In the first such study to be conducted, Mac Donald et al. assess US military personnel 0–7 days after blast-related concussion in Afghanistan and 6–12 months later in the United States. Military personnel who remain on duty after concussion frequently show poor outcomes, driven largely by impaired mental health.
Keywords: traumatic brian injury, post-traumatic stress, clinical outcome, concussion
In the first such study to be conducted, Mac Donald et al. assess US military personnel 0–7 days after blast-related concussion in Afghanistan and 6–12 months later in the United States. Military personnel who remain on duty after concussion frequently show poor outcomes, driven largely by impaired mental health.
Abstract
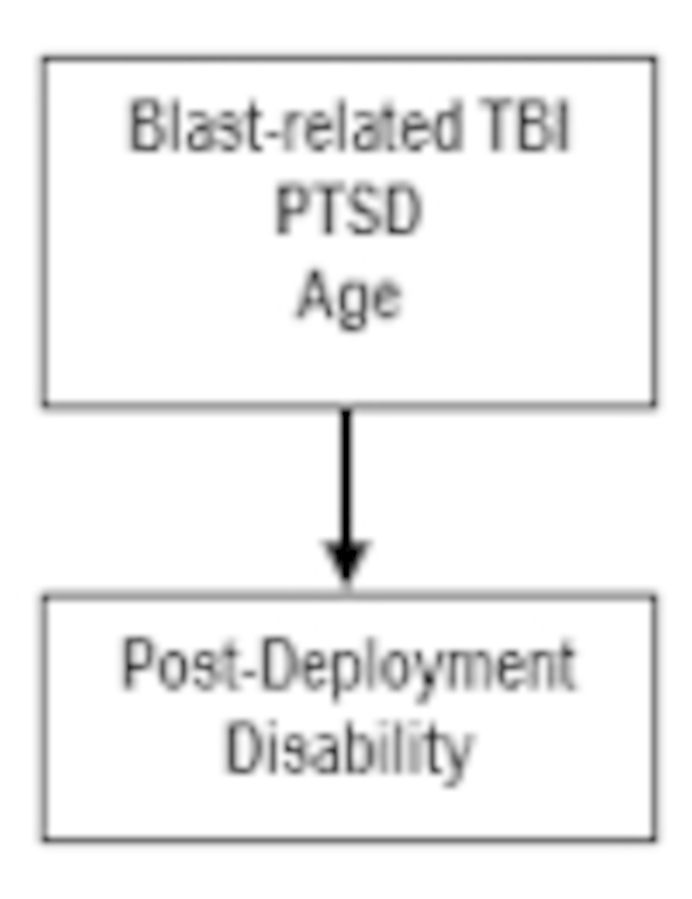
High rates of adverse outcomes have been reported following blast-related concussive traumatic brain injury in US military personnel, but the extent to which such adverse outcomes can be predicted acutely after injury is unknown. We performed a prospective, observational study of US military personnel with blast-related concussive traumatic brain injury (n = 38) and controls (n = 34) enrolled between March and September 2012. Importantly all subjects returned to duty and did not require evacuation. Subjects were evaluated acutely 0–7 days after injury at two sites in Afghanistan and again 6–12 months later in the United States. Acute assessments revealed heightened post-concussive, post-traumatic stress, and depressive symptoms along with worse cognitive performance in subjects with traumatic brain injury. At 6–12 months follow-up, 63% of subjects with traumatic brain injury and 20% of controls had moderate overall disability. Subjects with traumatic brain injury showed more severe neurobehavioural, post-traumatic stress and depression symptoms along with more frequent cognitive performance deficits and more substantial headache impairment than control subjects. Logistic regression modelling using only acute measures identified that a diagnosis of traumatic brain injury, older age, and more severe post-traumatic stress symptoms provided a good prediction of later adverse global outcomes (area under the receiver-operating characteristic curve = 0.84). Thus, US military personnel with concussive blast-related traumatic brain injury in Afghanistan who returned to duty still fared quite poorly on many clinical outcome measures 6–12 months after injury. Poor global outcome seems to be largely driven by psychological health measures, age, and traumatic brain injury status. The effects of early interventions and longer term implications of these findings are unknown.
Introduction
In the US military, it is estimated that ∼20% of the deployed force suffered a head injury (Taniellian and Jaycox, 2008) in the wars in Iraq and Afghanistan. Of these, 83.3% endured a mild, uncomplicated traumatic brain injury (TBI) or concussion (Casscells, 2007; DVBIC, 2013), the long-term impact of which is just beginning to be appreciated. Previous studies have reported that 78% of all combat casualties can be accounted for by explosive mechanisms (Owens et al., 2008) and 88% of all patients referred to second echelon treatment centres for further care were due to blast exposure (Warden, 2006).
Previous work has attempted to understand the sequelae of these blast-related ‘mild’/concussive brain injuries but it has been predominantly limited to later stage evaluations (Verfaellie et al., 2013; Fischer et al., 2014), retrospective review (Galarneau et al., 2008; Cooper et al., 2011; Eskridge et al., 2013; Kontos et al., 2013) or biased towards patients requiring medical evacuation (Mac Donald et al., 2014a, b), which may not be representative of the larger population of concussive TBI patients treated directly in the combat theatre. Few studies have prospectively examined patients acutely in theatre (Luethcke et al., 2011; Coldren et al., 2012; Norris et al., 2013), but none to our knowledge, have completed longitudinal evaluations to elucidate the relationship between acute characteristics and long-term outcomes. The objective of the current study was to clinically assess service members from the point of injury in Afghanistan and follow them to 6–12 month outcome back in the United States to determine if acute clinical measures could be used to predict brain injury sequelae and overall outcome.
Materials and methods
Participants were initially enrolled at Kandahar Air Field and Camp Leatherneck in Afghanistan between March and September 2012 as part of a prospective, observational, research study. Through this ongoing collaborative effort, a subset of these subjects were also enrolled in a 6–12 month follow-up at Washington University in Saint Louis, Missouri (Principal Investigator: D. Brody). This group was randomly selected from the larger cohort enrolled in Afghanistan from those who consented to participate in a long-term follow-up examination back in the USA. In total, 72 subjects, 34 controls and 38 TBI subjects completed both the initial study in Afghanistan and the follow-up evaluation at Washington University in Saint Louis 6–12 months later. Demographic characteristics were similar but not identical between groups (Table 1). Within each group, there were no significant differences in demographic information comparing those who followed up to those who only completed the initial study (Supplementary Table 1).
Table 1.
Participant characteristics
| Characteristic | Control (n = 34) | TBI (n = 38) | P-value |
|---|---|---|---|
| Age in years: median (range) | 28 (19–44) | 26 (20–41) | 0.02a |
| Education in years: median (range) | 15 (12–24) | 13 (12–18) | 0.0003a |
| Gender no (%) | |||
| Male | 27 (79%) | 36 (95%) | 0.05c |
| Female | 7 (21%) | 2 (5%) | |
| Race/ethnicity no (%) | |||
| White | 22 (65%) | 29 (77%) | 0.28b |
| African American | 5 (15%) | 2 (5%) | |
| Hispanic/Latino | 7 (20%) | 7 (18%) | |
| Asian | 0 | 0 | |
| Branch of Service no (%) | |||
| US Army | 13 (38%) | 32 (84%) | 0.0001b |
| US Air Force | 2 (6%) | 0 | |
| US Marine Corps | 3 (9%) | 6 (16%) | |
| US Navy | 16 (47%) | 0 | |
| Military Rank no (%) | |||
| Enlisted | 24 (71%) | 35 (92%) | 0.018c |
| Officer | 10 (29%) | 3 (8%) | |
| Enrolment Site (%) | |||
| Kandahar Airfield | 31 (91%) | 30(79%) | 0.15c |
| Camp Leatherneck | 3 (7%) | 8 (21%) | |
| Previous Deployments | |||
| median (range) | 2 (0–7) | 2 (0–8) | 0.99a |
| Previous Blast Exposures | |||
| median (range) | 0 (0–2) | 0 (0–6) | 0.0031a |
| Previous Concussions | |||
| median (range) | N/A | 2 (0–11) |
aMann-Whitney U-test.
bChi-square.
cFisher’s exact test.
