Abstract
Objective:
A ceiling-mounted robotic C-arm cone beam CT (CBCT) system was developed for use with a 190° proton gantry system and a 6-degree-of-freedom robotic patient positioner. We report on the mechanical design, system accuracy, image quality, image guidance accuracy, imaging dose, workflow, safety and collision-avoidance.
Methods:
The robotic CBCT system couples a rotating C-ring to the C-arm concentrically with a kV X-ray tube and a flat-panel imager mounted to the C-ring. CBCT images are acquired with flex correction and maximally 360° rotation for a 53 cm field of view. The system was designed for clinical use with three imaging locations. Anthropomorphic phantoms were imaged to evaluate the image guidance accuracy.
Results:
The position accuracy and repeatability of the robotic C-arm was high (<0.5 mm), as measured with a high-accuracy laser tracker. The isocentric accuracy of the C-ring rotation was within 0.7 mm. The coincidence of CBCT imaging and radiation isocentre was better than 1 mm. The average image guidance accuracy was within 1 mm and 1° for the anthropomorphic phantoms tested. Daily volumetric imaging for proton patient positioning was specified for routine clinical practice.
Conclusion:
Our novel gantry-independent robotic CBCT system provides high-accuracy volumetric image guidance for proton therapy.
Advances in knowledge:
Ceiling-mounted robotic CBCT provides a viable option than CT on-rails for partial gantry and fixed-beam proton systems with the added advantage of acquiring images at the treatment isocentre.
Introduction
Volumetric image guidance for photon therapy has been clinically implemented first with an in-room CT scanner1 and cone beam CT (CBCT) of megavoltage beams,2 followed by fan beam megavoltage CT3 and linear accelerator-mounted kilovoltage CBCT.4 It became widely adopted as dose distributions from intensity-modulated radiation therapy turned highly conformal and the radiation delivery less forgiving to patient setup errors. Volumetric image guidance also provides more information on tumour and patient circumference changes, thus facilitating better decision-making for adaptive re-planning.
For proton therapy, the advantages of volumetric image guidance for localizing soft tissue targets and detecting anatomic changes along the beam paths are well recognized.5–7 It is arguably more critical than photon therapy because of the steep dose gradient at the distal edge of the Bragg peak. To be able to monitor anatomy variations (target displacement, low and high density tissues moving in and out of the beam paths, patient surface deformation) for potential changes in water equivalent path length is also valuable. However, clinical adoption of volumetric image guidance for proton therapy has been slow compared to its adoption for photon therapy. Most operational proton treatment centres still rely on 2D radiographic imaging with or without implanted fiducial markers for patient setup verification. Only recently have gantry- and nozzle-mounted CBCT and CT-on-rails been implemented more widely in new facilities.
Fixed-beam treatment rooms and more recently partial gantry designs were favoured by some centres to save space and cost. In theory, 180° plus a cone angle is sufficient to acquire CBCT images of uncompromised quality for partial gantry systems. A novel rotate-plus-shift trajectory has also been proposed to enable C-arm–based CBCT to acquire a complete sampled dataset with less than 180° rotation.8 Nevertheless, implementation on a proton gantry is challenging and commercial solutions are limited. For nozzle-mounted CBCT, the mechanical interference between the imaging devices and the couch might limit the beam angles, even with the X-ray tube and flat-panel detectors in their retracted positions. The limited space inside treatment rooms and the constraints on clinical flow further complicate the design and placement of an image guidance system.
To accomplish volumetric image guidance in our compact 190° gantry and fixed-beam rooms, a ceiling-mounted robotic C-arm CBCT system was developed, installed, commissioned, and placed into clinical service. This CBCT system design is different from the mobile C-arm CBCT system used in interventional radiology and surgery,9,10 the existing robotic C-arm imaging systems used in some particle therapy centres,11 and the 360° gantry-mounted CBCT systems.12,13 The use of standalone robot arms on ceiling rails provides flexibility in imaging locations (at or off the treatment isocentre) in the room. Having the imaging equipment decoupled from the proton gantry and nozzle makes it easier to upgrade the system as technology advances than would otherwise be the case.
This paper describes the mechanical design, system components and other important features of the novel robotic CBCT system, along with the characterization of the system positioning accuracy, image quality and dose, and setup error correction. In addition, we present details of image acquisition, safety and collision avoidance, clinical use experience and the data flow in the supplemental data (see online supplementary materials).
Methods and materials
Facility
The robotic CBCT system was designed identically for each of our three treatment rooms (two gantries and one fixed beam). Each gantry room houses a 190° (± 95° from the horizontal plane) rotating gantry with a discrete scanning beam nozzle (PROBEAT V, Hitachi Ltd., Tokyo, Japan) and a 6-degree-of-freedom patient positioning system (PPS). The fixed-beam room contains a horizontal beam port with the same types of scanning nozzle and PPS.
