Gait disorders are untreatable in the elderly because the physiology of gait control is poorly understood. Lau et al. present electrophysiological evidence that the brainstem pedunculopontine nucleus is modulated by visual stimulation, voluntary movement and imaginary gait, suggesting that the brainstem controls gait in manners extending beyond driving pattern generation.
Keywords: pedunculopontine nucleus, subthalamic nucleus, Parkinson’s disease, deep brain stimulation, gait
Gait disorders are untreatable in the elderly because the physiology of gait control is poorly understood. Lau et al. present electrophysiological evidence that the brainstem pedunculopontine nucleus is modulated by visual stimulation, voluntary movement and imaginary gait, suggesting that the brainstem controls gait in manners extending beyond driving pattern generation.
Abstract
The brainstem pedunculopontine nucleus has a likely, although unclear, role in gait control, and is a potential deep brain stimulation target for treating resistant gait disorders. These disorders are a major therapeutic challenge for the ageing population, especially in Parkinson’s disease where gait and balance disorders can become resistant to both dopaminergic medication and subthalamic nucleus stimulation. Here, we present electrophysiological evidence that the pedunculopontine and subthalamic nuclei are involved in distinct aspects of gait using a locomotor imagery task in 14 patients with Parkinson’s disease undergoing surgery for the implantation of pedunculopontine or subthalamic nuclei deep brain stimulation electrodes. We performed electrophysiological recordings in two phases, once during surgery, and again several days after surgery in a subset of patients. The majority of pedunculopontine nucleus neurons (57%) recorded intrasurgically exhibited changes in activity related to different task components, with 29% modulated during visual stimulation, 41% modulated during voluntary hand movement, and 49% modulated during imaginary gait. Pedunculopontine nucleus local field potentials recorded post-surgically were modulated in the beta and gamma bands during visual and motor events, and we observed alpha and beta band synchronization that was sustained for the duration of imaginary gait and spatially localized within the pedunculopontine nucleus. In contrast, significantly fewer subthalamic nucleus neurons (27%) recorded intrasurgically were modulated during the locomotor imagery, with most increasing or decreasing activity phasically during the hand movement that initiated or terminated imaginary gait. Our data support the hypothesis that the pedunculopontine nucleus influences gait control in manners extending beyond simply driving pattern generation. In contrast, the subthalamic nucleus seems to control movement execution that is not likely to be gait-specific. These data highlight the crucial role of these two nuclei in motor control and shed light on the complex functions of the lateral mesencephalus in humans.
Introduction
Moving through the environment is a crucial and highly evolved ability in vertebrates, which humans primarily accomplish using the unique capacity to walk on two legs. This ability is controlled by neural networks linking automatic pattern generators in the spinal cord with supraspinal brainstem and forebrain structures responsible for selecting, initiating and regulating locomotor behaviour (Orlovsky et al., 1999). The brainstem lateral mesencephalus is a highly conserved supraspinal locomotor centre that has been identified in vertebrates ranging from lampreys to primates (Le Ray et al., 2011), and includes the pedunculopontine (PPN) and cuneiform nuclei. The PPN is a complex structure divided into a pars compacta containing mainly cholinergic neurons and a pars dissipata containing glutamatergic and GABAergic neurons (Olszewski and Baxter, 1954). The pedunculopontine and cuneiform nuclei receive direct cortical inputs, and are well positioned to influence locomotor behaviour via ascending outputs to the basal ganglia and thalamus (Pahapill and Lozano, 2000), as well as via descending outputs to the spinal cord (Rolland et al., 2011).
The brainstem region containing the pedunculopontine and cuneiform nuclei is commonly referred to as the mesencephalic locomotor region based on observations that electrical stimulation of this region can initiate and modulate locomotion in animals (Shik et al., 1966; Eidelberg et al., 1981; Skinner and Garcia-Rill, 1984; Mori et al., 1989), and that chemical modulation (Takakusaki et al., 2003) as well as lesioning cholinergic PPN neurons in monkeys (Karachi et al., 2010) impairs gait. The mesencephalic locomotor region is modulated by changes in imagined locomotion in healthy humans (Jahn et al., 2008; Karachi et al., 2012), which also modulates cortical networks similar to those involved during real gait (La Fougère et al., 2010). Further evidence implicating the mesencephalic locomotor region in locomotor control comes from pathophysiological studies of gait and balance disorders, which are major causes of chronic disability and mortality in the elderly (Snijders et al., 2007), suggesting that PPN dysfunction may be key to understanding the pathophysiology of gait disorders resistant to dopaminergic treatment (Pahapill and Lozano, 2000; Snijders et al., 2007; Demain et al., 2014).
