Sir,
We read with great interest the recent article by Mencacci et al. (2014) about the increased risk for Parkinson’s disease in carriers of GCH1 mutations. GCH1 mutations are the most common cause of DOPA-responsive dystonia (DRD), a disorder characterized typically by lower limb dystonia with onset in childhood, an excellent, sustained response to levodopa, absence of levodopa-induced dyskinesias, and absence of dopaminergic neurodegeneration as evidenced by normal dopamine transporter (DAT) imaging. Inheritance of GCH1-linked DRD is autosomal dominant and penetrance is incomplete (Charlesworth et al., 2013). Mencacci et al. (2014) demonstrated that some GCH1 mutation carriers who did not manifest DRD during childhood, developed adult-onset parkinsonism with imaging evidence of dopaminergic neurodegeneration. Thus, the same GCH1 mutations may result in two distinct phenotypes: a non-degenerative phenotype typically consisting of childhood-onset DRD, and a neurodegenerative phenotype consisting of parkinsonism with onset in later life (Parkinson’s disease). Intriguingly, none of the reported GCH1 mutation carriers with imaging evidence of dopaminergic degeneration (Kikuchi et al., 2004; Hjermind et al., 2006; Eggers et al., 2012; Ceravolo et al., 2013; Mencacci et al., 2014) had dystonia in childhood. Conversely, no childhood-onset DRD cases with GCH1 mutations have been reported to have abnormal nigrostriatal dopaminergic imaging in adult life (Snow et al., 1993; Turjanski et al., 1993; Jeon et al., 1998; Mencacci et al., 2014). Thus, currently available data suggest that the degenerative and non-degenerative GCH1-associated phenotypes are mutually exclusive and that GCH1 mutation carriers with dystonia during childhood might somehow be protected against dopaminergic degeneration in later life. It has been proposed that the initiation of levodopa treatment in childhood, as is common practice in GCH1 mutation carriers with DRD, might possibly prevent subsequent nigrostriatal degeneration in these patients (Mencacci et al., 2014; Weissbach and Klein, 2014). However, we here present the case of a GCH1 mutation carrier who developed DRD and started levodopa therapy as a child, but was found to have severely abnormal dopamine transporter imaging as an adult.
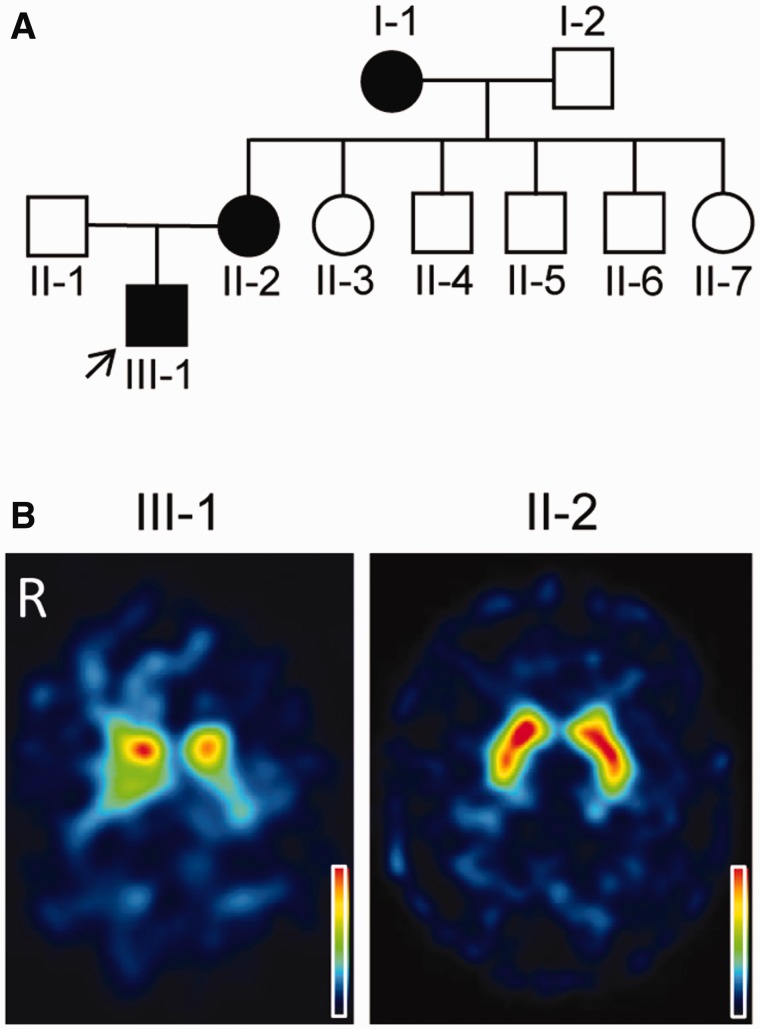
The proband (Case III-1; Fig. 1A), a 41-year-old Flemish male, developed bilateral foot dystonia with walking difficulties at the age of 8 years. At the age of 11 years levodopa therapy (300 mg/day) was initiated, resulting in complete resolution of the symptoms. The clinical diagnosis of DRD was confirmed by the finding of a heterozygous mutation (p.Y75S) in GCH1. The Y75S mutation has previously been reported in another Flemish family with DRD (Van Hove et al., 2006). We first assessed the proband in our adult neurology clinic at the age of 37. At that time he was taking 4 mg/kg of levodopa per day (150 mg at 6 am, 100 mg at noon, 150 mg at 7 pm) and was still doing well. Yet, he sometimes noticed a tense feeling in the legs, especially in the evening, and also reported that the dystonic postures of the feet returned within 6 h after inadvertently skipping a levodopa dose. He never experienced levodopa-induced dyskinesias. Clinical examination performed 5 h after his morning dose of 150 mg levodopa showed no clear abnormalities (Supplementary Video 1). We were intrigued by his report of relatively rapid recurrence of dystonia after missing a levodopa dose, because a previous study in three patients with DRD found that dystonia did not recur until 29 h or more after levodopa withdrawal (Dewey et al., 1998). We decided to examine him