Mechanical design and X-ray system
The CBCT system is mechanically separate from the gantry, nozzle and PPS. The ceiling rails provide a sliding mechanism for the first arm link of the robot, enabling the system to reach multiple spatially separated imaging and parking positions. As shown in Figure 1 (lower left photograph), the C-arm (87 cm inner diameter, 81 cm C-opening) is mounted to the wrist of the robotic arm. Another concentric C-shaped structure (the C-ring), where the kV X-ray tube and flat panel imager are mounted on, is coupled to the C-arm. The X-ray source-to-axis-distance and source-to-imager-distance (SID) are 100 cm and 150 cm, respectively. Projection images for CBCT are acquired by rotating the C-ring clockwise with flex correction applied. Both the C-arm and the robotic arm remain stationary during imaging to maintain high position accuracy for the X-ray tube and imager except minor adjustments to compensate for the flex. This is the major difference between our system and the conventional C-arm CBCT systems used in interventional radiology, as well as the other C-arm CBCT systems used in other particle therapy centres. Two cylindrical fields of view (FOVs) were designed: a small field with a diameter of 28 cm and an axial length of 28 cm (full fan mode), and a large field with a diameter of 53 cm and an axial length of 28 cm (half fan mode). Additional descriptions on X-ray tube, imager and image acquisition are provided in supplemental data.
Figure 1.
Photographs on the top row show the three imaging positions with the C-arm in place and the head phantom experimental setup. Positions 0 and 1 are 27 cm apart. Lower left photograph illustrates the major components of the CBCT system. Serial photographs with arrows in the lower right figure illustrate the deployment of the robotic C-arm from one of the four parking positions above the nozzle to the imaging position 2. C-arm retraction follows the same path in reverse. CBCT, cone beam CT.
Figure 1 illustrates three imaging positions at the treatment isocentre (position 0), 27 cm from the treatment isocentre on the axis perpendicular to the gantry rotation axis in the horizontal plane (position 1), and 100 cm from the treatment isocentre on the gantry rotation axis (position 2). Position 2 offers greater clearance of the nozzle for patient setup and imaging, especially for abdomen and pelvis sites. Figure 1 also shows the deployment of the C-arm for position 2. After imaging and setup correction at position 2, the PPS is programmed to move 100 cm back to the treatment isocentre. Acquiring images consecutively at positions 0 and 1 by translating the C-arm while keeping the PPS and patient stationary may offer a larger longitudinal coverage of 55 cm.
Mechanical performance measurements
The mechanical performance of the CBCT positioning system was assessed at three imaging positions with respect to the positioning accuracy of the robotic arm, the repeatability of the robotic arm movement, the isocentric accuracy of the C-ring rotation, the rotation speed of the C-ring, emergency stopping, and collision detection by the sensors. The positioning accuracy of the robotic arm was assessed by first moving the CBCT system from a parking position to an imaging position then acquiring 360° projection images around a 2-mm stainless-steel sphere in an apparatus mounted to the table top. Initially, the sphere was positioned at the radiation isocentre (position 0). It was then moved by offsets of 27 and 100 cm, respectively, to positions 1 and 2 with the guidance of a laser tracker (T3, Automated Precision Inc., Rockville, MD) and a reflector on the apparatus. The average deviation of the centre of the 2-mm sphere on projection images from the centre of the flat-panel imager was calculated for two orthogonal axes on the projection image plane. For the repeatability of the robotic arm movement, measurements were performed using a laser tracker and a reflector attached to the C-ring. The deviations of the actual stopping position from the mean position derived from 3 measurements were calculated for each imaging position. For the isocentric accuracy of the C-ring rotation, the maximum deviation of the centres of the 2-mm spheres on projection images from their average position was calculated for two orthogonal axes of the projection image plane.
Image-quality measurements
The image quality of the CBCT was evaluated for low-contrast visibility, high-contrast spatial resolution, CT-number uniformity, CT-number accuracy, spatial accuracy, and the presence of image artefact by using a Catphan 600 phantom (The Phantom Laboratory, Salem, NY). CT-number accuracy was evaluated on seven insert materials, including air, Teflon, Delrin, acrylic, polystyrene, low-density polyethylene, and polymethylpentene. For the full-fan mode, the scan settings were 100 kV, 20 mA, 20 ms pulse length, 10 frames per second, 512 × 512 reconstruction matrix, 3 × 3 pixel smoothing, 0.55-mm pixel size, and 0.4-mm reconstructed slices. For the half-fan mode, the settings were 125kV, 42 mA, 20 ms pulse length, 10 frames per second, 1024 × 1024 reconstruction matrix, 3 × 3 pixel smoothing, 0.52 mm pixel size and 0.4 mm reconstructed slices. CBCT images were also acquired on anthropomorphic phantoms for visual inspection and artefact detection. For quantifying spatial accuracy, we used CTP404 module of the Catphan phantom which has 4 rods forming a 5-cm square in the centre and an in-house slab phantom with 9 embedded 2-mm spheres forming 20-cm squares. The measured distances were compared to the true physical distances.
