Neural responses to violations of global regularities are thought to require consciousness. However, Tzovara et al. show that some comatose patients can also detect deviations in sequences composed of repeated groups of sounds, suggesting that the unconscious brain has a greater capacity to track sensory inputs than previously believed.
Keywords: coma, EEG, hypothermia, auditory system, consciousness
Neural responses to violations of global regularities are thought to require consciousness. However, Tzovara et al. show that some comatose patients can also detect deviations in sequences composed of repeated groups of sounds, suggesting that the unconscious brain has a greater capacity to track sensory inputs than previously believed.
Abstract
The neural response to a violation of sequences of identical sounds is a typical example of the brain’s sensitivity to auditory regularities. Previous literature interprets this effect as a pre-attentive and unconscious processing of sensory stimuli. By contrast, a violation to auditory global regularities, i.e. based on repeating groups of sounds, is typically detectable when subjects can consciously perceive them. Here, we challenge the notion that global detection implies consciousness by testing the neural response to global violations in a group of 24 patients with post-anoxic coma (three females, age range 45–87 years), treated with mild therapeutic hypothermia and sedation. By applying a decoding analysis to electroencephalographic responses to standard versus deviant sound sequences, we found above-chance decoding performance in 10 of 24 patients (Wilcoxon signed-rank test, P < 0.001), despite five of them being mildly hypothermic, sedated and unarousable. Furthermore, consistently with previous findings based on the mismatch negativity the progression of this decoding performance was informative of patients’ chances of awakening (78% predictive of awakening). Our results show for the first time that detection of global regularities at neural level exists despite a deeply unconscious state.
Introduction
We are constantly immersed in a stream of incoming sensory stimuli, though only a portion of them turns into a conscious percept. The mechanism that underlies conscious access to incoming stimuli relies on a complex pattern of neural activation, including implicit sensory representation, attention related processes, and retrieval of past experience and associations (Dehaene and Changeux, 2011).
When studying perception, one main challenge is being able to disentangle the contributions of implicit processing of sensory stimuli from their conscious processing. In the auditory domain, one proposition has been based on an experimental paradigm where two embedded auditory regularities are established at the level of single and groups of sounds (local and global regularity, respectively) (Bekinschtein et al., 2009). Electrophysiological and haemodynamic studies have shown that detecting neural responses to violations of global regularities depends on subjects’ awareness of the sequences (Bekinschtein et al., 2009; Faugeras et al., 2011, 2012; Wacongne et al., 2011; Chennu et al., 2013; King et al., 2013). Particularly, EEG studies suggest that a global discrimination signifies a conscious perception, mainly supported by the absence of evidence for a global regularity detection in patients in a vegetative state (Bekinschtein et al., 2009; Wacongne et al., 2011; King et al., 2013). Neural correlates of violations to global regularities have been detected in some cases, even when subjects were distracted by visual stimuli (Bekinschtein et al., 2009), and in monkeys tested in a passive context (Uhrig et al., 2014). This could be the consequence of the subjects becoming accidentally aware of the stimulus regularities, or it could represent an implicit mechanism subtending the detection of stimulus sequences. This latter hypothesis is also supported by recent evidence of significant decoding performance when classifying EEG responses to global standard and global deviant sounds in patients clinically diagnosed as vegetative (Faugeras et al., 2012; King et al., 2013). These results could be explained by assuming that patients were erroneously diagnosed, or by considering the possibility that global regularity detection can take place in absence of consciousness. In the present study, we examined comatose patients treated with mild therapeutic hypothermia (Holzer, 2010) and sedation. In this setting, given post-anoxic coma, mild therapeutic hypothermia and induced pharmacological coma based on sedation and analgesia, consciousness is deeply suppressed. Using EEG decoding analyses, we provide evidence that violations of global regularities, established over a scale of seconds, can be detected despite a deeply unconscious state.
Materials and methods
Comatose patients
We included data from 24 comatose patients, all of whom had an acute anoxic-ischaemic coma defined as a Glasgow Coma Scale < 6 (Teasdale and Jennett, 1974) at admission (Nielsen et al., 2013; Oddo and Rossetti, 2014) following prolonged cardiac arrest, and were admitted to the Department of Critical Care Medicine at the Lausanne University Hospital (three females, mean age: 65 ± 2, range: 45–87, Table 1). All procedures were approved by the Ethics Committee of the Faculty of Biology and Medicine of the University of Lausanne.