clinically after levodopa withdrawal and to perform 123I-FP-CIT single-photon emission computed tomography (SPECT). Clinical exam after 22 h of levodopa withdrawal showed prominent dystonia of the lower limbs with inversion of the left foot, eversion of the right foot and extension of the right knee (Supplementary Video 2). Gait was difficult due to lower limb dystonia. The right arm had a slightly dystonic posture during gait (Supplementary Video 3). Arm swing was preserved bilaterally. Muscle tone was increased in the dystonic limbs without cogwheeling. There was no hypokinesia, as illustrated by the absence of decrement during repetitive finger tapping (Supplementary Videos 4 and 5). There was no tremor, postural instability or freezing of gait. Speech and facial expression were normal. There were no pyramidal signs or other clinical abnormalities. 123I-FP-CIT SPECT revealed strong, bilateral reduction of tracer uptake, especially in the putamen and more marked on the left side, a pattern typical of Parkinson’s disease (Fig. 1B). After this SPECT result genetic analysis of PARK2, PINK1 and PARK7 was performed, but this did not show any mutations, exonic deletions or duplications. The dose of levodopa was slightly increased to 450 mg/day, and the patient has been free of clinical symptoms and signs in the 3 years since the 123I-FP-CIT SPECT. He is still employed as a factory worker.
Figure 1.
Family tree and dopamine transporter imaging. (A) Squares and circles denote males and females, respectively. Black symbols indicate individuals with childhood-onset dystonia and excellent levodopa response. The arrow indicates the proband. (B) Transversal 123I-FP-CIT SPECT images acquired in Case III-1 at the age of 37 years and Case II-2 at the age of 60 years. The colour scale indicates relative radioactivity uptake (0–100%).
The 63-year-old mother of the proband (Case II-2; Fig. 1A) developed cramping pain in the calves and dystonic inversion of the feet at the age of 8 years. Levodopa treatment (300 mg per day) was started at the age of 20, with an excellent response ever since. She carried the same heterozygous p.Y75S GCH1 mutation as the proband. Clinical exam during levodopa treatment was repeatedly normal. 123I-FP-CIT SPECT performed at the age of 60 years showed no abnormalities (Fig. 1B). According to Case II-2, her mother (Case I-1) also had childhood-onset dystonia with excellent response to levodopa, but Case I-1 was not available for evaluation.
The proband’s mother (Case II-2) had typical DRD, but the proband himself did not fit neatly into one of the two GCH1-linked phenotypic categories (i.e. non-degenerative DRD versus Parkinson’s disease). The features of his condition were typical of DRD except for the abnormal DAT imaging and the relatively rapid recurrence of dystonic symptoms after missing a levodopa dose. Clinically, he did not have Parkinson’s disease, because examination after levodopa withdrawal showed pure dystonia without hypokinesia, tremor or postural instability. Juvenile Parkinson’s disease can cause prominent dystonia and sometimes initially mimics the clinical picture of DRD, but the proband’s complete lack of levodopa-induced dyskinesias after 30 years of levodopa treatment, in addition to his lack of parkinsonism, argued against a diagnosis of juvenile Parkinson’s disease (Tassin et al., 2000). Moreover, PARK2, PINK1 and PARK7 mutations, the main known causes of juvenile Parkinson's disease, were excluded. A plausible scenario is that he had non-degenerative dystonia since childhood, developed nigrostriatal neurodegeneration more recently and is now in a preclinical stage of Parkinson’s disease.