Isocentre coincidence and image guidance accuracy
The alignment of the radiation isocentre and the centre of the projection radiographic image was assessed by measuring the distance between the centre of a 2-mm sphere gauge positioned at the radiation isocentre on every projection (45° apart) and the displayed cross-hair. In addition, the isocentre coincidence was tested alternatively by first relying on CBCT to bring a 1-cm diameter ceramic ball taped to a radiochromic film to the imaging isocentre and then delivering a 10 × 10-cm proton irradiation field normal to the film. The centre of the ball shadow on the film was compared with the centre of the irradiation field for evaluating the isocentricity.
The image guidance accuracy was evaluated in all three imaging positions by using the 3D-3D image registration tool in the Positioning Image Analysis System software (Hitachi Ltd., Tokyo, Japan). The phantom placement is illustrated in Figure 2. Planning CT slices of 1 mm and the same scan protocols in image quality measurements with 0.6–1 mm slices were used. Additional information on image guidance tests is provided in supplemental data.
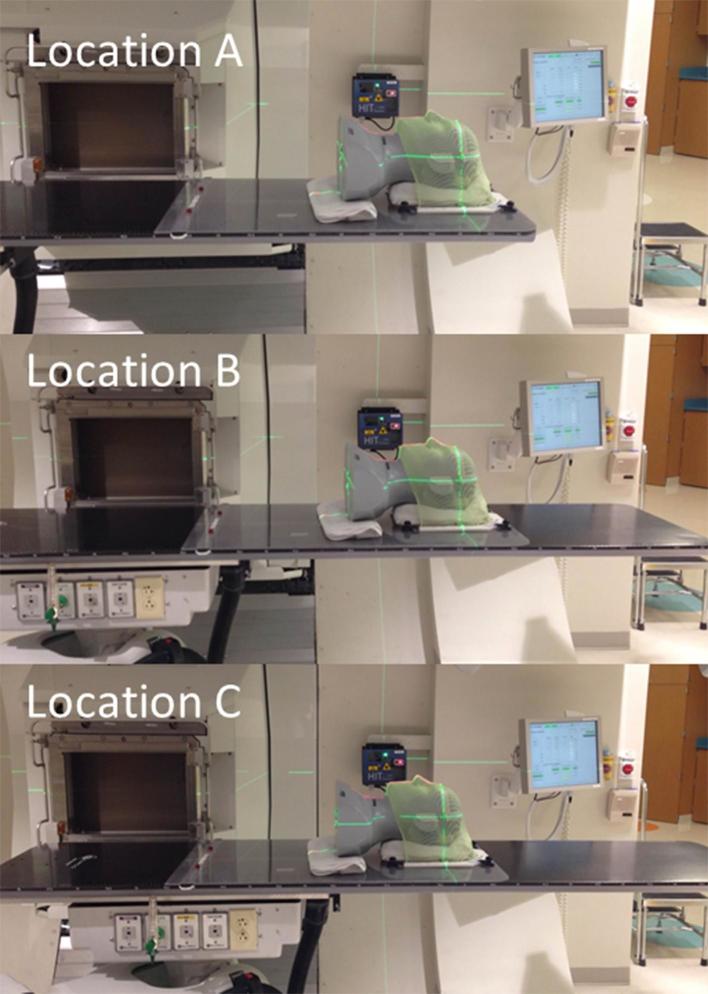
Figure 2.
Photographs showing the three phantom locations on the table top that were used to test image guidance accuracy at imaging position 2. Location B was at the centre of the 100-cm long calibrated volume of the PPS. Location A and location C were 50 cm superior and 30 cm inferior to location B, respectively. This was designed to test if residual errors after PPS setup correction are larger for objects placed away from the centre of the calibrated volume, which is 100 cm from the wrist flange of the robot.
CBCT imaging dose
The CT dose index (CTDI) was measured with a pencil ionization chamber (having an active length of 10 cm) without collimation on two uniform polymethyl methacrylate (PMMA) cylindrical phantoms with diameters of 16 cm and 32 cm and a length of 14 cm. CTDI values were measured for three standard head, pelvis and thorax protocols.
