponding to the lesion histological demonstrated as MALT lymphoma; while two patients (7%) had negative 18F-FDG PET/CT (Table 1). The patients with negative 18F-FDG PET/CT were a 74-year-old male with an isolated nodule of 12 mm of diameter in the right middle lobe (Figure 2) and a 64-year-old female with a GGO in the left upper lobe without other lung lesions. On a per-lesion based analysis, considering all 57 pulmonary lesions, 18F-FDG PET/CT showed avid lesions in 47/57 (82%) cases: 34/37 (92%) among areas of consolidation, 4/4 (100%) among masses; 6/12 (50%) considering nodules and 3/4 (75%) evaluating GGOs.
Figure 2.
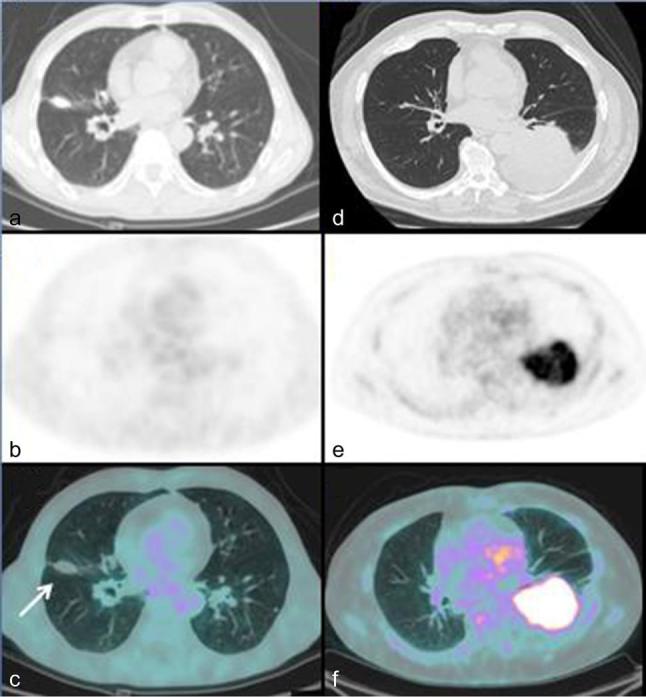
Comparison between FDG negative and positive lung MALT lymphoma. Axial CT (a), axial PET (b) and axial fused PET/CT images (c) in a 74-year-old male with a 12-mm nodule in the upper lobe of right lung showing no pathological FDG uptake corresponding to the pulmonary lesion. Axial CT (d), axial PET (e) and (f) axial PET/CT images in a 72-year-old male showing an intense FDG uptake in a big area of consolidation in lower lobe of left lung with a SUVmax of 11.9 lesion-to-liver SUVmax ratio of 3.2 and lesion-to-blood pool SUVmax ratio of 4.4.
18F-FDG PET/CT positive lung lesions had larger average maximum diameter than those not 18F-FDG avid (average diameter maximum 40 vs 11 mm, p = 0.002), this correlation was also confirmed in the multivariate regression analysis (p = 0.005) (Table 3). Considering a arbitrarily maximum diameter threshold of 2 cm, recognized as a value which widely overcomes the limits related to PET/CT resolution power and breathing variation, 8/17 (47%) lesions with a diameter less than 2 cm were 18F-FDG avid, whereas 38/38 (100%) lesions with diameter >2 cm were 18F-FDG avid. Evaluating every specific radiological pattern of appearance, in the six 18F-FDG not avid nodular lesions average maximum diameter was significantly lower compared to 18F-FDG positive nodular lesions (9.8 vs 18.2 mm, p = 0.025); a similar evidence was present also in consolidation pattern (13.3 vs 41 mm, p = 0.049). Comparing the metabolic and morphological pattern of presentation, laterality, presence of bronchiectasis and air bronchogram there were no significant differences between positive and negative 18F-FDG PET/CT (Table 3).
Table 3.