Subjects
Inclusion criteria for the TBI group were as follows: (i) clinical diagnosis of ‘mild’/concussive TBI from a blast exposure within the past 7 days made by a trained, board-certified neurologist or neurosurgeon based on the criteria from the American Congress of Rehabilitation Medicine 1993; (ii) injury from blast exposure within 7 days of enrolment; (iii) US military; (iv) ability to provide informed consent in person; (v) no contraindications to MRI such as retained metallic fragments; (vi) no prior history of moderate to severe TBI based on Department of Defense criteria; and (vii) agreement to communicate by telephone or email and then travel to Washington University in Saint Louis for in-person follow-up. Inclusion criteria for the control group were the same except for a negative assessment for TBI and no history of blast exposure.
The research protocol was approved by the Human Research Protection Office at Washington University. This study was conducted under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board and in accordance with the approved protocol. Written informed consent was obtained from all subjects in person; no surrogate consent was allowed. All subjects had a Glasgow Coma Scale of 15 at the time of consent and competence to provide informed consent was assessed in a standardized fashion based on responses to questions regarding the purpose of the study, expected requirements for participation, and potential risks. Additional written consent was obtained from the subjects at the time of follow-up at Washington University. Active duty military subjects were not paid for participation, though travel expenses to St Louis were covered. Subjects not on active military duty status at the time of follow-up in St Louis were paid $240 plus travel expenses for participation.
For the TBI group, no intracranial abnormalities were detected on non-contrast head CT. All TBI subjects met the Department of Defense criteria for uncomplicated mild TBI. All clinical histories were verified by study personnel taking additional clinical history and reviewing medical records. None that screened positive for TBI at initial enrolment in Afghanistan were determined not to have had a TBI at follow-up. Mean time from injury to enrolment was 3.76 ± 1.74 days with a total range of 0–7 days.
Initial clinical assessments
At the time of enrolment in Afghanistan, the following battery of assessments were completed: TBI subjects completed the Military Acute Concussion Evaluation (MACE) (Dempsey et al., 2009), which is a brief cognitive test to evaluate orientation, immediate verbal memory, concentration, and short term delayed verbal memory. Both TBI and control participants also completed the Rivermead Post-Concussion Symptom Questionnaire (RPCSQ) (King et al., 1995), Post-traumatic Stress Disorder Check List Military (PCL-M; Yeager et al., 2007), Beck Depression Inventory (Beck et al., 1961; Homaifar et al., 2009), Combat Exposures Scale (CES; Keane et al., 1989), Balance Error Scoring System (Guskiewicz et al., 2001), Automated Neurocognitive Assessment Metrics – Traumatic Brain Injury Military Version 4 (ANAM; Cernich et al., 2007) and the Test of Memory Malingering (TOMM; Tombough, 1996).
The severity of post-concussive symptoms was measured by the Rivermead Post-Concussion Symptom Questionnaire (King et al., 1995), a self-administered questionnaire assessing 16 post-concussive symptoms on a scale of 0 (none) to 4 (severe) covering three domains: cognitive (cognitive and concentration difficulties), emotional (anxiety, restlessness and depression) and somatic (fatigue, headache, dizziness, nausea, sleep disturbance and changes in vision).
Symptoms of post-traumatic stress disorder (PTSD), anxiety and mood changes were assessed using the PCL-M (Yeager et al., 2007) and Beck Depression Inventory (Homaifar et al., 2009). The PCL-M is a 17-item self-administered questionnaire tying symptom ratings to events experienced during military service, using a scale of 1 (not at all) to 5 (extremely). The BDI is a self-administered 21-item questionnaire corresponding to symptoms of depression rated on a severity scale of 0 (no symptoms) to 3 (severe symptoms).
Reports of wartime stressors experienced by combatants were measured using the Combat Exposure Scale (Keane et al., 1989), a 7-item scale with five response points (1 is ‘no’, 2 is ‘1 to 3 times’, 3 is ‘4 to 12 times’, 4 is ‘13 to 50 times’, and 5 is ‘51+ times’), each item being weighted differently based on the severity of the experience, the total scores ranging from 0 to 41.
Severity of balance impairment was tested using the Balance Error Scoring System (BESS) (Guskiewicz et al., 2001). The Balance Error Scoring System is a clinician administered balance test that includes single, double and tandem stance assessment on firm and foam (unstable) surfaces, each held for 20 s, with the participant’s hands on the hips and eyes closed. The final score is a representation of cumulative errors.
The ANAM (Cernich et al., 2007) is sanctioned by the Department of Defense for baseline neurocognitive assessment in all deploying troops and it is also available in the deployed setting. The ANAM includes a collection of cognitive modules. The simple reaction time and repeat simple reaction time for basic neural processing are expressed in milliseconds, lower scores indicating a faster reaction time. Code substitution learning for associative learning, procedural reaction time for processing speed, mathematical processing for working memory, matching to sample for visual spatial memory and code substitution delayed for delayed memory are expressed as percentage of correct answers in 60 s, higher scores indicating better performance. The cognitive modules are preceded by sleepiness and mood scales.
Level of examination effort was measured using the TOMM (Tombough, 1996), which is a clinician administered tool designed to assist in determining effort (Tombough, 1996). The testing paradigm involved a single TOMM trial for subjects with a score ≥45 and a second trial for subjects with a first TOMM score <45. Subjects with TOMM score <45 on both consecutive TOMM trials were excluded from analysis for possible poor effort during testing.
All exams and questionnaires were administered in a quiet, private room. Total examination time took ∼1 h 15 min.
Follow-up clinical assessments
The in-person clinical evaluations at Washington University included a standardized neurological exam, neuropsychological test battery, and psychiatric evaluation. Both controls and subjects with TBI were screened at follow-up for interim head injuries or blast exposure associated with alteration or loss of consciousness or amnesia. None of the participants in either group were found to have suffered additional TBI between the initial enrolment and follow-up visit. Overall clinical outcome was assessed using the Glasgow Outcome Scale Extended (GOS-E) (Wilson et al., 1998; Pettigrew et al., 2003). The GOS-E is scored from 1–8: 1 = dead, 2 = vegetative, 3–4 = severe disability, 5–6 = moderate disability, 7–8 = good recovery. Moderate disability (GOS-E = 5–6) is defined as one or more of the following: (i) inability to work to previous capacity; (ii) inability to resume the majority of regular social and leisure activities outside the home; and (iii) psychological problems which have frequently resulted in ongoing family disruption or disruption of friendships. Severe disability is defined as reduced ability to perform activities of daily living such that supervision is required. Standardized, structured interviews were performed according to published guidelines (Wilson et al., 1998).
The neurological evaluation included Neurobehavioural Rating Scale-Revised (Levin et al., 1987), a structured interview designed for TBI patients, two headache interviews to capture recent headache frequency and intensity, Migraine Disability Assessment (MIDAS) and Headache Impact Test (HIT-6) (Stewart et al., 1999; Kosinski et al., 2003), and the Neurological Outcome Scale for TBI (McCauley et al., 2010; Wilde et al., 2010a, b), a structured neurological examination targeting deficits frequently experienced by TBI patients. The Neurobehavioural Rating Scale – Revised was scored using a previously published five-subdomain model (McCauley et al., 2001).