For more detailed information on X-ray tube and imager, image acquisition and reconstruction, mechanical safety and collision avoidance, workflow and image guidance time, scan protocols and clinical operation, please see online supplemental materials.
Results
Mechanical performance
The position accuracy of the C-arm was high for all three designated imaging positions. The average deviations of the centre of the 2-mm sphere located at the radiation isocentre or with a 27 or 100 cm offset on projection images from the centre of the flat-panel imager were 0.06, 0.03 and 0.07 mm at positions 0, 1 and 2, respectively. The repeatability of the robotic arm movement was 0.05, 0.01 and 0.03 mm at positions 0, 1 and 2, respectively. The rotation speed of the C-ring was 6° s–1, intentionally slowed down for safety reason. The vibration of the C-ring during rotation was within ±0.417 mm, when evaluated on the flat panel detector plane. The gravity-induced flex of the C-ring due to the weights of X-ray tube and flat panel detector was angle-dependent (maximum 5 mm, average 3 mm). Flex correction was implemented by adjusting the C-arm position during image acquisition to compensate for the flex rather than re-aligning projection images after acquisition. For the isocentric accuracy of the C-ring rotation after flex correction, the maximum deviation from the average was 0.56 mm in the rotation-axis direction and 0.48 mm in the rotation direction for all imaging positions.
Image quality
For low-contrast visibility, a 7 mm disk on 1% supra-slice targets of Catphan phantom was visible with the half-fan mode. For the full-fan mode, a higher tube current of 42 mA and a higher degree of pixel smoothing (3 × 9 vs standard 3 × 3) in the longitudinal direction of the CBCT were needed to achieve the same visibility. For high-contrast resolution, 8 line pairs/cm were identifiable on CBCT images. The CT numbers were more uniform on the full-fan–mode images (28–49 HU in five circular regions of interest distributed throughout the image) than on the half-fan–mode images (45–74 HU). For CT number accuracy, as evaluated on seven insert materials, the deviation from the predicted value in the phantom manual ranged from −19 HU to +48 HU for the full-fan mode and −18 HU to +44 HU for the half-fan mode. The maximum deviation was observed in the Delrin material (388 HU for the full-fan–mode image and 384 HU for the half-fan–mode image vs the expected 340 HU). The spatial inaccuracy was less than 0.5 mm near the centre of the FOV, but it approached 1 mm at radial distances of 10 cm (full-fan mode) and 20 cm (half-fan mode). Beam-hardening artefacts were observed on anthropomorphic phantom images.
Isocentr coincidence and image guidance accuracy
The difference between the isocentre and the centre of the projection image was 0.25 mm or less for all the C-ring angles we tested. Positioning the 1 cm ball with CBCT followed by proton irradiation showed ≤0.5 mm difference between CBCT imaging centre and the proton field centre.
Tables 1 and 2 summarize the results for image guidance accuracy for anthropomorphic head and pelvis phantoms when using automatic 3D-3D registration. The differences between the actual and calculated shifts were all less than 1 mm and 1° for 3D vectors, except in the scenario with a 2 cm and 2° shift at position 1. Residual errors calculated by the PIAS software based on the verification CBCT images after PPS couch shifts were less than 0.5 mm for 3D vectors in most cases and less than 1 mm in all cases.
Table 1.
Results of image guidance accuracy for head phantom
| CBCT imaging position | Phantom location on couch top | Actual physical shifts | Difference between actual and calculated shifts |
Residual errors based on verification CBCT images |
||
|---|---|---|---|---|---|---|
| Translation (mm) | Rotation (°) | Translation (mm) | Rotation (°) | |||
| 0 | CCV + 50 | 5 mm & 0.5° | 0.33 | 0.30 | 0.44 | 0.30 |
| 0 | CCV + 50 | 10 mm & 1° | 0.59 | 0.60 | 0.36 | 0.30 |
| 0 | CCV + 50 | 20 mm & 2° | 0.47 | 0.20 | 0.36 | 0.40 |
| 1 | CCV + 50 | 5 mm & 0.5° | 0.30 | 0.20 | 0.14 | 0.10 |
| 1 | CCV + 50 | 10 mm & 1° | 0.64 | 0.30 | 0.59 | 0.40 |
| 1 | CCV + 50 | 20 mm & 2° | 1.24 | 0.30 | 0.66 | 1.20 |
| 2 | CCV + 50 | 5 mm & 0.5° | 0.46 | 0.30 | 0.62 | 0.20 |
| 2 | CCV + 50 | 10 mm & 1° | 0.63 | 0.50 | 0.02 | 0.30 |
| 2 | CCV + 50 | 20 mm & 2° | 0.20 | 0.50 | 0.22 | 0.50 |
| 2 | CCV | 5 mm & 0.5° | 0.46 | 0.40 | 0.33 | 0.20 |
| 2 | CCV | 10 mm & 1° | 0.47 | 0.30 | 0.35 | 0.30 |
| 2 | CCV | 20 mm & 2° | 0.75 | 0.50 | 0.36 | 0.30 |
| 2 | CCV−30 | 5 mm & 0.5° | 0.49 | 0.40 | 0.54 | 0.10 |
| 2 | CCV−30 | 10 mm & 1° | 0.67 | 0.50 | 0.20 | 0.10 |
| 2 | CCV−30 | 20 mm & 2° | 0.51 | 0.30 | 0.14 | 0.70 |
CBCT, cone beam CT. CCV, the centre of the PPS calibrated volume; CCV +50, the location of the phantom was 50 cm from the CCV toward the superior end of the couch top; CCV−30, the location of the phantom was 30 cm from the CCV toward the PPS flange.