In Parkinson’s disease, dopamine replacement therapy and deep brain stimulation (DBS) of the subthalamic nucleus (STN) effectively treat tremor, rigidity and akinesia caused by dopamine cell death (Limousin et al., 1998). These interventions can also effectively treat DOPA-sensitive gait and balance disorders, but as Parkinson’s disease advances gait disorders can become resistant to dopamine medication and STN-DBS (Grabli et al., 2012), which may be related to degeneration of cholinergic neurons in the PPN (Hirsch et al., 1987; Jellinger, 1988; Zweig et al., 1989). This is associated with decreased thalamic cholinergic activity, and both brainstem cholinergic loss and decreases in thalamic cholinergic activity correlate with the history of falls in patients with advanced Parkinson’s disease (Bohnen et al., 2009; Karachi et al., 2010). Functional imaging in patients with Parkinson’s disease indicates that PPN area activity depends on the severity of gait disorders, where blood oxygenation level-dependent enhancement during imaginary gait may reflect compensation for brainstem atrophy observed in patients with gait disorders (Snijders et al., 2011; Demain et al., 2014; Maillet et al., 2015).
Deep brain stimulation of the PPN has recently been tested for treating severe dopamine-resistant gait disorders in patients with Parkinson’s disease, using stimulation parameters thought to stimulate the remaining neurons (Mazzone et al., 2005; Plaha and Gill, 2005; Stefani et al., 2007; Ferraye et al., 2010; Moro et al., 2010). Electrophysiological recordings performed in these patients during DBS surgery have revealed that some PPN neurons respond to behavioural manipulations intended to elicit locomotor contexts, including alternating leg movements (Piallat et al., 2009) and imaginary gait (Tattersall et al., 2014). While these results are consistent with a role for the mesencephalic locomotor region in controlling locomotion, it remains unclear how neural activity in the human mesencephalic locomotor region relates to sensory perception and the selection, initiation and modulation of motor programs supporting locomotion.
We sought to improve understanding of the role of the PPN in gait control by recording neural activity in this nucleus in patients with Parkinson’s disease undergoing DBS surgery for intractable gait disorders. We used a validated imaginary gait task (Bakker et al., 2007; Karachi et al., 2012) and compared PPN neural activity to STN recordings made in a second group of patients with Parkinson’s disease performing this same task. We provide the first comparative analysis of the neural activity of these two nuclei in a locomotor context, and our results suggest distinct roles for the PPN and STN in locomotor control.
Materials and methods
Patients
Fourteen patients with idiopathic Parkinson’s disease were recruited at the Pitié-Salpêtrière Hospital. We included eight patients using standard criteria for STN-DBS (Welter et al., 2002). For PPN-DBS, we included six patients using the same criteria except that gait and balance disorders measured by freezing of gait and/or falling subscores were ≥2 ON DOPA (Tables 1 and 2). Thus, patients in the PPN-DBS group exhibited significantly more DOPA-resistant gait disorders compared to the STN-DBS group. Patients in each group participated in separate clinical trials that were approved by the local ethical committee of the Salpêtrière Hospital and the INSERM-DGOS (PPN trial #NCT02055261, STN trial #NCT01682668). Randomization was not used to assign patients, and the authors were not blinded to the group allocations. All patients agreed to participate and signed a written consent.
Table 1.