Comparison between qualitative, semiquantitative features at 18F-FDG PET/CT and clinical-morphological characteristics
| PET/CT posn 47 | PET/CT negn 10 | p value | Av SUVmax | p value | Av L-to-L SUVmax ratio | p value | Av L-t-BP SUVmax ratio | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Pattern | 0.778 | 0.839 | 0.667 | 0.713 | |||||
| Consolidation | 34 | 3 | 5.1 | 2.1 | 2.7 | ||||
| Mass | 4 | 0 | 7.8 | 2.8 | 3.8 | ||||
| Nodule | 6 | 6 | 3.8 | 1.8 | 2.2 | ||||
| GGO | 3 | 1 | 5.7 | 2.5 | 3.1 | ||||
| Laterality | 0.820 | 0.498 | 0.392 | 0.622 | |||||
| Unilateral | 10 | 2 | 5.7 | 2.5 | 2.9 | ||||
| Bilateral | 37 | 8 | 5 | 2.1 | 2.7 | ||||
| Air brochogram | 0.051 | 0.986 | 0.453 | 0.648 | |||||
| Positive | 30 | 3 | 5.2 | 2.3 | 2.8 | ||||
| Negative | 17 | 7 | 5.2 | 2 | 2.6 | ||||
| Bronchiectasis | 0.136 | 0.541 | 0.578 | 0.877 | |||||
| Positive | 17 | 1 | 4.8 | 2.3 | 2.8 | ||||
| Negative | 30 | 9 | 5.4 | 2.1 | 2.7 |
av, average; L-t-L, lesion to liver; L-t-BP, lesion to blood pool: neg, negative; Pos, positive.
Between paranthesis p value of multivariate analysis.
All thoracic lymph nodes detected by chest CT were 18F-FDG-avid; not further thoracic lymphadenopathies were discovered by 18F-FDG PET/CT. In three patients 18F-FDG PET/CT showed additional pathological uptakes corresponding to extrapulmonary localizations of MALT lymphoma: in two cases detecting gastric 18F-FDG avid lesions (Figure 3; confirmed to be a MALT lymphoma by histological examination after biopsy) and in one patient detecting kidney involvement. Thus 18F-FDG PET/CT changed tumour stage in these three cases identifying new pathological uptakes related to MALT lymphoma and upstaging to stage IV from stage I in two cases and from stage II in one. The other two cases of extrapulmonary MALT localizations (gastric and hepatic involvement) were already known before 18F-FDG PET/CT and it simply confirmed pathological findings.
Figure 3.
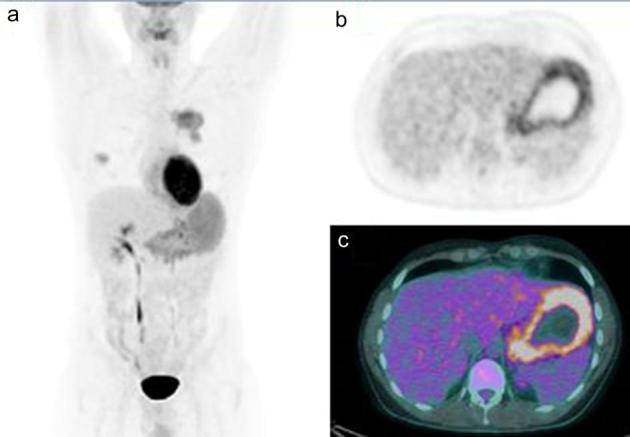
(a) MIP, showing the presence of bilateral hypermetabolic lung lesions and an increased diffuse FDG uptake in the stomach. Axial PET (b) and axial PET/CT (c) scan revealing an increased FDG uptake corresponding to the gastric thickening wall.