At positions 0 and 1, only the location at the superior boundary of the calibrated volume (50 cm superior to the CCV) was tested because of interference between the PPS and the 90° nozzle at 2 other locations.
Actual physical shifts were applied to all translations and rotations. For example, the 5 mm & 0.5° shift represents the shifts of 5 mm in x, 5 mm in y, 5 mm in z, 0.5° in pitch, 0.5° in roll and 0.5° in yaw.
Translation error = , where , and Δx, y, and Δz represent differences in translational shifts on 3 orthogonal axes. Rotation error = max (Δpitch, Δroll, Δyaw), where Δpitch, Δroll, and Δyaw represent differences in the rotational angles on the 3 orthogonal axes.
The scan protocol parameters were 200° arc acquisition, 100 kV, 20 mA, 20 ms, 10 frames per seconds, 512 × 512 reconstruction matrix, 0.55 mm pixel size and 0.6 mm slice thickness.
Table 2.
Results of image guidance accuracy for pelvis phantom
| CBCT imaging position | Phantom location on couch top | Actual physical shifts | Difference between actual and calculated shifts | Residual errors based on verification CBCT images | ||
|---|---|---|---|---|---|---|
| Translation (mm) | Rotation (°) | Translation (mm) | Rotation (°) | |||
| 2 | CCV | 5 mm & 0.5° | 0.75 | 0.40 | 0.41 | 0.20 |
| 2 | CCV | 10 mm & 1° | 0.42 | 0.30 | 0.37 | 0.40 |
| 2 | CCV | 20 mm & 2° | 0.41 | 0.30 | 0.22 | 0.20 |
| 2 | CCV−30 | 5 mm & 0.5° | 0.22 | 0.10 | 0.22 | 0.10 |
| 2 | CCV−30 | 10 mm & 1° | 0.62 | 0.10 | 0.00 | 0.10 |
| 2 | CCV−30 | 20 mm & 2° | 0.51 | 0.30 | 0.76 | 0.00 |
CBCT, cone beam CT; CCV, centre of the calibrated volume; CCV, CCV−30, translation error, and rotation error are defined as in Table 1. The CCV +50 location was not tested, because it is unlikely to position the patient’s pelvis near the superior end of the couch top.
Scan protocol parameters were 360° acquisition, 125 kV, 42 mA, 20 ms, 10 frames per seconds, 1024 × 1024 reconstruction matrix, 0.52-mm pixel size, and 1-mm slice thickness.
Scan protocols and CBCT imaging dose
Table 3 summarizes the scan parameters and measured CTDI numbers for three initial institutional CBCT protocols (for the head, pelvis and thorax). The asymmetry in the peripheral doses for head scans was primarily due to the use of partial arc acquisition. The angular range was chosen in order to reduce the doses to the eye lenses. Efforts are ongoing to reduce the mAs per projection for paediatric patients by using a stage-wise approach. So far, the tube current in the head protocol has been reduced to 15 mA for paediatric patients older than 7 years and to 10 mA for patients aged 7 years or younger. Example CBCT images of the head and pelvis, acquired using the protocols in Table 3, are shown in Figure 3.
Table 3.