Table 1.
Clinical description of comatose patients
| Patients with significant global decoding | Patients without significant global decoding | t-value | P-value | ||
|---|---|---|---|---|---|
| Hypothermia | |||||
| n = 5 | n = 19 | ||||
| Patients alive at 3 months (%) | 80% | 47% | |||
| Age (years) | 56 ± 6 | 67 ± 3 | 1.99 | 0.056 | |
| Aetiology | Cardiac | 100% | 79% | ||
| Pulmonary | 0% | 21% | |||
| Consciousness | Glasgow Coma Scale | N.A. | ≤3 (n = 9) | ||
| Sedation-Agitation Scale | 1 (n = 5) | ≤2 (n = 10) | |||
| Spontaneous EEG | Reactive | 80% | 63% | ||
| Burst Suppression | 20% | 42% | |||
| Myoclonous | N.A. | N.A. | |||
| Background EEG frequency | Delta - Theta | 80% | 58% | ||
| Alpha - Beta | – | 10% | |||
| N.A. | 20% | 32% | |||
| Time to ROSC (min) | 23 ± 6 | 20 ± 3 | 0.50 | 0.6204 | |
| Normothermia | |||||
| n = 7 | n = 17 | ||||
| Patients alive at 3 months (%) | 43 % | 65% | |||
| Age (years) | 62 ± 6 | 66 ± 2 | 0.75 | 0.461 | |
| Aetiology | Cardiac | 71% | 88% | ||
| Pulmonary | 29% | 12% | |||
| Consciousness | Glasgow Coma Scale | ≤7 (n = 6) | ≤10 (n = 14) | ||
| Sedation-Agitation Scale | 1 (n = 1) | 1 (n = 3) | |||
| Spontaneous EEG | Reactive | 57% | 82 % | ||
| Burst Suppression | N.A. | N.A. | |||
| Myoclonous | 0% | 6% | |||
| Backgorund EEG frequency | Delta - Theta | 71% | 76% | ||
| Alpha - Beta | – | 6% | |||
| N.A. | 29% | 18% | |||
| Time to ROSC (min) | 28 ± 5 | 18 ± 2 | 2.09 | 0.0483 | |
Description of comatose patients according to whether they showed significant results in decoding global standards versus global deviant sequences, in therapeutic hypothermia and normothermia recordings separately. Two patients had significant results in both recordings. N.A. = not available; ROSC = return of spontaneous circulation.
Patients were treated with mild therapeutic hypothermia (33°C and sedation for 24 h, see Supplementary material, ‘Clinical care of comatose patients’). During the first 48 h after coma onset, their level of consciousness was regularly evaluated using the Glasgow Coma Scale, and the Sedation-Agitation Scale (Riker et al., 1999). To relate decoding results to their outcome (awake with return of consciousness, or dead at 3 months), patients were split in two cohorts: the first 12 were part of a pilot group and the remaining 12 of a second independent group. Analyses in the pilot group were exploratory and in relation to the patients’ outcome. Analyses in the second group were blinded to their outcome.
Auditory stimuli
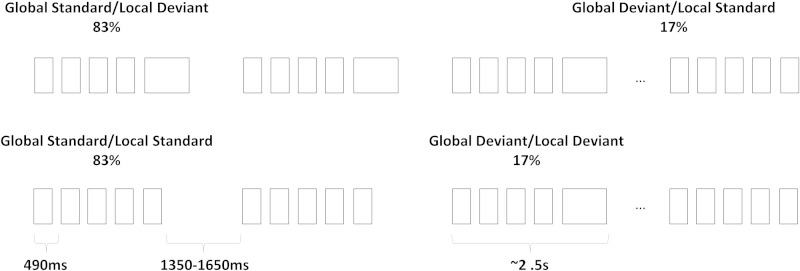
Groups of five auditory stimuli were presented via in-ear headphones (Fig. 1). This paradigm induces repetition at a global (repeating groups of sounds) and at a local (repeating single-sounds) level and it allows studying neural processing of repeated versus rare auditory sequences. Previous studies have implemented this paradigm by manipulating the pitch of the stimuli (Bekinschtein et al., 2009; Faugeras et al., 2011; King et al., 2013), whereas here we manipulated their duration (Supplementary material, ‘Auditory stimuli’).