The neuropsychological test battery consisted of the Conner’s Continuous Performance Test II (Conners and Staff., 2000), a computer-based assessment of attention, impulsivity, reaction time, and vigilance; California Verbal Learning Test II (Delis et al., 2000), an assessment of verbal declarative memory; 25-hole grooved pegboard test (Matthews and Kløve, 1964), an assessment of upper extremity motor speed and coordination; timed 25-foot walk: the Trail Making test (Reitan, 1992), an assessment of visual scanning, coordination and mental flexibility; the Controlled Oral Word Association test (Benton et al., 1983), an assessment of verbal fluency; the Wechsler Test of Adult Reading (Wechsler, 2001) as an estimate of pre-injury verbal intelligence; the Iowa Gambling Test (Bechara et al., 1994), a computer-based assessment of impulsivity and decision making; the Delis–Kaplan Executive Function System Colour-Word Interference Test (Delis, 2001), an multi-domain assessment of executive function similar to the Stroop Test; and the Ruff-Light Trail Learning Test (Ruff et al., 1996), an assessment of visual-spatial learning and memory. A relatively easy forced choice test embedded in the California Verbal Learning Test was used to assess adequacy of effort.
The psychiatric evaluation included the Clinician-Administered PTSD Scale for DSM-IV (CAPS) (Weathers et al., 2001), Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979; Snaith et al., 1986), Combat Exposure Scale (Keane et al., 1989), and the Michigan Alcohol Screening Test (Selzer, 1971). The CAPS was scored using standard scoring rules from the Blake et al., National Centre for Post-traumatic Stress Disorder, July 1998 revision.
The standardized neurological evaluation required ∼1 h per subject. The psychiatric assessments required ∼2 h per subject, and the neuropsychological battery required ∼2 h per subject. Subjects took all medications as prescribed by their clinical providers. All tests were performed between 9 am and 5 pm in private, quiet, well-lighted rooms. All examiners were blinded to other clinical information and imaging results, though in the course of the interviews it often became clear whether the subjects were in the TBI or control group based off their endorsements of prior events. All examiners were clinicians who underwent standardized training in administering the assessments.
Safety and data monitoring
Subjects were assigned a random four-digit code number to protect confidentiality and all research data were identified by code number only. A board-certified psychiatrist (Dr Nelson) was immediately available in case the CAPS examination exacerbated PTSD symptoms. No exacerbations requiring medical intervention occurred, though additional support from study staff was required on several occasions.
Statistical analyses
All data were analysed using Statistica 10.0 (Statsoft Inc). Continuous variables have been summarized as mean ± standard deviation (SD) unless otherwise specified. The normal distribution of each continuous variable was assessed using the Shapiro-Wilk test. For normally distributed variables, Student’s t-tests were used to compare groups. For non-normally distributed variables, Mann-Whitney U-tests were used. Although we pre-specified the hypothesis that TBI subjects would have worse outcomes than controls, we have reported results of two-sided tests throughout to be conservative. Nominal P-values have been reported, but only considered significant using a Bonferroni correction for multiple comparisons with a significance level of 0.05. The number of tests within each domain of assessment was used to calculate the Bonferroni correction.
Data from neuropsychological testing were further analysed for the expected occurrence by chance that a subject would have multiple abnormal evaluations. The binomial distribution was used with P = 0.02275 for the (n = 18) neuropsychological variables examined. Prior to this analysis, all neuropsychological variables were confirmed to be statistically independent as is required by the assumptions of this approach.
Correlation analysis was employed to investigate the relationship between initial and follow-up data. Non-parametric rank-based Spearman correlations were used. Pearson correlations were attempted, but the residuals were not normally distributed as determined by the Shapiro Wilk test.
Logistic regression analysis were used to explore the relationship between a dichotomized measure of clinical outcome (GOS-E), clinical measures 6–12 months post-injury, and acute clinical data collected at 0–7 days. The Statistica 10.0 ‘generalized linear/non-linear model building’ algorithm was used with the selection of the ‘logit’ link function for logistic regression. The algorithm generated a distinct model for each possible subset of quantitative measures of specific symptoms and impairments. Models were then ranked by Akaike information criterion. Receiver-operating characteristic curves were generated for the top ranked models.
Results
Acute clinical measures
Assessments performed 0–7 days post-injury indicated more severe symptoms and worse performance in the TBI group than in controls (Fig. 1 and Supplementary Fig. 1). TBI subjects had significantly higher scores on the Rivermead Post Concussion Symptoms Questionnaire (Fig. 1A) than controls (P = 0.0000002, Mann-Whitney U-test). In contrast, there were no significant differences in performance on the Balance Error Scoring System assessment (Fig. 1B), a test of postural stability (P = 0.36, Mann-Whitney U-test). Both groups, control and TBI subjects, performed worse than the normative performance score of college varsity athletes (Fig. 1B). TBI subjects also endorsed significantly worse symptoms on measures of PTSD (P = 0.000002, Mann-Whitney U-test) and depression (P = 0.0006, Mann-Whitney U-test) during this acute phase following injury (Fig. 1C and D).
Figure 1.
Initial clinical assessments indicate more severe concussion, PTSD and depression symptoms in TBI patients versus controls. (A) Rivermead Post-Concussion Symptoms Questionnaire (Max 64). (B) Balance Error Scoring System (Max 60), an assessment of balance and postural stability. Dashed line indicates average score of normal performance by college varsity athletes. (C) PCL-M (Max 85). Dashed line indicates minimum score of 17 on questionnaire. (D) Beck Depression Inventory (Max 63). Uncorrected P-values reported. *Significance after Bonferroni correction for multiple comparisons at *P < 0.05/4 = 0.0125.
In addition, TBI subjects generally performed worse than controls on the ANAM test after exposure to brain injury in Afghanistan compared to their individual pre-deployment baseline (Supplementary Fig. 1). This assessment was completed before they deployed to combat theatre and then repeated in theatre 0–7 days post-injury or at the point of enrolment for the control subjects. Scores represent the ‘delta’ of each subject’s performance compared to his or her own baseline testing before deployment. Specifically, performance on simple reaction time (P = 0.002, Supplementary Fig. 1A), procedural reaction time (P = 0.004, Supplementary Fig. 1D), code substitution learning (P = 0.0012, Supplementary Fig. 1E), mathematical processing (P = 0.002, Supplementary Fig. 1G) and match to sample (P = 0.0006, Supplementary Fig. 1H) were significantly worse in TBI subjects 0–7 days post-injury (Mann-Whitney U-test). The acute clinical symptoms and deficits in this subgroup of subjects who participated in the complete longitudinal study were similar to those in the entire cohort. Self-reported sleep deprivation was also substantially worse in the TBI subjects than controls (P = 0.01, Supplementary Fig. 1C).
Other injuries sustained at the time of mild TBI were all minimal and all subjects were returned to duty. Median time to return to duty was 7 days (range 2–23). All subjects had an Injury Severity Score (Baker et al., 1974) of zero meaning there were no injuries to the head and neck, face, chest, abdomen, extremities, or general body as scored by a clinician.