Scan parameters and CTDI of 3 initial CBCT protocols
| Head | Pelvis | Thorax | |
|---|---|---|---|
| Tube voltage (kVp) | 100 | 125 | 110 |
| Tube current (mA) | 20 | 42 | 20 |
| Exposure time per projection (ms) | 20 | 20 | 20 |
| C-ring rotation range (°) | 200 | 360 | 360 |
| Frame rate (fps) | 10 | 10 | 10 |
| Number of projections | 340 | 600 | 600 |
| Fan mode | Full | Half | Half |
| Reconstruction matrix | 512 × 512 | 1024 × 1024 | 1024 × 1024 |
| Bow-tie filter | Full | Half | Half |
| Pixel size (mm) | 0.55 | 0.52 | 0.52 |
| Slice spacing (mm) | 0.6 | 1 | 1 |
| Phantom diameter (cm) | 16 | 32 | 32 |
| CTDI (centre) (mGy) | 3.4 | 6.5 | 2.0 |
| CTDI (top) (mGy) | 1.9 | 9.7 | 3.2 |
| CTDI (right) (mGy) | 4.2 | 9.3 | 3.1 |
| CTDI (bottom) (mGy) | 5.1 | 8.8 | 2.9 |
| CTDI (left) (mGy) | 3.5 | 11.5 | 3.8 |
| Weighted CTDI (mGy) | 3.6 | 8.7 | 2.9 |
CBCT, cone beam CT; CTDI, CT dose index.
Figure 3.
Example CBCT images of the head and pelvis (window width/level = 2000/–200 HU). CBCT, cone beam CT.
Discussion
The development of the reported image guidance system represents a progress in particle therapy by providing high image quality and positioning accuracy previously achieved by on-board kV CBCT systems for linear accelerators.14–16 For example, the spatial resolution of 8 line pairs/cm is within the range of 6‒9 line pairs cm–1 reported for existing kV CBCT systems of photon therapy.17 The CT number accuracy of ±50 HU, CT number uniformity of ±40 HU, and measured CTDI of 3 (head and thorax protocols)‒9 mGy (pelvis protocol) for our system are comparable to the specifications of Varian TrueBeam STx system (Varian Medical Systems, Palo Alto, CA).18 The reported CBCT system provides another viable option than CT on rails for partial gantry and fixed-beam proton systems with the added advantage of acquiring images at the treatment isocentre. Although our CBCT system is mechanically separated from the proton gantry and nozzle, the coincidence between CBCT imaging isocentre and proton beam isocentr is high (<1 mm).
Compared to a floor-mounted robotic CBCT for proton and carbon ion therapy,11 our system has advantages of multiple imaging location options (at or off the treatment isocentre) and a larger FOV (maximum 53 cm in diameter vs 30 cm). Both systems achieved sub-millimeter and sub-degree positioning accuracy with anthropomorphic phantoms. Unfortunately, scarce literature reports on image guidance system performance of gantry- and nozzle-mounted CBCT prevent direct comparisons. Our CBCT system has a smaller SID and smaller acceleration and deceleration effects of the gantry (i.e. non-uniformly spaced projections due to slower rotation speeds near the start and end angles). For example, the SAD/SID is 200/300 cm for Varian ProBeam systems and 288.4/347 cm for IBA Proteus systems.12,13,19 Although the scatter effect theoretically becomes less severe as the cone angle decreases and the patient-to-imager distance increases, as in many gantry-mounted systems, the sensitivity to mechanical accuracy and stability also plays an important part in the resultant image quality. The larger SID of gantry-mounted CBCT systems may also place a greater burden on the X-ray tube if the same photon flux to the detector is expected as in the C-arm CBCT system with a smaller SID; even in the absence of an anti-scatter grid, the mAs exposure is typically much higher.
In our centre, patients with brain tumours and head and neck cancers received CBCT scans predominantly at the treatment isocentre (positon 0). Pelvis and abdomen sites as well as craniospinal irradiation patients were imaged mostly at the off-treatment isocentre position (position 2) because the patient couch would not interfere with the 90° nozzle. Currently the nozzle needs to be rotated to 90° (horizontal) before the robotic C-arm deployment to prevent potential collision. Lower neck and thorax sites were imaged at one of the two off-treatment isocentre positions (either position 1 or 2). For patients who were CBCT scanned at off-isocentre locations, a set of floor-mounted kV X-ray units pointing toward the treatment isocentre can be used to confirm the patient setup before proton beam delivery.
Because of the ceiling mounting mechanism and the robotic arms that sweep across the room to reach different imaging positions, the CBCT system occupies a significant portion of the ceiling space. This required a careful design of the robot movement paths to prevent physical interference with other devices in the room. It also limits the space available for installing ceiling-mounted optical surface-tracking cameras. A recessed ceiling to accommodate infrared cameras may be one solution to the problem; however, this would have to be included in the early design phase for the facility and in the radiation-shielding calculation. Other areas for improvement include the visibility of low-contrast anatomy such as the ventricles of the brain, CT number accuracy and consistency for dose calculation in CBCT, image reconstruction and transfer speed, and image artefacts. Ongoing efforts to reject or correct scatter, correct beam hardening, and reduce charge trapping in irradiated silicon detectors are needed to address these common issues with CBCT.