Figure 1.
Experimental protocol. The experimental paradigm included four blocks, two of which are shown here. Sounds had duration of 100 or 150 ms (short and long rectangles, respectively) and were presented in groups of five. The five stimuli could be either identical or of different duration and play the role of standard or deviant stimuli at global or at local level. In the other two blocks of the experiment the 100 ms and 150 ms duration sounds are swapped with respect to what is shown here. This experimental design was similar to the one in Fig. 1 of Bekinschtein et al. (2009).
EEG acquisition and preprocessing
We performed two EEG recordings per patient, the first within 24 h after coma onset, under therapeutic hypothermia and sedation and the second after rewarming at >35°C and within 48 h after coma onset (normothermia). All patients were intubated and with eyes closed. We used a clinical EEG system (Viasys Neurocare), with 19 electrodes positioned based on the international 10-20 system, referenced to the Fpz electrode and with 1024 Hz sampling rate.
Continuous EEG was acquired for all patients prior to the auditory stimulation for both therapeutic hypothermia and normothermia. This EEG was examined by trained electroencephalographers for the presence of EEG reactivity to stimuli, evidence of discontinous (or burst-suppression) background EEG activity and the presence of epileptiform transients (Table 1; Rossetti et al., 2012). This evaluation was part of the clinical routine examination (Rossetti et al., 2012).
We extracted EEG responses to the fifth sound of the series, spanning from 100 ms before and up to 500 ms after stimulus onset. An artefact rejection criterion of ± 100 mV was applied to all electrodes offline. Raw EEG data were filtered offline to 0.1–40 Hz. Before the decoding analysis, data were normalized by their instantaneous EEG power, which corresponds to the mean of the squared values across electrodes.
Multivariate decoding of single-trial EEG
Our main aim was to investigate whether single patients showed evidence of differential EEG responses to global standard versus global deviant sounds. We applied a multivariate decoding analysis for each recording, which has been previously validated (Tzovara et al., 2012a, b) and implemented in auditory evoked potentials studies in healthy controls (Bernasconi et al., 2011; De Lucia et al., 2012) and comatose patients (Tzovara et al., 2013; Cossy et al., 2014). The decoding algorithm consists of modelling voltage topographies of single-trial EEG activity, with a mixture of Gaussians model, in an n-dimensional space, where n is the total number of electrodes. These models were used for decoding a separate set of single-trials, randomly extracted from all the experimental blocks (validation data set).
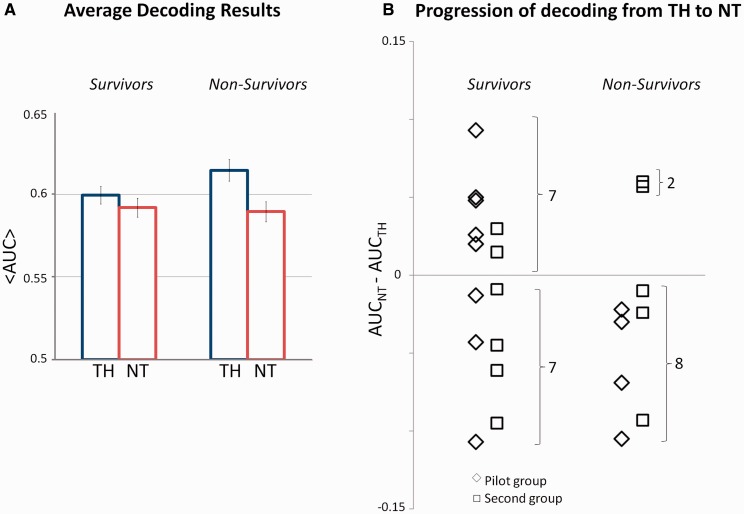
Decoding performance was measured as the average area under the receiver operating characteristic curve (Green and Swets, 1966) and was statistically compared to chance, computed by randomly permuting the labels of all trials used for training the algorithm and recomputing the mixture of Gaussians models 500 times for each recording. The actual decoding performance on the validation data set was compared to the distribution of the decoding values based on the random permutations (Wilcoxon signed-rank test, P < 0.001). In the following, significant results always refer to the validation data set. The link between decoding performance and outcome was based on all the decoding results irrespective of their significance at single patient level. The validity of outcome prediction was tested by first finding a relation between decoding performance and outcome in a pilot group of 12 patients, and then confirming this relation in the remaining patients (Fig. 2).