Demographic and clinical features of patients
| Gender, Age | Disease duration | Activities of daily living (UPDRS II) |
Motor disability (UPDRS III) |
Levodopa motor complications | Levodopa-equivalent dosage | Cognitive status |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | MMS | MDRS | Frontal score | WCST | |||||
| PPN-DBS | ||||||||||||
| S01 | M, 69 | 19 | 21 | 19 | 44 | 18 | 4 | 1700 | 28 | 132 | 47 | 12 |
| S02 | F, 70 | 18 | 26 | 9 | 45 | 16 | 9 | 570 | 25 | 140 | 52 | 15 |
| S03 | F, 68 | 23 | 28 | 19 | 61 | 30 | 9 | 700 | 29 | 130 | 51 | 12 |
| S04 | F, 64 | 10 | 22 | 6 | 38 | 19 | 4 | 1050 | 25 | 141 | 52 | 12 |
| S05 | M, 63 | 14 | 11 | 11 | 38 | 16 | 5 | 585 | 29 | 139 | 54 | 20 |
| S06 | M, 46 | 12 | 22 | 5 | 50 | 19 | 10 | 1300 | 27 | 139 | 58 | 20 |
| Mean (SD) | 63 (8.9) | 16 (4.9) | 22 (5.9) | 12 (6.2) | 46 (8.6) | 20 (5.2) | 7 (2.8) | 984 (453) | 27 (1.9) | 137 (4.6) | 52 (3.6) | 15 (3.9) |
| STN-DBS | ||||||||||||
| S07 | M, 55 | 9 | 17 | 14 | 18 | 3 | 5 | 1000 | 30 | 143 | 59.5 | 20 |
| S08 | F, 53 | 7 | 33 | 7 | 46 | 11 | 8 | 1130 | 27 | 143 | 60 | 20 |
| S09 | F, 51 | 13 | 15 | 1 | 32 | 1 | 6 | 450 | 30 | 144 | 60 | 20 |
| S10 | M, 55 | 11 | 24 | 16 | 53 | 11 | 7 | 625 | 25 | 122 | 46 | 12 |
| S11 | F, 57 | 10 | 24 | 2 | 26 | 4 | 9 | 875 | 29 | 143 | 54 | 15 |
| S12 | M, 60 | 10 | 25 | 11 | 53 | 27 | 8 | 1080 | 28 | 144 | 58 | 20 |
| S13 | F, 58 | 11 | 35 | 11 | 58 | 17 | 7 | 400 | 30 | 144 | 58 | 20 |
| S14 | F, 67 | 10 | 18 | 2 | 37 | 15 | 5 | 1125 | 28 | 129 | 44 | 9 |
| Mean (SD) | 57 (4.9) | 10 (1.7)* | 24 (7.3) | 8 (5.9) | 40 (14.3) | 11 (8.6)* | 7 (1.5) | 836 (303) | 28 (1.8) | 139 (8.6) | 55 (6.4) | 17 (4.4) |
*P < 0.05 unpaired t-test comparing STN-DBS patients with PPN-DBS; MMS = Mini-Mental Status (range 0–30, higher score indicates better cognitive function); MDRS = Mattis Dementia Rating Scale (range 0–144, higher score indicates better cognitive function); UPDRS = Unified Parkinson’s Disease Rating Scale (part II: range 0–52; part III: range 0–108; part IV: range 0–44; higher scores indicate worse parkinsonian status); WCST = Wisconsin Card Sorting Test (range 0–20; higher scores indicate better cognitive status). OFF and ON refer to dopaminergic medication.
Table 2.
Gait and balance disorders of patients
| Falling (item 13-UPDRS II) |
FOG (item 14-UPDRS II) |
Axial motor score (UPDRS III) |
Postural stability (item 29-UPDRS III) |
Gait (item 30-UPDRS III) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | OFF | ON | OFF | ON | OFF | ON | |
| PPN-DBS | ||||||||||
| S01 | 4 | 4 | 4 | 4 | 8 | 2 | 2 | 1 | 3 | 1 |
| S02 | 2 | 2 | 3 | 2 | 12 | 6 | 3 | 2 | 3 | 2 |
| S03 | 2 | 2 | 3 | 3 | 11 | 8 | 3 | 1 | 3 | 3 |
| S04 | 2 | 2 | 3 | 1 | 8 | 3 | 2 | 1 | 3 | 1 |
| S05 | 2 | 2 | 3 | 3 | 4 | 4 | 2 | 1 | 2 | 2 |
| S06 | 2 | 2 | 3 | 2 | 9 | 1 | 2 | 2 | 3 | 0 |
| Mean (SD) | 2 (0.8) | 2 (1.2) | 3 (0.4) | 2 (1.0) | 9 (2.8) | 4 (2.6) | 2 (0.5) | 1 (0.5) | 3 (0.4) | 2 (1.0) |
| STN-DBS | ||||||||||
| S07 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S08 | 0 | 0 | 3 | 0 | 14 | 2 | 2 | 0 | 4 | 0 |
| S09 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 |
| S10 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 |
| S11 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 1 | 0 |
| S12 | 2 | 0 | 3 | 1 | 11 | 5 | 2 | 0 | 4 | 1 |
| S13 | 1 | 0 | 4 | 1 | 11 | 3 | 1 | 0 | 3 | 1 |
| S14 | 0 | 0 | 0 | 0 | 10 | 4 | 0 | 0 | 2 | 0 |
| Mean (SD) | 0 (0.7)* | 0 (0)* | 2 (1.8)* | 0 (0.5)* | 7 (5.2) | 2 (0.1) | 1 (0.8)* | 0 (0)* | 2 (1.5) | 0 (0.5)* |
*P < 0.05 unpaired t-test comparing STN-DBS patients with PPN-DBS; UPDRS = Unified Parkinson’s Disease Rating Scale [part II: Falling and freezing of gait (FOG) scores: range 0–4; part III: axial score: range 0–20; gait and postural instability scores: range 0–4; higher scores indicate worse parkinsonian status]. OFF and ON refer to dopaminergic medication.