The ranges of proton beams are sensitive to anatomical changes along the beam paths.20–22 Methods have been proposed in the literature to convert CBCT images to water-equivalent thickness plots and virtual proton-depth radiographs to better detect the areas of significant deviation than visual inspection and trigger adaptive proton therapy.23,24 The feasibility has also been demonstrated of recalculating the proton dose distribution by using the deformed planning CT that matches the CBCT of the day.25,26 Because traditional anatomy-based patient alignment may not always be sufficient to meet the target coverage constraints for proton therapy, a dose-based positioning method has been suggested as an alternative to replanning. In this approach, after performing fast dose calculation on the treatment-day CT or corrected CBCT for all patient shifts in the search space, the optimal isocentre shift that maximizes the target coverage can be made.27
Conclusion
A gantry- and nozzle-independent, ceiling-mounted robotic CBCT system can be implemented to provide high-accuracy volumetric image guidance for proton therapy.
Acknowledgments
We thank Forte Automation Systems., Inc. for manufacturing the robotic system, the Hitachi Central Research Laboratory for developing the image reconstruction algorithms, the Hitachi engineers for the installation and technical commissioning of the system, and Dr Keith A. Laycock for scientific editing of the manuscript.
Contributor Information
Chiaho Hua, Email: chia-ho.hua@stjude.org.
Takenori Nishimura, Email: takenori.nishimura.hq@hitachi.com.
Tatsuya Fujisawa, Email: tatsuya.fujisawa.ka@hitachi.com.
Ryousuke Shinagawa, Email: ryosuke.shinagawa.vh@hitachi.com.
Thomas E Merchant, Email: thomas.merchant@stjude.org.
REFERENCES
- 1.Uematsu M, Fukui T, Shioda A, Tokumitsu H, Takai K, Kojima T, et al. A dual computed tomography linear accelerator unit for stereotactic radiation therapy: a new approach without cranially fixated stereotactic frames. Int J Radiat Oncol Biol Phys 1996; 35: 587–92. DOI: https://doi.org/10.1016/S0360-3016(96)80022-8 [DOI] [PubMed] [Google Scholar]
- 2.Mosleh-Shirazi MA, Evans PM, Swindell W, Webb S, Partridge M. A cone-beam megavoltage CT scanner for treatment verification in conformal radiotherapy. Radiother Oncol 1998; 48: 319–28. DOI: https://doi.org/10.1016/S0167-8140(98)00042-5 [DOI] [PubMed] [Google Scholar]
- 3.Mackie TR, Kapatoes J, Ruchala K, Lu W, Wu C, Olivera G, et al. Image guidance for precise conformal radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56: 89–105. DOI: https://doi.org/10.1016/S0360-3016(03)00090-7 [DOI] [PubMed] [Google Scholar]
- 4.Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys 2002; 53: 1337–49. DOI: https://doi.org/10.1016/S0360-3016(02)02884-5 [DOI] [PubMed] [Google Scholar]
- 5.Mori S, Zenklusen S, Knopf AC. Current status and future prospects of multi-dimensional image-guided particle therapy. Radiol Phys Technol 2013; 6: 249–72. DOI: https://doi.org/10.1007/s12194-013-0199-0 [DOI] [PubMed] [Google Scholar]
- 6.Engelsman M, Schwarz M, Dong L. Physics controversies in proton therapy. Semin Radiat Oncol 2013; 23: 88–96. DOI: https://doi.org/10.1016/j.semradonc.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 7.Seco J, Spadea MF. Imaging in particle therapy: state of the art and future perspective. Acta Oncol 2015; 54: 1254–8. DOI: https://doi.org/10.3109/0284186X.2015.1075665 [DOI] [PubMed] [Google Scholar]
- 8.Ritschl L, Kuntz J, Fleischmann C, Kachelrieß M. The rotate-plus-shift C-arm trajectory. Part I. Complete data with less than 180° rotation. Med Phys 2016; 43: 2295–302. DOI: https://doi.org/10.1118/1.4944785 [DOI] [PubMed] [Google Scholar]
- 9.Orth RC, Wallace MJ, Kuo MD. Technology Assessment Committee of the Society of Interventional Radiology. C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 2008; 19: 814–20. [DOI] [PubMed] [Google Scholar]
- 10.Floridi C, Radaelli A, Abi-Jaoudeh N, Grass M, Grass M, Lin M, et al. C-arm cone-beam computed tomography in interventional oncology: technical aspects and clinical applications. Radiol Med 2014; 119: 521–32. DOI: https://doi.org/10.1007/s11547-014-0429-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattori G, Riboldi M, Pella A, Peroni M, Cerveri P, Desplanques M, et al. Image guided particle therapy in CNAO room 2: implementation and clinical validation. Phys Med 2015; 31: 9–15. DOI: https://doi.org/10.1016/j.ejmp.2014.10.075 [DOI] [PubMed] [Google Scholar]