Figure 2.
Summary of the decoding results across the group of 24 post-anoxic comatose patients. (A) Average decoding performance of global standard versus global deviant sequences in patients during hypothermia and normothermia across 14 survivors and 10 non-survivors. (B) Difference in the decoding performance between normothermia (NT) and therapeutic hypothermia (TH) for all the patients. Rhombi indicate values measured in the pilot group, squares those measured in a second group of patients. All non-survivors but two had a decrease in decoding performance from therapeutic hypothermia to normothermia. By contrast, an improvement in auditory discrimination from therapeutic hypothermia to normothermia was associated to survival, providing an overall positive predictive value of 78%. AUC = area under the curve.
Results
Global auditory discrimination
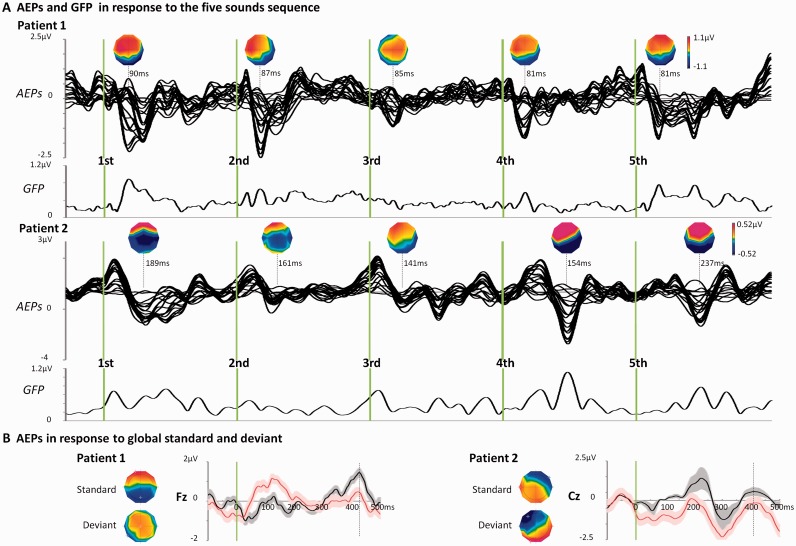
Global auditory discrimination was significant for 10 of 24 patients; five were during therapeutic hypothermia (average auditory evoked potentials for two exemplar patients are shown in Fig. 3) and seven during normothermia (two patients had significant results during therapeutic hypothermia and normothermia). The average decoding performance in a validation data set in these 10 patients (six survivors) was 0.59 ± 0.02 in therapeutic hypothermia and 0.60 ± 0.02 in normothermia. The same analysis in age-matched healthy control subjects gave significant results for 4 of 11 active and 2 of 10 passive controls, with an average decoding performance of 0.72 ± 0.05 and 0.56 ± 0.03, respectively (Supplementary material, ‘Description of control subjects’). Importantly, our results in comatose patients were not trivially driven by the local context, as an above-chance level decoding could also be observed in blocks in which the global and local regularities were not concurrent (first block of Fig. 1; Supplementary material, ‘Experimental factors influencing the decoding results’).
Figure 3.