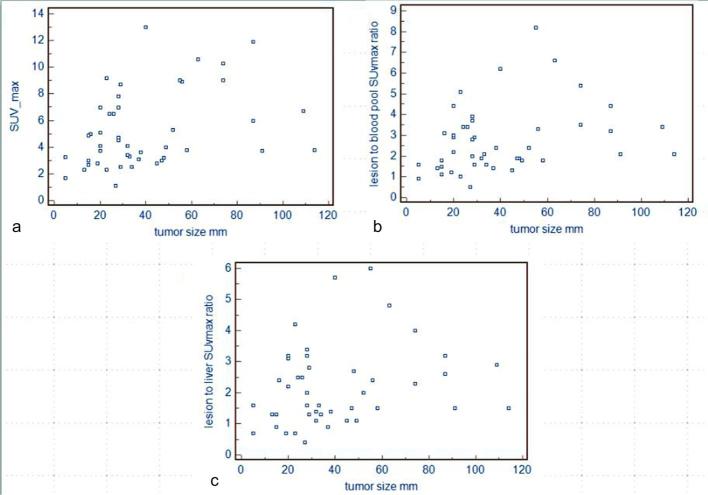
In patients with positive 18F-FDG PET/CT, average SUVmax of the lesions was 5.3 ± 2.9 (range 1.1–13); lesion-to-liver SUVmax ratio was 2.3 ± 1.3 (range 0.4–6) and lesion-to-blood pool SUVmax ratio 2.8 ± 1.7 (range 0.5–8.2) (Table 1). SUVmax, lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio in consolidation areas were 5.1 ± 2.9 (range 1.1–13), 2.1 ± 1.3 (0.4–6), 2.7 ± 1.6 (0.5–8.2), respectively; in masses were 7.8 ± 2.5 (4.9-10-6), 2.8 ± 1.5 (1.3–4.8), 3.8 ± 2 (1.8–6.6), respectively; in nodules were 3.8 ± 1.9 (2.3–7), 1.8 ± 0.9 (0.9–3.2), 2.2 ± 1.2 (1.1–3.9), respectively and in GGO were 5.7 ± 3.2 (2.8–9.2), 2.5 ± 1.5 (1.1–4.2), 3.1 ± 1.9 (1.3–5.1), respectively. SUVmax, lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio were not correlated with morphological pattern of disease, laterality and secondary radiological findings (Table 3). Only SUVmax was correlated significantly with tumour size (Figure 4).
Figure 4.
(a) Relationship between tumour size in millimeter and SUVmax value (r = 0.477; p = 0.002). (b) Relationship between tumour size in millimeter and lesion to blood pool SUVmax ratio (r = 0.283, p = 0.061). (c) Relationship between tumour size in millimeter and lesion to liver SUVmax ratio (r = 0.221; p = 0.136). SUV, standardized uptake value.
Discussion
Lung MALT lymphoma is a rare extranodal low-grade B-cell lymphoma and it is considered to originate from MALT of the bronchus. It is the most common subtype of NHL of the lungs, accounting for up to 90% of all primary pulmonary lymphoma.1–6 The CT imaging findings of pulmonary MALT lymphoma are various and include solitary or multifocal, round or segmental lesions that may be located centrally or peripherally with no lobular predilection28; the most frequent imaging findings are consolidations, followed by nodules and masses. Other rarer appearances include GGO and interstitial change. The most common concomitant findings are air bronchogram and bronchiectasis.29–32 Cavitation, calcification, pulmonary cystic lesions and pleural effusion are less common. Also in our study, we demonstrated that consolidation (65%) is the most frequent morphological pattern of disease presentation, followed by nodule (21%), mass (7%) and GGO (7%). Air bronchogram was detected in more than half of the patients and bronchiectasis was present in about a third, frequently present with areas of consolidation. Lung MALT lymphoma is often observed as multiple and bilateral disease31–33 ; this result was confirmed also in our work with a rate of multiple lesions of 64% and bilaterality of 57%. Similar patterns on CT scans can be seen in many other entities, both neoplastic and non neoplastic disease like eosinophilic pneumonia, sarcoidosis, adenocarcinoma, and other lung lymphoproliferative disorders; thus differential diagnosis can be very difficult.
There are conflicting reports about 18F-FDG uptake in MALT lymphoma and also in lung MALT lymphoma. Hoffmann et al34,35 firstly described lung MALT lymphoma as not 18F-FDG avid tumours and did not recommend 18F-FDG PET. However in those two works, only a small number of patients were included and patients were scanned without whole-body CT attenuation correction resulting in lower imaging quality. Besides, PET scans were performed 40 min following the injection of 18F-FDG, probably too early. Subsequently, other authors36–43 investigated the metabolic behaviour of pulmonary MALT achieving a high detection rate (Table 4). In 2005 Beal et al (33 reported 11 cases of pulmonary MALT lymphoma avid of 18F-FDG. Moreover, other authors38 demonstrated that 18F-FDG uptake differences in MALT may be due to different histological features, showing that MALT lymphoma with plasmacytic differentiation had more frequently 18F-FDG avid lesions. This result was controverted by Albano et al in a larger sample of patients studied.23
Table 4.