Imagery tasks
Imaginary gait task
Patients performed an imaginary gait task previously described (Karachi et al., 2012). Prior to surgery, patients were trained to walk along an 8-m long segment of a corridor. On separate trials they were instructed to walk at normal speed and then 30% faster. After practicing walking at each speed, patients were seated in front of a computer screen and trained to perform the imaginary gait task. Each trial began with the presentation of a white cross at the centre of the screen. After a delay, an image showing the corridor that the patient had walked along was displayed, along with instructions indicating the speed at which to imagine gait (normal or rapid, pseudo-randomized). When the patient was ready, he closed his eyes and initiated imaginary gait by pressing a button. Finally, the patient terminated imagery with a second button press, and then opened his eyes.
Imaginary object movement task
Patients in the PPN-DBS group were also trained on an imaginary object movement task where they imagined an object moving at two different speeds along the same 8-m corridor segment. These conditions were indicated by ‘normal object’ and ‘rapid object’. The trials were otherwise identical in terms of stimulus presentation, times and the sequence of button pressing and eye closing the subject was instructed to perform to initialize and terminate imagery.
During surgery, patients only performed the imaginary gait task due to time limitations. In postsurgical sessions, four PPN patients performed both imaginary gait and object movement tasks (conditions pseudo-randomly interleaved).
Surgical procedure and localization of neurons and definitive DBS electrodes
We targeted the PPN and STN using a combination of direct MRI targeting (Bejjani et al., 2000; Zrinzo et al., 2008) and a 3D histological atlas of the basal ganglia that were deformed to the preoperative T1 MRI of each patient’s brain obtained the day before surgery (Yelnik et al., 2007). We obtained a set of coordinates with each method, aiming for the lowest contact to be just outside the lower limit of the nucleus, and defined a target for each side by averaging these coordinates. We chose trajectories that avoided ventricles, the caudate nuclei, and blood vessels, and accounted for the size of the brainstem (for PPN surgeries). During surgery, we used a Leksell frame (Elekta Instruments, Inc.) with X-ray imaging to control the position of the electrodes. For PPN recordings, we used only two microelectrodes (instead of three to five) per side to reduce haemorrhagic risk. For STN recordings, we used three microelectrodes for both sides. Individual neurons were localized within the structures using preoperative MRI and perioperative X-rays to validate microelectrode trajectories and depths. We localized the definitive DBS electrodes for each patient using a postoperative helicoidal CT scan registered to the preoperative T1-weighted MRI (Bardinet et al., 2009).
Data collection
The patient was brought out of anaesthesia, and tungsten microelectrodes (1 MΩ impedance; FHC Inc.) were lowered together using a microdrive. We started physiological recordings 5 mm above the target. We reminded patients of the imaginary gait task, which was presented on a mounted LCD screen. When neural activity could be stably recorded, the patients performed the task for ∼15 min, using the hand contralateral to the recorded PPN or STN to press the button. Patients were free to discontinue the experiment at any time. After finishing the task and the clinical assessments, the patient was re-anaesthetized and the definitive DBS electrode was implanted. This entire procedure was repeated for the other hemisphere.
Neuronal activity was amplified, filtered and written to disk for offline analysis (Guideline 4000, FHC Inc. or Leadpoint, Medtronic). Action potentials were isolated using manual clustering on the basis of several waveform parameters including principal components, peak and trough amplitudes, as well as the presence of a refractory period (Offline Sorter, Plexon Instruments). We characterized over half of the unit activities as single-unit, with signal-to-noise ratios >4 (Tattersall et al., 2014) (PPN, n = 27, mean signal-to-noise ratio = 6.01, min signal-to-noise ratio = 4.14; STN, n = 36, mean signal-to-noise ratio = 6.34, min signal-to-noise ratio = 4.30). When multiple action potentials could not be separated into well-defined clusters, we thresholded the raw voltage signal at ∼3 standard deviations (SD) from the mean and labelled this multi-unit activity (PPN, n = 22, mean signal-to-noise ratio = 3.43, min signal-to-noise ratio = 3.01; STN, n = 31, mean signal-to-noise ratio = 3.39, min signal-to-noise ratio = 2.81). There was no difference in the proportion of single-units isolated in each area (P > 0.5), nor in the signal-to-noise ratios between areas (P-values > 0.4).