Chronic clinical measures
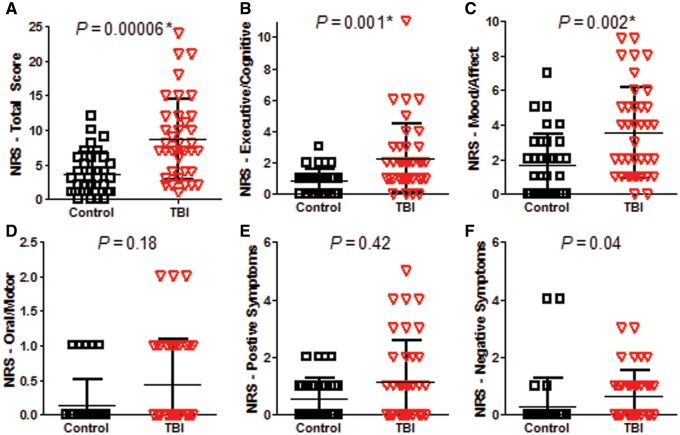
At 6–12 months post-injury, global outcomes as measured by the GOS-E were significantly worse in TBI subjects than in controls (P = 0.0001, Mann-Whitney U, Fig. 2). The majority of TBI subjects had moderate disability (GOS-E = 5–6). This was surprising given the relatively mild TBI sustained in these non-medically evacuated service members, but in line with previous work reporting GOS-E disability in service members medically evacuated from the combat theatre for blast plus impact complex concussive TBI (Macdonald et al., 2014a, b). In a similar fashion, subjects with TBI were found to have worse impairment than controls on the Neurobehavioural Rating Scale (P = 0.00006, Mann-Whitney U, Fig. 3A). Significant impairments were observed in the executive/cognitive (P = 0.001, Fig. 3B) and mood/affect (P = 0.002, Fig. 3C) subdomains of the Neurobehavioural Rating Scale. In contrast, there were no significant differences in neurological examination by the Neurological Outcome Scale for TBI (P = 0.81, Chi-square 0 versus 1 or more, Supplementary Fig. 2). Most subjects in both groups (25 of 34 controls and 27 of 38 TBI subjects) had no abnormalities on neurological examination. In controls, six subjects had olfactory deficits, one had a partial visual field deficit, one had a partial gaze deficit, and one had partial sensory loss of a lower limb. In TBI subjects, six had olfactory deficits and five had partial hearing loss, one of whom also had a partial gaze deficit.
Figure 2.
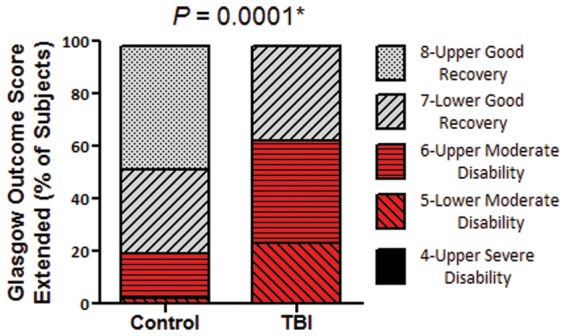
Global measure of outcome 6–12 months after enrolment indicate worse outcomes in TBI subjects versus controls. *Mann-Whitney U-test.
Figure 3.
Neurobehavioural outcome indicates worse outcomes in TBI subjects versus controls. (A) Total Neurobehavioural Rating Scale (NRS) Total Score (Max 87). (B) Executive/cognitive domain (Max 24). (C) Mood/affect domain (Max 15). (D) Oral/motor domain (Max 12). (E) Positive symptoms domain (Max 21). (F) Negative symptoms domain (Max 12). Higher scores on all of the measures indicate worse impairment. Uncorrected P-values reported. *Significance after Bonferroni correction for multiple comparisons at *P < 0.05/6 = 0.0083.
TBI subjects had significantly worse impact of headache in comparison to controls, as identified by two measures, the Migraine Disability Scale and Headache Impact Test. Headache impairment as assessed by the Migraine Disability Scale (Supplementary Fig. 3) was significantly worse for TBI subjects on total impact (Supplementary Fig. 3A, P = 0.0001), overall severity (Supplementary Fig. 3B, P = 0.00012), and frequency (Supplementary Fig. 3C, P = 0.000002) (all Mann-Whitney U-test). Headache Impact Test results (Supplementary Fig. 4) also indicated overall worse impairment based on the total score (Supplementary Fig. 4A, P = 0.00004), and frequency of severe headache pain (Supplementary Fig. 4B, P = 0.004), frequency of limitations of abilities due to headache (Supplementary Fig. 4C, P = 0.001), frequency of being tired because of headache (Supplementary Fig. 4D, P = 0.0008), frequency of being irritated because of headache (Supplementary Fig. 4E, P = 0.0000002), and frequency of having reduced concentration due to headache (Supplementary Fig. 4F, P = 0.000008) (all Mann-Whitney U-test).
Examination of neuropsychological test results identified trends towards worse performance in the TBI group 6-12 months post-injury in comparison to controls although none that were significant after correction for multiple comparisons (Supplementary Table 2). There was concern that these results may have been skewed by the disproportionate number of controls who were older and had higher education (Table 1). Secondary analysis restricted to enlisted service members only from each group revealed that these trends in neurocognitive test performance were likely due to subject mismatch as most of the differences were not present in this subgroup analysis (Supplementary Table 3). The only exception was the California Verbal Learning Test long-delay free recall assessment of delayed verbal memory, where performance remained significantly worse in the TBI group (P = 0.004, Mann-Whitney U-test). There was no significant difference between groups (P = 0.46, Mann-Whitney U-test) on a forced choice test embedded in the California Verbal Learning Test that was used to assess adequacy of effort; all subjects performed adequately on this measure.
However, evaluation at the single-subject level revealed subsets of TBI subjects with impaired cognitive test battery performance (Fig. 4). Abnormal performance on each individual assessment was defined as a subject’s score that fell two standard deviations worse than the mean of the control group for that exam. For each subject, the number of tests with abnormal performance was then summed. The number of subjects per group was then compared to what would be expected by chance. For 18 variables, 66% of subjects per group would be expected to have abnormal performance on 0 exams, 28% on one exam, and 5% on two or more exams. The TBI group had a greater number of subjects with two or more abnormal exams than what would have been expected by chance for the group size, with 15 of 38 performing abnormally on two or more tests (Fig. 4A, P = 0.0003, chi-square). No significant difference was observed in the control group compared to what would have been expected by chance (P = 0.3925, chi-square). Secondary evaluation of enlisted subjects only (Fig. 4B) confirmed this finding in a better matched sample (TBI: P = 0.0012, control: P = 0.2543; chi-square). There was a heterogeneous distribution of which assessments were found to be abnormal for each subject. These findings are in line with previously published work on medically-evacuated ‘mild’/concussive TBI subjects (Mac Donald et al., 2014a, b).
Figure 4.
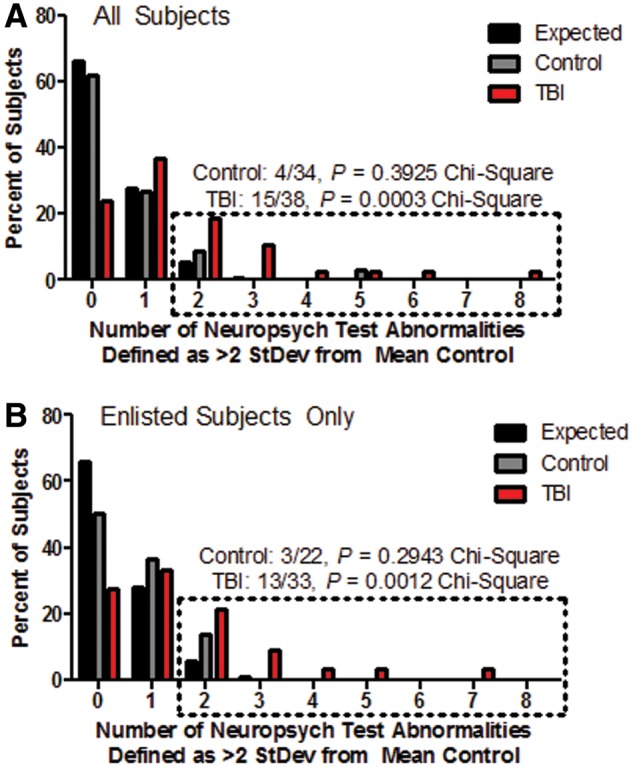
Larger numbers of TBI subjects than controls with two or more neuropsychological performance abnormalities at follow-up. (A) All subjects. (B) Enlisted subjects only. The number of subjects with neuropsychological test abnormalities are displayed by group in comparison to what would be expected by chance (black bars). Per cent of subjects is displayed to account for the differences in the number of subjects in each group. Dotted box indicates the group of subjects who had poor performance on two or more of the 18 neuropsychological assessments. Poor performance is defined as a score that is >2 SD away from the mean of the control group in the direction of worse performance. P-value calculated using the chi-square test by group in comparison to the expected distribution for that group size.