The proband’s case adds further complexity to the phenotypic spectrum of GCH1 mutations and suggests that some GCH1 mutation carriers may have an overlap phenotype, combining elements of DRD and Parkinson’s disease. Furthermore, this case shows that early treatment of GCH1 mutation carriers with levodopa does not necessarily prevent nigrostriatal degeneration.
Acknowledgements
We thank Dr G. Matthijs for genetic analysis.
Funding
W.V. and K.V.L. are Senior Clinical Investigators of the Research Foundation Flanders (FWO).
Supplementary material
Supplementary material is available at Brain online.
References
- Ceravolo R, Nicoletti V, Garavaglia B, Reale C, Kiferle L, Bonuccelli U. Expanding the clinical phenotype of DYT5 mutations: is multiple system atrophy a possible one? Neurology. 2013;81:301–2. doi: 10.1212/WNL.0b013e31829bfd7c. [DOI] [PubMed] [Google Scholar]
- Charlesworth G, Bhatia KP, Wood NW. The genetics of dystonia: new twists in an old tale. Brain. 2013;136:2017–37. doi: 10.1093/brain/awt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RB, Muenter MD, Kishore A, Snow BJ. Long-term follow-up of levodopa responsiveness in generalized dystonia. Arch Neurol. 1998;55:1320–23. doi: 10.1001/archneur.55.10.1320. [DOI] [PubMed] [Google Scholar]
- Eggers C, Volk AE, Kahraman D, Fink GR, Leube B, Schmidt M, et al. Are dopa-responsive dystonia and Parkinson’s disease related disorders? A case report. Parkinsonism Relat Disord. 2012;18:666–8. doi: 10.1016/j.parkreldis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Hjermind LE, Johannsen LG, Blau N, Wevers RA, Lucking CB, Hertz JM, et al. Dopa-responsive dystonia and early-onset Parkinson’s disease in a patient with GTP cyclohydrolase I deficiency? Mov Disord. 2006;21:679–713. doi: 10.1002/mds.20773. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jeong JM, Park SS, Kim JM, Chang YS, Song HC, et al. Dopamine transporter density measured by [123I]beta-CIT single-photon emission computed tomography is normal in dopa-responsive dystonia. Ann Neurol. 1998;43:792–800. doi: 10.1002/ana.410430614. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Takeda A, Fujihara K, Kimpara T, Shiga Y, Tanji H, et al. Arg(184)His mutant GTP cyclohydrolase I, causing recessive hyperphenylalaninemia, is responsible for dopa-responsive dystonia with parkinsonism: a case report. Mov Disord. 2004;19:590–3. doi: 10.1002/mds.10712. [DOI] [PubMed] [Google Scholar]
- Mencacci NE, Isaias IU, Reich MM, Ganos C, Plagnol V, Polke JM, et al. Parkinson’s disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137:2480–92. doi: 10.1093/brain/awu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BJ, Nygaard TG, Takahashi H, Calne DB. Positron emission tomographic studies of dopa-reponsive dystonia and early-onset idiopathic parkinsonism. Ann Neurol. 1993;34:733–8. doi: 10.1002/ana.410340518. [DOI] [PubMed] [Google Scholar]
- Tassin J, Dürr A, Bonnet AM, Gil R, Vidailhet M, Lücking CB, et al. Levodopa-responsive dystonia. GTP cyclohydrolase I or parkin mutations? Brain. 2000;123:1112–21. doi: 10.1093/brain/123.6.1112. [DOI] [PubMed] [Google Scholar]
- Turjanski N, Bhatia K, Burn DJ, Sawle GV, Marsden CD, Brooks DJ. Comparison of striatal 18F-dopa uptake in adult-onset dystonia-parkinsonism, Parkinson’s disease, and dopa-responsive dystonia. Neurology. 1993;43:1563–8. doi: 10.1212/wnl.43.8.1563. [DOI] [PubMed] [Google Scholar]
- Van Hove JL, Steyaert J, Matthijs G, Legius E, Theys P, Wevers R, et al. Expanded motor and psychiatric phenotype in autosomal dominant Segawa syndrome due to GTP cyclohydrolase deficiency. J Neurol Neurosurg Psychiatry. 2006;77:18–23. doi: 10.1136/jnnp.2004.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A, Klein C. Hereditary dystonia and parkinsonism: two sides of the same coin? Brain. 2014;137:2402–4. doi: 10.1093/brain/awu181. [DOI] [PubMed] [Google Scholar]