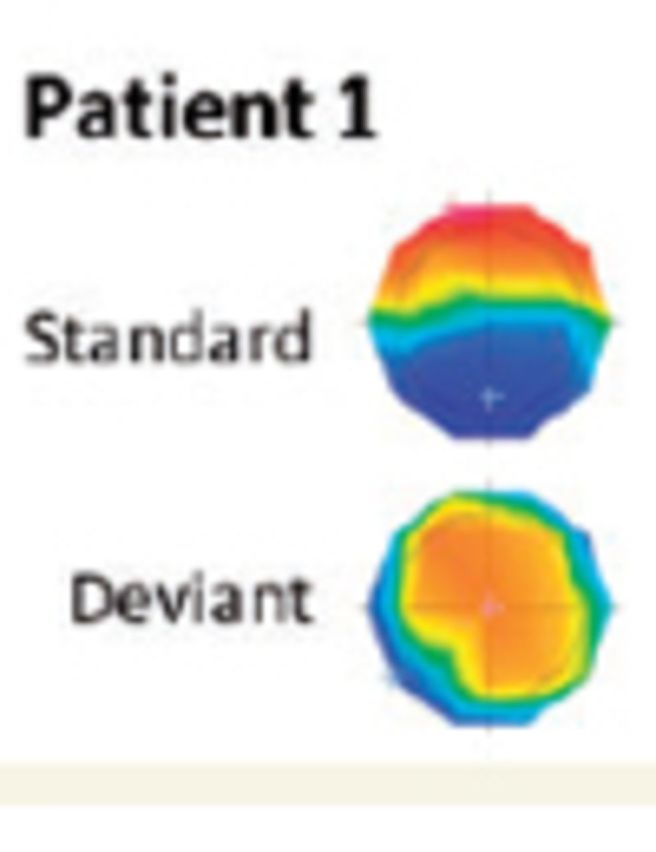
Exemplar auditory evoked potentials in two patients with significant decoding during therapeutic hypothermia. (A) Auditory evoked potentials (AEPs) across the electrodes montage and averaged across all the global standard sequences (∼400 trials) and corresponding GFP (Global Field Power). The periodicity of the response and the stability of the voltage topographies at the first negative peak of the auditory evoked potentials (latencies indicated) for each sound reflect the regularity of the stimulus sequence. (B) Average auditory evoked potentials in response to the fifth sound in global standard and global deviant sequences (∼80 trials in each condition) at fronto-central electrodes. Voltage topographies measured at ∼400 ms post-stimulus onset are shown.
The level of consciousness of patients exhibiting a significant global effect during therapeutic hypothermia was evaluated with the Sedation-Agitation Scale and was in all cases equal to 1, corresponding to an ‘unarousable’ state (Table 1). In normothermia, six of seven patients with a significant global effect had a Glasgow Coma Scale ≤ 7 (median = 3, with individual scores ranging across patients from 3 to 7), corresponding to deeply unconscious state (Table 1). The seventh patient with a significant global effect was evaluated with the Sedation-Agitation Scale and had a score of 1 (‘unarousable’). None of the available clinical variables significantly differed between patients who had significant discrimination results and those who did not (Table 1). These results suggest that accurate discrimination of complex sound sequences can be observed in the acute resuscitation phase despite a deep unconscious state and independently of patients’ outcome (evidence also confirmed with classic auditory evoked potentials analyses, Supplementary material).
The same analysis applied to decode responses to local standard versus local deviant sounds revealed significant results only in four patients (two in therapeutic hypothermia and two in normothermia).
Progression of global auditory discrimination
We investigated whether global discrimination was related to patients’ outcome. We evaluated a first group of pilot patients including 12 individuals (four of whom died). In this pilot group, the average decoding performance was 0.59 ± 0.01 during therapeutic hypothermia and 0.61 ± 0.02 during normothermia in survivors. The average decoding performance for non-survivors was 0.64 ± 0.02 and 0.59 ± 0.02 during therapeutic hypothermia and normothermia, respectively. The difference in the decoding performance for individual patients from therapeutic hypothermia to normothermia was informative of the chance of surviving, as all the patients who improved their auditory discrimination at the global level from therapeutic hypothermia to normothermia eventually woke up from coma (Fig. 2B). We repeated the same analysis in the remaining 12 patients (six died). The mean decoding performance in survivors was 0.61 ± 0.01 during therapeutic hypothermia and 0.58 ± 0.02 during normothermia and in non-survivors 0.60 ± 0.02 during therapeutic hypothermia and 0.58 ± 0.02 during normothermia. The observation based on the pilot group was also confirmed in this second group with the exception of two patients (Fig. 2B). These results across all patients provided a predictive value of 78% for awakening. The same analysis based on decoding local standard/deviant sounds did not relate to patients’ outcome.
Discussion
We show for the first time systematic evidence that a rule violation in auditory sequences of repeated groups of sounds can be detected at the neural level in comatose patients. This phenomenon was observed in 10 of 24 post-anoxic comatose patients, and even during induced hypothermia and sedation.