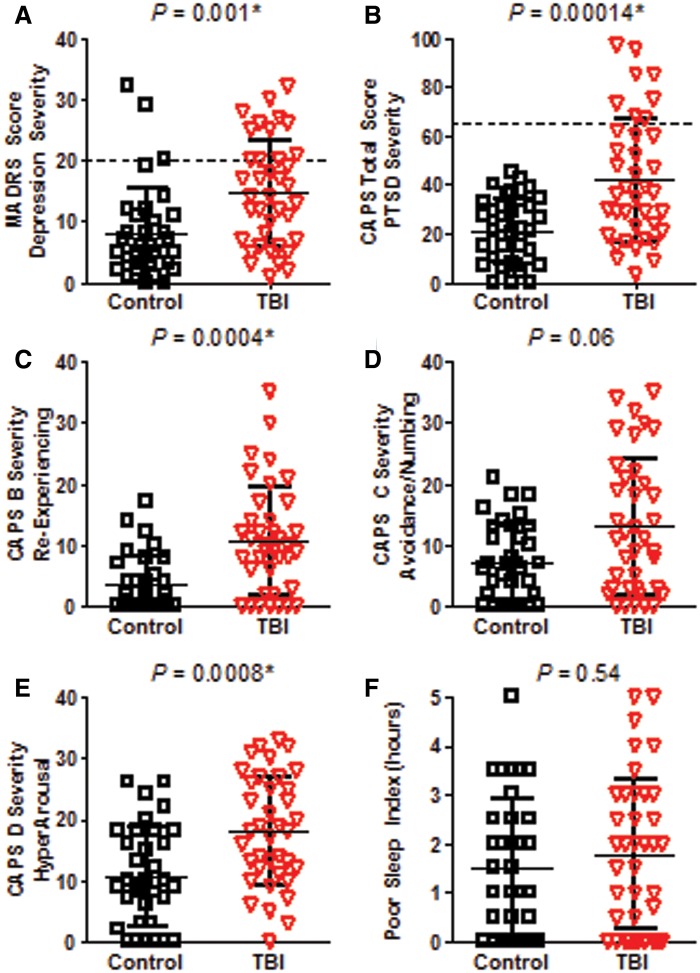
Clinical evaluations for PTSD and depression revealed a greater severity of symptoms in TBI subjects than in controls 6–12 months post-injury (Fig. 5). Symptoms of depression as measured by the MADRS were more severe in TBI subjects (P = 0.001, Mann-Whitney U-test, Fig. 5A). Twenty-four per cent of TBI subjects and 6% of controls were found to have moderate to severe depression (Snaith et al., 1986). Total PTSD symptom severity was also significantly worse in TBI subjects than in controls as determined by the CAPS for DSM-IV (P = 0.00014, Mann-Whitney U-test, Fig. 5B). Twenty-one per cent of TBI subjects were found to have moderate to severe PTSD whereas no control subjects exceeded this threshold (Weathers et al., 2001). CAPS subdomain B, which quantifies symptoms of re-experiencing or reliving traumatic events, (Fig. 5C, P = 0.0004) and CAPS subdomain D, which quantifies feelings of hyperarousal or hypervigilance, (Fig. 5E, P = 0.0008) were also significantly worse in TBI subjects than in controls. Importantly, there was no significant difference in the poor sleep index, a submeasure of CAPS-D which assesses the difference between the number of hours of sleep desired versus the number of hours of sleep reported. This is in contrast to previous reports of medically evacuated non-blast complex concussive TBI in which TBI subjects were found to have worse self-reported sleep than controls on this assessment (MacDonald et al., 2014a).
Figure 5.
Greater depression and PTSD severity in TBI subjects versus controls at follow-up. (A) Depression severity assessed by the MADRS (Max 60). Dashed line indicates cut-off for moderate to severe symptoms (Snaith et al., 1986). (B) PTSD severity assessed by the CAPS for DSM IV (Max 136). Dashed line indicates cut-off for moderate to severe symptoms (Weathers et al., 2001). (C) CAPS B Severity–Re-experiencing (Max 40). (D) CAPS C Severity–Avoidance and Numbing (Max 56). (E) CAPS D Severity–Increased Arousal and hypervigilance (Max 40). (F) Poor sleep index, taken from CAPS D1, defined as the self-reported number of desired hours of sleep minus the number of hours reported. Higher scores on all of the measures indicate worse impairment. Uncorrected P-values reported. *Significance after Bonferroni correction for multiple comparisons for the three CAPS subdomains at P < 0.05/3 = 0.0167.
Self-reported alcohol use was not significantly different across groups as evidenced by the Michigan Alcohol Screening Test (Supplementary Fig. 5) suggesting that alcohol abuse at follow-up did not contribute to the disability observed in these TBI subjects.
Relationship between acute and chronic clinical measures
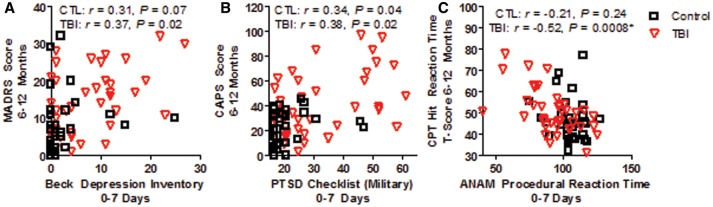
Only very modest correlations were observed between clinical data acquired 0–7 days post-injury and comparable measures collected at 6–12 months follow-up (Fig. 6). Correlations between acute self-report symptom measures of depression (Fig. 6A) and PTSD (Fig. 6B) modestly correlated with results from structured interviews administered for the comparable measure in the chronic phase post-injury. This modest correlation was observed for both controls (r = 0.31, P = 0.07 for depression measures; r = 0.34, P = 0.04 for PTSD measures) and TBI subjects (r = 0.37, P = 0.02 for depression measures; r = 0.38, P = 0.02 for PTSD measures).
Figure 6.
Modest correlations observed between acute and chronic measures of depression, PTSD and Neuropsychological Test Performance-Reaction Time. (A) Very modest correlations for both control and TBI subjects were observed between the self-report symptom checklist for depression collected 0–7 days post-injury and a structured interview administered by trained research staff at 6–12 month follow-up. (B) Similar modest correlations were observed for controls and TBI subjects comparing acute self-report to chronic structured interview data for PTSD symptoms. (C) Metrics of reaction time collected as part of the neuropsychological examination in both the acute (ANAM procedural reaction time) and chronic phase (Conners’ Continuous Performance Test II reaction time) significantly correlated in TBI subjects but not in controls using the Bonferonni corrected criterion of P < 0.05/6 = 0.0083.