Summary of main studies in literature including patients with lung MALT lymphoma and 18F-FDG PET/CT
| Authors | Year | Device | 18F-FDG avidity per patient (%) | Mean SUVmax (range) |
|---|---|---|---|---|
| Beal et al 33 | 2005 | PET/CT | 11/11 (100%) | 6.8 (2.6–26) |
| Alinari et al 37 | 2006 | PET | 8/8 (100%) | 7.5 (3–18) |
| Hoffmann et al 38 | 2006 | PET | 2/4 (50%) | na |
| Perry et al 39 | 2007 | PET/CT | 5/5 (100%) | na |
| Enomoto et al 40 | 2008 | PET | 2/2 (100%) | 3.8 (3.2–4.5) |
| Bae et al 36 | 2008 | PET/CT | 6/6 (100%) | 4.2 (2.2–6.3) |
| Zhang et al 28 | 2011 | PET/CT | 8/8 (100%) | 4.9 (2.8–9.4) |
| Zinzani et al 41 | 2013 | PET | 11/11 (100%) | 3.6 (2.8–6) |
| Yoon et al 42 | 2013 | PET/CT | 4/5 (80%) | 3.3 (2.3–5.7) |
| Park et al 43 | 2015 | PET/CT | 7/7 (100%) | 4.4 (2.4–6.3)a |
| Albano et al 23 | 2017 | PET/CT | 24/24 (100%) | na |
| Present study | 2017 | PET/CT | 26/28 (93%) | 5.3 (1.1–13) |
FDG, fluorodesoxyglucose; na, not available; MALT, mucosa associated lymphoid tissue; PET, positron emission tomography; SUV, standardizeduptake value.
SULmax, standardized uptake lean body mass.
In a recent work23 focused on the role of 18F-FDG-PET/CT, Ki-67 score and plasmacytic differentiation in extragastric MALT lymphoma, we demonstrated a per-patient detection rate of 100% in 24 patient with pulmonary MALT lymphoma; in the present study we have increased the number of pulmonary MALT lymphoma adding four patients with histological diagnosis and showing a slightly reduction of detection rate (93%).
In our study, per-patient lung MALT lymphomas detection rate of 18F-FDG PET/CT was anyway very high and similar to that of all the other studies available33,36–43 (Table 4); only two patients had not 18F-FDG avid lung MALT lymphoma. From a per-lesion detection 18F-FDG avidity was present in 82% of cases. A possible limit of previous works was that the presence of a per-patient analysis only, not considering the frequent bilaterality and multiplicity of lung MALT lymphoma and the heterogeneous morphological and metabolic features of this disease. In our analysis the only variable significantly correlated with 18F-FDG PET/CT positivity was the maximum diameter of the lesion; lung MALT lymphoma with small size lesions were less 18F-FDG avid compared to larger lesion. This evidence could be related with resolution power and partial volume effect. The crucial role of tumour size is confirmed also in the analysis of each type of morphological pattern of disease. On the other hand, no correlations were demonstrated between semi-quantitative 18F-FDG PET/CT characteristics (SUVmax, lesion to liver SUVmax ratio and lesion to blood pool SUVmax ratio) and tumour size. Morphological pattern and other radiological features didn’t seem to influence 18F-FDG avidity.
In the literature average SUVmax of lung MALT lymphoma ranges from 3.3 to 7.5 (Table 4); in our study, we had a mean SUVmax of 5.3 very similar to those aforementioned.28 So far, in the literature no articles considering the other semiquantitative parameters (lesion-to-liver SUVmax ratio and lesion-to-blood pool SUVmax ratio) are available as well as works with a number of patients like us.
In three patients 18F-FDG PET/CT detected more lesions than CT upstaging the patient and contributing to a better clinical management. This point is in agreement with the results of Carrillo-Cruz et al,44 who demonstrated a high sensitivity of 18F-FDG PET/CT in staging 17 marginal zone B cell lymphoma.
About this topic, more and larger studies are desirable and needed to confirm our evidences being aware that one important advantage of 18F-FDG PET-CT is that it is a whole body technique allowing a the whole body in a single scan.
Our present study shows several limitations: the retrospective type of analysis, the small sample of patients analysed (due to the rarity of this disease) and the heterogeneous features of our sample (for example not all patients have made the same imaging scans).
In conclusion, lung MALT lymphoma can manifest diverse patterns of lung abnormality at CT, but single or multiple bilateral areas of consolidation or nodules are the main patterns. Our data confirm that lung MALT lymphomas are 18F-FDG avid in most cases, indicating that 18F-FDG PET/CT should be part of the diagnostic process of this particular disease. Maximum diameter of the lesion is the only factor which influences 18F-FDG avidity.