- 12.Veiga C, Janssens G, Teng CL, Baudier T, Hotoiu L, McClelland JR, et al. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol Biol Phys 2016; 95: 549–59. DOI: https://doi.org/10.1016/j.ijrobp.2016.01.055 [DOI] [PubMed] [Google Scholar]
- 13.Veiga C, Janssens G, Baudier T, Hotoiu L, Brousmiche S, McClelland J, et al. A comprehensive evaluation of the accuracy of CBCT and deformable registration based dose calculation in lung proton therapy. Biomed Phys Eng Express 2017; 3: 015003 DOI: https://doi.org/10.1088/2057-1976/3/1/015003 [Google Scholar]
- 14.Oelfke U, Tücking T, Nill S, Seeber A, Hesse B, Huber P, et al. Linac-integrated kV-cone beam CT: technical features and first applications. Med Dosim 2006; 31: 62–70. DOI: https://doi.org/10.1016/j.meddos.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 15.Chan M, Yang J, Song Y, Burman C, Chan P, Li S. Evaluation of imaging performance of major image guidance systems. Biomed Imaging Interv J 2011; 7: e11 DOI: https://doi.org/10.2349/biij.7.2.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan K, Mohammadi M, Shepherd J. Applications of linac-mounted kilovoltage Cone-beam computed tomography in modern radiation therapy: a review. Pol J Radiol 2014; 79: 181–93. DOI: https://doi.org/10.12659/PJR.890745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bissonnette JP, Balter PA, Dong L, Langen KM, Lovelock DM, Miften M, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys 2012; 39: 1946–63. DOI: https://doi.org/10.1118/1.3690466 [DOI] [PubMed] [Google Scholar]
- 18.Varian TrueBeam STx system specification. Table 14: kV CBCT specifications. 2015. Available at: https://varian.force.com
- 19.Kaissl W, Benner A, Vuriti M, Timmer J, Abel E. First images from ProBeam CBCT with integrated workflow on at Scripps proton therapy center Poster presentation at the 54th Annual Conference of the Particle Therapy Co-Operative Group (PTCOG. San Diego, CA: May 2015. [Google Scholar]
- 20.Hui Z, Zhang X, Starkschall G, Li Y, Mohan R, Komaki R, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys 2008; 72: 1385–95. DOI: https://doi.org/10.1016/j.ijrobp.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lassen-Ramshad Y, Vestergaard A, Muren LP, Høyer M, Petersen JB. Plan robustness in proton beam therapy of a childhood brain tumour. Acta Oncol 2011; 50: 791–6. DOI: https://doi.org/10.3109/0284186X.2011.590149 [DOI] [PubMed] [Google Scholar]
- 22.Li Z. Toward robust proton therapy planning and delivery. Transl Cancer Res 2012; 1: 217–26. [Google Scholar]
- 23.Wang P, Yin L, Zhang Y, Kirk M, Song G, Ahn PH, et al. Quantitative assessment of anatomical change using a virtual proton depth radiograph for adaptive head and neck proton therapy. J Appl Clin Med Phys 2016; 17: 5819–440. DOI: https://doi.org/10.1120/jacmp.v17i2.5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Park YK, Sharp G, Busse P, Winey B. Water equivalent path length calculations using scatter-corrected head and neck CBCT images to evaluate patients for adaptive proton therapy. Phys Med Biol 2017; 62: 59–72. DOI: https://doi.org/10.1088/1361-6560/62/1/59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landry G, Nijhuis R, Dedes G, Handrack J, Thieke C, Janssens G, et al. Investigating CT to CBCT image registration for head and neck proton therapy as a tool for daily dose recalculation. Med Phys 2015; 42: 1354–66. DOI: https://doi.org/10.1118/1.4908223 [DOI] [PubMed] [Google Scholar]
- 26.Kurz C, Nijhuis R, Reiner M, Ganswindt U, Thieke C, Belka C, et al. Feasibility of automated proton therapy plan adaptation for head and neck tumors using cone beam CT images. Radiat Oncol 2016; 11: 64 DOI: https://doi.org/10.1186/s13014-016-0641-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung JP, Park PC, Court LE, Ronald Zhu X, Kudchadker RJ, Frank SJ, et al. A novel dose-based positioning method for CT image-guided proton therapy. Med Phys 2013; 40: 051714 DOI: https://doi.org/10.1118/1.4801910 [DOI] [PMC free article] [PubMed] [Google Scholar]