In addition, comparisons of early and chronic cognitive performance measures were explored. Only measures of reaction time were found to be significantly correlated in TBI subjects (Fig. 6C). No correlation was observed in control subjects across any of the neuropsychological test measures.
Of interest, there were no correlations between the Military Acute Concussion Evaluation scores and any chronic clinical outcome measure.
Acute and chronic multivariate predictors of dichotomized global outcome
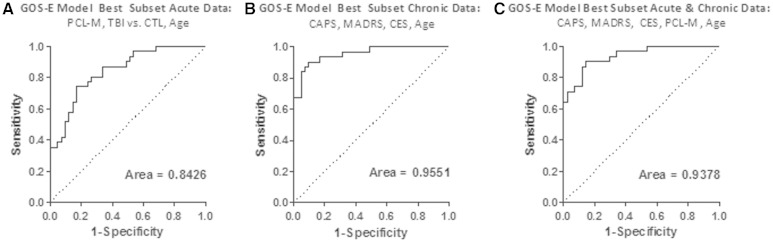
Predictors of dichotomized global outcome were examined using the acute clinical data only, chronic clinical data only, and combined acute and chronic clinical data. Global outcome was defined by the dichotomized GOS-E, with scores of 7 or 8 categorized as good outcome, and scores of ≤6 defined as disabled. Candidate variables for the model using acute data only included total scores on the PCL-M, Beck Depression Inventory, Balance Error Scoring System, Rivermead Post-Concussion Symptoms Questionnaire, the ‘delta’ scores from the eight domains of the ANAM-Traumatic Brain Injury Military Version 4, Combat Exposure Scale, number of previous deployments, age, education, and group distinction (Control versus TBI). Acute PTSD symptoms as assessed by the PCL-M, group distinction (Control versus TBI), and age were the variables in the best fit logistic regression model. Higher likelihood of disability was observed in older TBI subjects with more severe PTSD symptoms (Fig. 7 and Supplementary Table 4). The receiver-operator curve (Fig. 7A) indicated a good prediction of dichotomized GOS-E with a receiver-operating characteristic area under the curve (AUC) of 0.8426. This multivariate model performed substantially better than any single variable; the AUC for PCL-M alone was 0.76, for control versus TBI alone was 0.72, and for age alone was 0.56. There was also no apparent relationship between time to return to duty and 6–12 month global outcome. In addition, there was no relationship between a dichotomized measure of the GOS-E defined as good outcome (GOSE 7–8) or moderate disability (GOS-E ≤6) and the mild TBI subject’s concussion history or history of previous blast exposure [P = 0.56 blast history versus no blast history, P = 0.39 previous concussions (0–1) versus previous concussions (≥2), chi-square].
Figure 7.
Logistic regression models predict global outcome moderately based on acute data and strongly based on chronic data. (A) Receiver-operator curve for best fit model of overall disability defined as the dichotomized GOS-E of 7 or 8 (good outcome), and ≤6 (disabled) using acute clinical data. The best model included the PCL-M, group distinction of control versus TBI, and age. (B) Receiver-operator curve for model of overall disability using chronic clinical data. The best fit model consisted of the CAPS for DSM IV, MADRS, Combat Exposure Scale and Age. This model showed improvement over the acute data best fit model. (C) Receiver-operator curve for best fit model of overall disability using both acute and chronic clinical data. The model consisted of PCL-M, CAPS for DSM IV, MADRS, Combat Exposure Scale and age.
Candidate variables for the model using chronic clinical data only included total scores on the CAPS for DSM IV, MADRS, Michigan Alcohol Screening Test, Migraine Disability Scale, Headache Impact Test, Neurological Outcome Scale for TBI, group distinction (Control versus TBI), Combat Exposure Scale, age, education, and the number of neuropsychological abnormalities. Using chronic clinical measures only, the best fit logistic regression model contained the CAPS, MADRS, Combat Exposure Scale and age (Fig. 7B). The chronic model provided an excellent reflection of global outcome with an AUC of 0.9551.
Using combined acute and chronic measures, the best fit model from logistic regression contained the PCL-M, CAPS for DSM IV, MADRS, Combat Exposure Scale and age (Fig. 7C). This model performed comparably to the chronic only model with an AUC of 0.9378.
Discussion
In summary, non-medically evacuated concussive blast-related TBI subjects fared more poorly than controls in the chronic phase (6–12 months) following injury. Early clinical assessments revealed heightened PTSD and depression symptoms along with worse cognitive performance on the ANAM in comparison to pre-deployment baseline testing. At 6–12 months follow-up, TBI subjects showed persistent and more severe neurobehavioural, PTSD and depression symptoms along with more significant headache impairment in comparison to controls. Worse performance on neurocognitive exams largely resolved at the group level; however, analysis at the single-subject level revealed subsets of TBI subjects with lasting abnormal test performance in two or more assessments.
Interestingly, results from logistic regression utilizing either acute measures or chronic data identified that a diagnosis of TBI, age, and measures of psychological health contributed most strongly to the best predictive models of adverse 6–12 month overall outcomes. Of no surprise, the model generated by the acute data left a larger amount of the variance in 6–12 month outcome unaccounted for in comparison to the best model generated from chronic data. This could be due to many factors including the validity of the self-report measures (Beck Depression Inventory, PCL-M) used acutely versus structured interviews (MADRS, CAPS) used at the chronic time point, the consideration of both ‘current’ and ‘lifetime’ psychological trauma on the CAPS versus only ‘current-military’ trauma on the PCL-M, or other factors. Balance, neurological deficits, headache impact, cognitive performance, and alcohol use did not seem to contribute substantially to prediction of overall outcomes.
It is important to point out that the acute measures captured less of the determinants of global outcome than the specific chronic assessments, and that acute and chronic measures of the same domains correlated only modestly. This lends support to the need for new measures to be used in the early evaluation of these patients that could better predict the long-term impact of concussive brain injuries. However, irrespective of the measures tested, poor global outcome as evidenced by the GOS-E in this population seems to be largely driven by psychological health measures, TBI status, and age. This finding adds to the growing body of literature underscoring the very high risk of disability in patients with both psychological health impairments and TBI in the military (Hoge et al., 2008; Lippa et al., 2010; Polusny et al., 2011; Drag et al., 2012; Maguen et al., 2012; Ruff et al., 2012; Scheibel et al., 2012; Vanderploeg et al., 2012; Eskridge et al., 2013; Kontos et al., 2013; Verfaellie et al., 2013; Yurgil et al., 2014; Mac Donald et al., 2014a, b). An alternative explanation for our findings could be that TBI status acts as a surrogate marker for greater combat stress, which is difficult to directly measure in the acute phase following injury, and that it is this combat stress that is the primary driver of adverse outcomes. Previous work in civilian studies has shown particular vulnerability to poor outcome following TBI in older patients (Senathi-Raja et al., 2010), those who report use of maladaptive coping strategies (Anson and Ponsford, 2006; Spitz et al., 2013), and those with pre-injury psychiatric history (Gould et al., 2011a, b) suggesting that baseline psychiatric condition, environmental stress surrounding brain injury, and age may negatively influence overall disability. In the absence of direct measures of both combat stress and of structural brain injury, these alternative explanations cannot be resolved directly.