In previous studies, vegetative and minimally conscious state patients provided evidence that detection of global novelties depends on the level of consciousness. In particular, in a vegetative state, the detection of global regularities is very rare and possibly related to a clinical misdiagnosis (Cruse et al., 2011; Faugeras et al., 2012). Our results do not confirm this interpretation, as we found robust evidence that global violations were detected in patients for which any conscious access to external stimuli can be reasonably excluded. Our data therefore provide a novel view and support the hypothesis that neural detection of complex sound sequences is possible even in the absence of consciousness.
A possible reconciliation between previous evidence and ours comes from the observation that progression of the global auditory discrimination between the first two days of coma was linked to patients’ chance of surviving. This suggests that auditory discrimination relies on a neural process that is impaired over time in patients with a poor clinical evolution. It is thus likely that previous studies focusing on a much later time from coma onset (Bekinschtein et al., 2009; Faugeras et al., 2011) have uncovered the tail of this phenomenon, i.e. the disappearance of any sensitivity to sequence regularities.
Other possible explanations for observing this global discrimination can be found in the experimental design. Previous works used differences in pitch between the fourth and the fifth sounds of the sequence; here we manipulated the sounds’ duration. Irrespective of these differences in the auditory features, it is worth noting that our experimental paradigm seems more challenging than those previously considered, as it is built using longer sound sequences and intertrial intervals (and therefore implies a longer memory trace). In this perspective, our experimental evidence showing the sensitivity to global structure in the absence of consciousness seems to be even more striking.
The present study complements previous evidence regarding the evolution of auditory regularities at a local level (Tzovara et al., 2013). Having found a similar relation between the progression of auditory discrimination over time and patients’ outcome, we speculate that the detection of unexpected deviant sounds relies on a common mechanism regardless of whether they occur after repeating single or groups of sounds. One could argue that these results reflect a general property of EEG signals under acute coma, which is not directly related to the regularity violation detection. However, in a previous EEG experiment, we showed evidence of auditory semantic categorization in the same type of patients, but without any link to patients’ outcome (Cossy et al., 2014). Based on this evidence we can exclude the contribution of non-specific features of the EEG signal to the progressive impairment of novelty detection in patients who later die.
One benefit of the implemented protocol is the possibility to explore two embedded regularities. While this remains a major advantage it should still be noted that the detection of the local and global effect might have a different sensitivity. Indeed, the novelty at a global level is always random, whereas at the local level, violation arises both in a context where the novelty is within a repeated structure (Block 1 of Fig. 1) as well as in situations when the violation detection may rely on a surprise effect (Block 2 of Fig. 1). Given this sequence structure, it is not surprising that the significance of the results of the local violation detection is much less pronounced than for the global (List et al., 2007; Sussman et al., 2013).
Our study is not the first to implement a decoding method for quantifying the EEG responses to global standard and global deviant stimuli (King et al., 2013). King et al. (2013) analysed EEG signals recorded in vegetative and minimally conscious state patients by combining time periods of fixed length or based on single time-samples. Here, not only did we focus on a different population, but we also employed a crucially different decoding algorithm, which allows a flexible and data-driven estimation of the most discriminative time-windows.
In summary, our results complement previous findings in patients with disorders of consciousness exhibiting a reliable EEG response to novel stimuli (Perrin et al., 2006) and offer a novel perspective on the mechanism underlying detection of rule violation. In particular, we show that a memory trace of a complex auditory structure, over a scale of seconds, can be detected in unconscious patients. Future studies will investigate the point at which the temporal information to be stored becomes too long to be traced in the absence of consciousness, and what features within a series of sounds are most effective at creating such a trace.
Acknowledgements
We thank Christine Stähli and Natacha Cossy for help in clinical data acquirement, Olivier Jaccard for technical support, Tamarah Suys for help in collecting patients’ clinical description and Andrew Coristine for his suggestions on the manuscript.
Funding
This work was supported by the Swiss National Science Foundation (grant number CR32I3_143780 to A.O.R) and the ‘Service Projets et Organisation Stratégiques’ of the University Hospital of Lausanne (Project number 29062-1144 to M.D.L.).
Supplementary material
Supplementary material is available at Brain online.