Contributor Information
Domenico Albano, Email: doalba87@libero.it.
Andrea Borghesi, Email: andrea.borghesi@unibs.it.
Giovanni Bosio, Email: giovanni.bosio@asst-spedalicivili.it.
Mattia Bertoli, Email: mattia.bertoli@gmail.com.
Roberto Maroldi, Email: roberto.maroldi@unibs.it.
Raffaele Giubbini, Email: raffaele.giubbini@unibs.it.
Francesco Bertagna, Email: francesco.bertagna@unibs.it.
REFERENCES
- 1.Ferraro P, Trastek VF, Adlakha H, Deschamps C, Allen MS, Pairolero PC. Primary non-Hodgkin’s lymphoma of the lung. Ann Thorac Surg 2000; 69: 993–7.https://doi.org/10.1016/S0003-4975(99)01535-0 [DOI] [PubMed] [Google Scholar]
- 2.Parissis H. Forty years literature review of primary lung lymphoma. J Cardiothorac Surg 2011; 6: 23https://doi.org/10.1186/1749-8090-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordier JF, Chailleux E, Lauque D, Reynaud-Gaubert M, Dietemann-Molard A, Dalphin JC, et al. Primary pulmonary lymphomas. A clinical study of 70 cases in nonimmunocompromised patients. Chest 1993; 103: 201–8. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Hansmann ML, Zwingers T, Lennert K. Primary lymphomas of the lung: morphological, immunohistochemical and clinical features. Histopathology 1990; 16: 519–31.https://doi.org/10.1111/j.1365-2559.1990.tb01157.x [DOI] [PubMed] [Google Scholar]
- 5.Ahmed S, Siddiqui AK, Rai KR. Low-grade B-cell bronchial associated lymphoid tissue (BALT) lymphoma. Cancer Invest 2002; 20(7-8): 1059–68.https://doi.org/10.1081/CNV-120005924 [DOI] [PubMed] [Google Scholar]
- 6.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol 2000; 13: 193–207.https://doi.org/10.1038/modpathol.3880035 [DOI] [PubMed] [Google Scholar]
- 7.Bienenstock J, Johnston N, Perey D. Bronchial lymphoid tissue. Lab Invest 1973; 28: 686–92. [PubMed] [Google Scholar]
- 8.Kurtin PJ, Myers JL, Adlakha H, Strickler JG, Lohse C, Pankratz VS, et al. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol 2001; 25: 997–1008.https://doi.org/10.1097/00000478-200108000-00003 [DOI] [PubMed] [Google Scholar]
- 9.Restrepo CS, Carrillo J, Rosado de Christenson M, Ojeda Leon P, Lucia Rivera A, Koss MN, et al. Lymphoproliferative lung disorders: a radiologic-pathologic overview. Part II: neoplastic disorders. Semin Ultrasound CT MR 2013; 34: 535–49.https://doi.org/10.1053/j.sult.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 10.Kligerman SJ, Franks TJ, Galvin JR. Primary extranodal lymphoma of the thorax. Radiol Clin North Am 2016; 54: 673–87.https://doi.org/10.1016/j.rcl.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Sirajuddin A, Raparia K, Lewis VA, Franks TJ, Dhand S, Galvin JR, et al. Primary pulmonary lymphoid lesions: radiologic and pathologic findings. Radiographics 2016; 36: 53–70.https://doi.org/10.1148/rg.2016140339 [DOI] [PubMed] [Google Scholar]
- 12.Wislez M, Cadranel J, Antoine M, Milleron B, Bazot M, Mayaud C, et al. Lymphoma of pulmonary mucosa-associated lymphoid tissue: CT scan findings and pathological correlations. Eur Respir J 1999; 14: 419–22.https://doi.org/10.1183/09031936.99.14242399 [DOI] [PubMed] [Google Scholar]