This study is the first to our knowledge to provide longitudinal assessments that include both acute clinical information collected 0–7 days post-injury in the combat theatre and chronic data collected 6–12 months post-injury in the USA. Limitations include a modest sample size, mismatch in age and education across the groups, no information collected regarding treatment during the interval between injury and evaluation, enrolment of subjects only from two concussion care centre treatment facilities in Afghanistan and lack of matched assessments completed at both 0–7 days and 6–12 months. None of the participants had a known history of PTSD, depression or other mental health disorders, which would have precluded them from being deployed to a war zone based on pre-deployment health screening. However, pre-deployment medical records were not accessible at the time of enrolment for confirmation. In addition, the evaluations collected at 0–7 days and 6–12 months post-injury were designed to assess many relevant domains in an efficient manner so that subject test fatigue would not be a major problem. Nonetheless, it is possible that increased fatigue in the mild TBI subjects relative to controls could have contributed to the results. A notable strength is that evaluation and treatment of service members with concussion acutely in theatre was conducted based on established, standardized Department of Defense protocols (DTM 09-033).
As this study did not include a comparable cohort of non-blast-related brain injured participants, no conclusions can be drawn regarding any specificity that may be present due to injury mechanism. However, recent findings in medically-evacuated blast and non-blast service members suggest that clinical outcome may not be differentially related to injury mechanism (Mac Donald et al., 2014a).
In conclusion, this study found that US military personnel with concussive blast-related TBI mild enough to remain in theatre still fared quite poorly on clinical outcome measures acquired 6–12 months following injury. It was surprising that these concussive injuries, perceived by many as trivial, seemed to result in significantly worse global outcomes and psychological health symptoms. Most notably, the percentage of subjects with poor global outcome was much higher than what has been previously reported in comparable civilian studies of ‘mild’ traumatic brain injury or sports concussion (Alexander, 1995; Thornhill et al., 2000; Mosenthal et al., 2004; Sigurdardottir et al., 2009; Benedictus et al., 2010; Jacobs et al., 2010; Lannsjo et al., 2013; Yuh et al., 2013; McMahon et al., 2014) and much more in line with recent studies of service members with ‘mild’/concussive TBI that required medical evacuation from the combat theatre (Mac Donald et al., 2014a, b). The incongruity between time to return to duty and the outcome measures is likely the result of the fact that the return to duty decision is based on overall clinical assessments. These are performed acutely and may not be an accurate reflection of subsequent disability associated with mild TBI, which may be better predicted by poor psychological health. Most importantly, the observation that the best predictive models using acute data provided a good but incomplete account of global outcome suggests that further research will be necessary to identify additional determinants of adverse outcomes. Identification of these determinants of outcome may in turn allow a rational approach to revising protocols for the care and management of these patients (Conaton, 2012). It remains to be determined whether early interventions focused on psychological health symptoms in high risk subjects will improve outcomes. Likewise, the longer term implications of concussive blast-related military TBI are currently unknown and are an active area of ongoing research.
Funding
The study was funded by the Congressionally Directed Medical Research Program (PT090444 Supplement, PI: D. Brody). The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Navy, Department of Defense, or U.S. Government. The principal investigators O. Adam, D. Brody and study director C. Mac Donald had full access to all of the data and take full responsibility for the integrity of the data and the accuracy of the analysis. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank the service members, their families, commanding officers, and clinical providers at Kandahar Airfield and Camp Leatherneck in Afghanistan for making this study possible including John Ritter, MD, Todd May, DO, Maria Barefield, OT, Josh Duckworth, MD, Donald LaBarge, MD, Dean Asher, MD, Benjamin Drinkwine, MD, Yvette Woods, PhD, and Michael Connor, PysD. We are grateful for the assistance of the Washington University clinical assessment team including Leslie French, PhD, Justin Hampton, LCSW, Eric Shumaker, PhD, Kathryn Salmo, MS, Kathryn Stinson, MS, Danielle Marinucci, MSW, April Reupke, MS, Meghan Jenkins, MSW, Natasha Hilts, MSW, Christine Lakey, LCSW, Amanda Hiesele, MS and Laura Daigh, BS for whom compensation was provided for their contributions to the study.
Glossary
Abbreviations
- ANAM
Automated Neurocognitive Assessment Metrics
- CAPS
Clinician Administered PTSD Scale
- GOS-E
Glasgow Outcome Scale Extended
- MADRS
Montgomery Asberg Depression Rating Scale
- PCL-M
Post-traumatic Stress Disorder Check List Military
- TBI
traumatic brain injury
- PTSD
post-traumatic stress disorder
References
- Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–60. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- Anson K, Ponsford J. Coping and emotional adjustment following traumatic brain injury. J Head Trauma Rehabil. 2006;21:248–59. doi: 10.1097/00001199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benedictus MR, Spikman JM, van der Naalt J. Cognitive and behavioural impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med and Rehabil. 2010;91:1436–41. doi: 10.1016/j.apmr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Abigai BS. Multilingual aphasia examination. 3rd edn. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Casscells S. Traumatic brain injury: definition and reporting. Defense Aso, editor. Ft Detrick, Maryland: Department of Defense; 2007. [Google Scholar]
- Cernich A, Reeves D, Sun W, Bleiberg J. Automated neuropsychological assessment metrics sports medicine battery. Arch Clin Neuropsychol. 2007;22(Suppl 1):S101–14. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Coldren RL, Russell ML, Parish RV, Dretsch M, Kelly MP. The ANAM lacks utility as a diagnostic or screening tool for concussion more than 10 days following injury. Mil Med. 2012;177:179–83. doi: 10.7205/milmed-d-11-00278. [DOI] [PubMed] [Google Scholar]
- Conaton E. DoD policy guidance for management of mild traumatic brain injury/concussion in the deployed setting. Washington, DC: Department of Defense; 2012. [Google Scholar]
- Conners C, Staff M. Conners’ Continuous performance test II: computer program for windows technical guide and software manual. North Tonwanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- Cooper DB, Kennedy JE, Cullen MA, Critchfield E, Amador RR, Bowles AO. Association between combat stress and post-concussive symptom reporting in OEF/OIF service members with mild traumatic brain injuries. Brain Inj. 2011;25:1–7. doi: 10.3109/02699052.2010.531692. [DOI] [PubMed] [Google Scholar]
- Delis D, John F, Kramer J, Kaplan E. California verbal learning test manual: 2nd editor, Adult Version. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS): examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Dempsey KE, Dorlac WC, Martin K, Fang R, Fox C, Bennett B, et al. Landstuhl regional medical center: traumatic brain injury screening program. J Trauma Nurs. 2009;16:10–12. doi: 10.1097/01.JTN.0000348063.41099.a7. [DOI] [PubMed] [Google Scholar]
- Drag LL, Spencer RJ, Walker SJ, Pangilinan PH, Bieliauskas LA. The contributions of self-reported injury characteristics and psychiatric symptoms to cognitive functioning in OEF/OIF veterans with mild traumatic brain injury. J Int Neuropsychol Soc. 2012;18:576–84. doi: 10.1017/S1355617712000203. [DOI] [PubMed] [Google Scholar]
- DVBIC. DoD numbers for traumatic brain injury, Worldwide - Totals, 2000-2012 (Q1-Q3) 2013 Available from: http://www.dvbic.org/sites/default/files/uploads/dod-tbi-worldwide-2000-2013-Q3-as-of-05%20Nov-2013.pdf (12 Novermber 2013, date last accessed) [Google Scholar]
- Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, MacGregor AJ, et al. Influence of combat blast-related mild traumatic brain injury acute symptoms on mental health and service discharge outcomes. J Neurotrauma. 2013;30:1391–7. doi: 10.1089/neu.2012.2537. [DOI] [PubMed] [Google Scholar]