References
- Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L. Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci USA. 2009;106:1672–7. doi: 10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi F, De Lucia M, Tzovara A. Manuel AL, Murray MM, Spierer L. Noise in brain activity engenders perception and influences discrimination sensitivity. J Neurosci. 2011;31:17971–81. doi: 10.1523/JNEUROSCI.3715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennu S, Noreika V, Gueorguiev D, Blenkmann A, Kochen S, Ibanez A, et al. Expectation and attention in hierarchical auditory prediction. J Neurosci. 2013;33:11194–205. doi: 10.1523/JNEUROSCI.0114-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossy N, Tzovara A, Simonin A, Rossetti AO, De Lucia M. Robust discrimination between EEG responses to categories of environmental sounds in early coma. Front Psychol. 2014;5:155. doi: 10.3389/fpsyg.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernandez-Espejo D, Pickard JD, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet. 2011;378:2088–94. doi: 10.1016/S0140-6736(11)61224-5. [DOI] [PubMed] [Google Scholar]
- De Lucia M, Tzovara A, Bernasconi F, Spierer L, Murray MM. Auditory perceptual decision-making based on semantic categorization of environmental sounds. Neuroimage. 2012;60:1704–15. doi: 10.1016/j.neuroimage.2012.01.131. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–27. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein T, Galanaud D, Puybasset L, et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia. 2012;50:403–18. doi: 10.1016/j.neuropsychologia.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology. 2011;77:264–8. doi: 10.1212/WNL.0b013e3182217ee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D, Swets J. Signal detection theory and psychophysics. New York, NY: John Wiley and Sons Inc; 1966. [Google Scholar]
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med. 2010;363:1256–64. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- King JR, Faugeras F, Gramfort A, Schurger A, El Karoui I, Sitt JD, et al. Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage. 2013;83C:726–38. doi: 10.1016/j.neuroimage.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List A, Justus T, Robertson LC, Bentin S. A mismatch negativity study of local-global auditory processing. Brain Res. 2007;1153:122–33. doi: 10.1016/j.brainres.2007.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Watterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after Cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med. 2014;42:1340–7. doi: 10.1097/CCM.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Perrin F, Schnakers C, Schabus M, Degueldre C, Goldman S, Bredart S, et al. Brain response to one's own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch Neurol. 2006;63:562–9. doi: 10.1001/archneur.63.4.562. [DOI] [PubMed] [Google Scholar]
- Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–9. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- Rossetti AO, Carrera E, Oddo M. Early EEG correlates of neuronal injury after brain anoxia. Neurology. 2012;78:796–802. doi: 10.1212/WNL.0b013e318249f6bb. [DOI] [PubMed] [Google Scholar]
- Sussman ES, Chen S, Sussman-Fort J, Dinces E. The five myths of MMN: redefining how to use MMN in basic and clinical research. Brain Topogr. 2013;27:553–64. doi: 10.1007/s10548-013-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tzovara A, Murray M, Plomp G, Herzog M, Michel C, Lucia MD. Decoding stimulus-related information from single-trial EEG responses based on voltage topographies. 2012a; 45: 2109–22. [Google Scholar]
- Tzovara A, Murray MM, Michel CM, De Lucia M. A tutorial review of electrical neuroimaging from group-average to single-trial event-related potentials. Dev Neuropsychol. 2012b;37:518–44. doi: 10.1080/87565641.2011.636851. [DOI] [PubMed] [Google Scholar]
- Tzovara A, Rossetti AO, Spierer L, Grivel J, Murray MM, Oddo M, et al. Progression of auditory discrimination based on neural decoding predicts awakening from coma. Brain. 2013;136:81–9. doi: 10.1093/brain/aws264. [DOI] [PubMed] [Google Scholar]
- Uhrig L, Dehaene S, Jarraya B. A hierarchy of responses to auditory regularities in the macaque brain. J Neurosci. 2014;34:1127–32. doi: 10.1523/JNEUROSCI.3165-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C, Labyt E, van Wassenhove V, Bekinschtein T, Naccache L, Dehaene S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc Natl Acad Sci USA. 2011;108:20754–9. doi: 10.1073/pnas.1117807108. [DOI] [PMC free article] [PubMed] [Google Scholar]