- 13.Hare SS, Souza CA, Bain G, Seely JM, Frcpc, Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol 2012; 85: 848–64.https://doi.org/10.1259/bjr/16420165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Lee SH, Park J, Kim HY, Lee SI, Park JO, et al. Primary pulmonary non-Hodgkin's lymphoma. Jpn J Clin Oncol 2004; 34: 510–4.https://doi.org/10.1093/jjco/hyh095 [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32: 3059–67.https://doi.org/10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on Malignant lymphomas Imaging Working Group. J Clin Oncol 2014; 32: 3048–58.https://doi.org/10.1200/JCO.2013.53.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Specht L. 2-[18F]fluoro-2-deoxyglucose positron-emission tomography in staging, response evaluation, and treatment planning of lymphomas. Semin Radiat Oncol 2007; 17: 190–7.https://doi.org/10.1016/j.semradonc.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 18.Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl 6): vi144–vi148.https://doi.org/10.1093/annonc/mdt343 [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN). National Comprehensive Cancer Network guidelines on non-Hodgkin’s lymphomas, version 2.2014 2014. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
- 20.Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma. CA Cancer J Clin 2016; 66: 153–71.https://doi.org/10.3322/caac.21330 [DOI] [PubMed] [Google Scholar]
- 21.Treglia G, Zucca E, Sadeghi R, Cavalli F, Giovanella L, Ceriani L. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: a meta-analysis. Hematol Oncol 2015; 33: 113–24.https://doi.org/10.1002/hon.2152 [DOI] [PubMed] [Google Scholar]
- 22.Albano D, Bertoli M, Ferro P, Fallanca F, Gianolli L, Picchio M, et al. 18F-FDG PET/CT in gastric MALT lymphoma: a bicentric experience. Eur J Nucl Med Mol Imaging 2017; 44: 589–97.https://doi.org/10.1007/s00259-016-3518-y [DOI] [PubMed] [Google Scholar]
- 23.Albano D, Bosio G, Giubbini R, Bertagna F. 18F-FDG PET/CT and extragastric MALT lymphoma: role of Ki-67 score and plasmacytic differentiation. Leuk Lymphoma 2017; 58: 2328–34.https://doi.org/10.1080/10428194.2017.1298754 [DOI] [PubMed] [Google Scholar]
- 24.Albano D, Giubbini R, Bertagna F. 18F-FDG PET/CT and primary hepatic MALT: a case series. Abdom Radiol 2016; 41: 1956–9.https://doi.org/10.1007/s00261-016-0800-1 [DOI] [PubMed] [Google Scholar]
- 25.Albano D, Bosio G, Orlando E, Bertagna F. Role of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in evaluating breast mucosa-associated lymphoid tissue lymphoma: a case series. Hematol Oncol 2016; in presshttps://doi.org/10.1002/hon.2376 [DOI] [PubMed] [Google Scholar]
- 26.Jaffe ES, Harris NL, Stein H, Vardinam JW. Tumours of Haematopoietic and Lymphoid Tissues: World Health Organization Classification of Tumours, Pathology, and Genetics. Lyon, France: IARC Press; 2001. [Google Scholar]
- 27.Austin JH, Müller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996; 200: 327–31.https://doi.org/10.1148/radiology.200.2.8685321 [DOI] [PubMed] [Google Scholar]
- 28.Zhang WD, Guan YB, Li CX, Huang XB, Zhang FJ. Pulmonary mucosa-associated lymphoid tissue lymphoma: computed tomography and ¹⁸F fluorodeoxyglucose-positron emission tomography/computed tomography imaging findings and follow-up. J Comput Assist Tomogr 2011; 35: 608–13.https://doi.org/10.1097/RCT.0b013e318227a661 [DOI] [PubMed] [Google Scholar]
- 29.Kinsely BL, Mastey LA, Mergo PJ, Voytovich MC, Zander D, Almasri NM, et al. Pulmonary mucosa-associated lymphoid tissue lymphoma: CT and pathologic findings. AJR Am J Roentgenol 1999; 172: 1321–6.https://doi.org/10.2214/ajr.172.5.10227510 [DOI] [PubMed] [Google Scholar]
- 30.King LJ, Padley SP, Wotherspoon AC, Nicholson AG. Pulmonary MALT lymphoma: imaging findings in 24 cases. Eur Radiol 2000; 10: 1932–8.https://doi.org/10.1007/s003300000491 [DOI] [PubMed] [Google Scholar]
- 31.McCulloch GL, Sinnatamby R, Stewart S, Goddard M, Flower CD. High-resolution computed tomographic appearance of MALToma of the lung. Eur Radiol 1998; 8: 1669–73.https://doi.org/10.1007/s003300050609 [DOI] [PubMed] [Google Scholar]