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, et al. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma. 2014;31:169–79. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarneau MR, Woodruff SI, Dye JL, Mohrle CR, Wade AL. Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg. 2008;108:950–7. doi: 10.3171/JNS/2008/108/5/0950. [DOI] [PubMed] [Google Scholar]
- Gould KR, Ponsford JL, Johnston L, Schonberger M. The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychol Med. 2011a;41:2099–109. doi: 10.1017/S003329171100033X. [DOI] [PubMed] [Google Scholar]
- Gould KR, Ponsford JL, Johnston L, Schonberger M. Predictive and associated factors of psychiatric disorders after traumatic brain injury: a prospective study. J Neurotrauma. 2011b;28:1155–63. doi: 10.1089/neu.2010.1528. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263–73. [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Homaifar BY, Brenner LA, Gutierrez PM, Harwood JF, Thompson C, Filley CM, et al. Sensitivity and specificity of the Beck Depression Inventory-II in persons with traumatic brain injury. Arch Phys Med Rehabil. 2009;90:652–6. doi: 10.1016/j.apmr.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B, Beems T, Stulemeijer M, van Vugt AB, van der Vliet TM, Borm GF, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27:655–68. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- Keane T, Fairbank J, Caddell J, Zimering R, Taylor K, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychol Assess. 1989;1:53–5. [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–92. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kontos AP, Kotwal RS, Elbin RJ, Lutz RH, Forsten RD, Benson PJ, et al. Residual effects of combat-related mild traumatic brain injury. J Neurotrauma. 2013;30:680–6. doi: 10.1089/neu.2012.2506. [DOI] [PubMed] [Google Scholar]
- Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr, Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–74. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- Lannsjo M, Raininko R, Bustamante M, von Seth C, Borg J. Brain pathology after mild traumatic brain injury: an exploratory study by repeated magnetic resonance examination. J Rehabil Med. 2013;45:721–8. doi: 10.2340/16501977-1169. [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, Goethe KE, Sisson RA, Overall JE, Rhoades HM, et al. The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50:183–93. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa SM, Pastorek NJ, Benge JF, Thornton GM. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J Int Neuropsychol Soc. 2010;16:856–66. doi: 10.1017/S1355617710000743. [DOI] [PubMed] [Google Scholar]
- Luethcke CA, Bryan CJ, Morrow CE, Isler WC. Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. J Int Neuropsychol Soc. 2011;17:36–45. doi: 10.1017/S1355617710001207. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol. 2014a;71:994–1002. doi: 10.1001/jamaneurol.2014.1114. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Nelson EC, Werner NJ, Fang R, Flaherty SF, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma. 2014b;31:889–98. doi: 10.1089/neu.2013.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguen S, Madden E, Lau KM, Seal K. The impact of head injury mechanism on mental health symptoms in veterans: do number and type of exposures matter? J Trauma Stress. 2012;25:3–9. doi: 10.1002/jts.21669. [DOI] [PubMed] [Google Scholar]
- Matthews C, Kløve H. Instruction manual for the adult neuropsychology test battery. Madison, WI: University of Wisconsin Medical School; 1964. [Google Scholar]
- McCauley SR, Levin HS, Vanier M, Mazaux JM, Boake C, Goldfader PR, et al. The neurobehavioural rating scale-revised: sensitivity and validity in closed head injury assessment. J Neurol Neurosurg Psychiatry. 2001;71:643–51. doi: 10.1136/jnnp.71.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Kelly TM, Weyand AM, Yallampalli R, Waldron EJ, et al. The neurological outcome scale for traumatic brain injury (NOS-TBI): II. Reliability and convergent validity. J Neurotrauma. 2010;27:991–7. doi: 10.1089/neu.2009.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon P, Hricik A, Yue JK, Puccio AM, Inoue T, Lingsma HF, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J Neurotrauma. 2014;31:26–33. doi: 10.1089/neu.2013.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma. 2004;56:1042–8. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- Norris JN, Carr W, Herzig T, Labrie DW, Sams R. ANAM4 TBI reaction time-based tests have prognostic utility for acute concussion. Mil Med. 2013;178:767–74. doi: 10.7205/MILMED-D-12-00493. [DOI] [PubMed] [Google Scholar]
- Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi freedom and operation enduring freedom. J Trauma. 2008;64:295–9. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- Pettigrew LE, Wilson JT, Teasdale GM. Reliability of ratings on the Glasgow outcome scales from in-person and telephone structured interviews. J Head Trauma Rehabil. 2003;18:252–8. doi: 10.1097/00001199-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch Gen Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- Reitan R. Trail making test manual for administration and scoring. Tuscon, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Ruff R, Light R, Parker S. Visuospatial learning: Ruff Light trail learning test. Arch Clin Neuropsychol. 1996;11:313–27. [PubMed] [Google Scholar]
- Ruff RL, Riechers RG, II, Wang XF, Piero T, Ruff SS. A case-control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open. 2012;2:e000312. doi: 10.1136/bmjopen-2011-000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, et al. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc. 2012;18:89–100. doi: 10.1017/S1355617711001433. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test (MAST): the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Senathi-Raja D, Ponsford J, Schonberger M. Impact of age on long-term cognitive function after traumatic brain injury. Neuropsychol. 2010;24:336–44. doi: 10.1037/a0018239. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir S, Andelic N, Roe C, Schanke AK. Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J Int Neuropsychol Soc. 2009;15:740–50. doi: 10.1017/S1355617709990452. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Harrop FM, Newby DA, Teale C. Grade scores of the Montgomery-Asberg depression and the clinical anxiety scales. Br J Psychiatry. 1986;148:599–601. doi: 10.1192/bjp.148.5.599. [DOI] [PubMed] [Google Scholar]
- Spitz G, Schonberger M, Ponsford J. The relations among cognitive impairment, coping style, and emotional adjustment following traumatic brain injury. J Head Trauma Rehabil. 2013;28:116–25. doi: 10.1097/HTR.0b013e3182452f4f. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Whyte J, Dowson A, Kolodner K, Liberman JN, et al. An international study to assess reliability of the migraine disability assessment (MIDAS) score. Neurology. 1999;53:988–94. doi: 10.1212/wnl.53.5.988. [DOI] [PubMed] [Google Scholar]
- Taniellian T, Jaycox L. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Arlington, VA: RAND Corporation; 2008. [Google Scholar]
- Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320:1631–5. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombough T. The test of memory malingering. North Tonawanda, NY: Multi-Health Systems; 1996. [Google Scholar]
- Vanderploeg RD, Belanger HG, Horner RD, Spehar AM, Powell-Cope G, Luther SL, et al. Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch Phys Med Rehabil. 2012;93:1887–95. doi: 10.1016/j.apmr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Lafleche G, Spiro A, III, Tun C, Bousquet K. Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J Int Neuropsychol Soc. 2013;19:1–10. doi: 10.1017/S1355617712000902. [DOI] [PubMed] [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehab. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading (WTAR) manual. New York: Psychological Corporation; 2001. [Google Scholar]
- Wilde EA, McCauley SR, Kelly TM, Levin HS, Pedroza C, Clifton GL, et al. Feasibility of the neurological outcome scale for traumatic brain injury (NOS-TBI) in adults. J Neurotrauma. 2010a;27:975–81. doi: 10.1089/neu.2009.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Kelly TM, Weyand AM, Pedroza C, Levin HS, et al. The neurological outcome scale for traumatic brain injury (NOS-TBI): I. construct validity. J Neurotrauma. 2010b;27:983–9. doi: 10.1089/neu.2009.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15:573–85. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC. Performance characteristics of the posttraumatic stress disorder checklist and SPAN in Veterans Affairs primary care settings. Gen Hosp Psychiatry. 2007;29:294–301. doi: 10.1016/j.genhosppsych.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73:224–35. doi: 10.1002/ana.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgil KA, Barkauskas DA, Vasterling JJ, Nievergelt CM, Larson GE, Schork NJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2014;71:149–57. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]