- 32.Lee DK, Im JG, Lee KS, Lee JS, Seo JB, Goo JM, et al. B-cell lymphoma of bronchus-associated lymphoid tissue (BALT): CT features in 10 patients. J Comput Assist Tomogr 2000; 24: 30–4.https://doi.org/10.1097/00004728-200001000-00006 [DOI] [PubMed] [Google Scholar]
- 33.Beal KP, Yeung HW, Yahalom J. FDG-PET scanning for detection and staging of extranodal marginal zone lymphomas of the MALT type: a report of 42 cases. Ann Oncol 2005; 16: 473–80.https://doi.org/10.1093/annonc/mdi093 [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann M, Kletter K, Diemling M, Becherer A, Pfeffel F, Petkov V, et al. Positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol 1999; 10: 1185–9.https://doi.org/10.1023/A:1008312726163 [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann M, Kletter K, Becherer A, Jäger U, Chott A, Raderer M. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) for staging and follow-up of marginal zone B-cell lymphoma. Oncology 2003; 64: 336–40.https://doi.org/70290 [DOI] [PubMed] [Google Scholar]
- 36.Bae YA, Lee KS, Han J, Ko YH, Kim BT, Chung MJ, et al. Marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: imaging findings in 21 patients. Chest 2008; 133: 433–40.https://doi.org/10.1378/chest.07-1956 [DOI] [PubMed] [Google Scholar]
- 37.Alinari L, Castellucci P, Elstrom R, Ambrosini V, Stefoni V, Nanni C, et al. 18F-FDG PET in mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma 2006; 47: 2096–101.https://doi.org/10.1080/10428190600733499 [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann M, Wöhrer S, Becherer A, Chott A, Streubel B, Kletter K, et al. 18F-Fluoro-deoxy-glucose positron emission tomography in lymphoma of mucosa-associated lymphoid tissue: histology makes the difference. Ann Oncol 2006; 17: 1761–5.https://doi.org/10.1093/annonc/mdl295 [DOI] [PubMed] [Google Scholar]
- 39.Perry C, Herishanu Y, Metzer U, Bairey O, Ruchlemer R, Trejo L, et al. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur J Haematol 2007; 79: 205–9.https://doi.org/10.1111/j.1600-0609.2007.00895.x [DOI] [PubMed] [Google Scholar]
- 40.Enomoto K, Hamada K, Inohara H, Higuchi I, Tomita Y, Kubo T, et al. Mucosa-associated lymphoid tissue lymphoma studied with FDG-PET: a comparison with CT and endoscopic findings. Ann Nucl Med 2008; 22: 261–7.https://doi.org/10.1007/s12149-007-0125-9 [DOI] [PubMed] [Google Scholar]
- 41.Zinzani PL, Pellegrini C, Gandolfi L, Casadei B, Derenzini E, Broccoli A, et al. Extranodal marginal zone B-cell lymphoma of the lung: experience with fludarabine and mitoxantrone-containing regimens. Hematol Oncol 2013; 31: 183–8.https://doi.org/10.1002/hon.2039 [DOI] [PubMed] [Google Scholar]
- 42.Yoon RG, Kim MY, Song JW, Chae EJ, Choi CM, Jang S. Primary endobronchial marginal zone B-cell lymphoma of bronchus-associated lymphoid tissue: CT findings in 7 patients. Korean J Radiol 2013; 14: 366–74.https://doi.org/10.3348/kjr.2013.14.2.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SH, Lee JJ, Kim HO, Lee DY, Suh C, Jung HY, et al. 18F-Fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography in mucosa-associated lymphoid tissue lymphoma: variation in 18F-FDG avidity according to site involvement. Leuk Lymphoma 2015; 56: 3288–94.https://doi.org/10.3109/10428194.2015.1030640 [DOI] [PubMed] [Google Scholar]
- 44.Carrillo-Cruz E, Marín-Oyaga VA, de la Cruz Vicente F, Borrego-Dorado I, Ruiz Mercado M, Acevedo Báñez I, Banez A I, et al. Role of 18F-FDG-PET/CT in the management of marginal zone B cell lymphoma. Hematol Oncol 2015; 33: 151–8.https://doi.org/10.1002/hon.2181 [DOI] [PubMed] [Google Scholar]