Postsurgical recordings were made 4 days after surgery for PPN patients. Local field potentials were recorded bilaterally from the definitive DBS electrodes (model 3389, Medtronic Neurological Division), which had four cylindrical platinum-iridium cylindrical contacts (1.27 mm in diameter and 1.5 mm in length) separated by 0.5 mm. Signals were amplified, low pass filtered at 250 Hz and digitized at 512 Hz (Basis BE System, EB Neuro S.p.A.). Bipolar recordings were made between adjacent contacts of each electrode, yielding six recording channels per patient (three per side). Patients were on their dopaminergic medication, and were comfortably seated in front of a screen to perform the imagery tasks for ∼30 min. Five patients participated in the postsurgical local field potential recordings. We excluded data from two of these patients; one due to excessive signal artefacts, and the second patient could not perform the task due to postsurgical fatigue. Local field potentials were processed to remove power line noise by fitting a parametric function to the power spectral density of the raw voltage signal in the vicinity of 50 Hz (±2 Hz). The raw voltage signal was then filtered using the inverse of the fitted function so that the resulting spectrum at 50 Hz was similar to that of surrounding frequencies (Zanos et al., 2011). We excluded data from individual trials when the amplitude of the raw voltage signal exceeded 500 µV or 6 SD of the amplitude distribution across the session.
Data analysis
We performed all analyses using MATLAB (version 2013a, Mathworks Inc.) and R (version 3.10, R Core Development Team). We fit the linear mixed models described below using the R package lme4 (version 1.1), and assessed significance with mixed-effects ANOVAs using the R package lmerTest (version 2.0) with Satterthwaite’s approximation for the denominator degrees of freedom of the F-statistic.
Behavioural analyses
We analysed the durations between button presses initiating and terminating imagery. Trials where this duration was <2 or >30 s were excluded, leaving 1463 (97.5%) trials of intrasurgical data and 577 (96.7%) trials of postsurgical data for analysis. We modelled imagination duration using a linear mixed model, including fixed effects of target location (PPN or STN), speed (rapid or normal) and imagery type (gait or object movement), with an additive random effect for each patient. Imagination time distributions were positively skewed, and we log-transformed the data to symmetrize and stabilize residuals. Resulting model residuals were symmetrically distributed, but had heavier than Gaussian tails. We checked that this did not affect our conclusions by fitting robust versions of the models (robustlmm version 1.6), and confirmed that our conclusions were unchanged.
Analysis of spiking activity
We compared activity within a trial to baseline using a Wilcoxon rank sum test. The activity for every trial of each neuron was first smoothed using a Gaussian kernel density estimator with optimal bandwidth (Shimazaki and Shinomoto, 2010). We estimated baseline rate by averaging activity from −2.5 to −0.1 s before visual instruction onset for each trial, and compared activity at every millisecond to this baseline distribution. We adjusted for multiple comparisons using the procedure of Benjamini and Hochberg (1995) to control the false discovery rate (FDR).
We quantified modulation strength in three time windows (Visual, 0 to 0.5 s after visual instruction onset; Motor, −0.5 to 0.5 s relative to button presses; Imaginary gait, 2 to 4 s after imagery was initiated), z-scoring absolute responses by subtracting mean baseline activity and dividing by its standard deviation (baseline from −2.5 to −0.1 s before visual instruction onset). We modelled modulation strength using a linear mixed model, including fixed effects of target location (PPN or STN), recording side (right or left) and recording depth, with an additive random effect for each patient. Distributions of the resulting model residuals were positively skewed, and we checked that this did not affect our conclusions by power transforming modulation strength to symmetrize and stabilize residuals. This yielded residuals that were Gaussian (Lilliefors test) with homogeneous variance across patients (Levene’s test). The results from these fits agreed with those presented in the ‘Results’ section; PPN modulation strength was significantly greater than STN modulation strength in the Visual (P = 0.031) and Imaginary gait (P = 0.037) epochs, but not the Motor epoch (P = 0.367).
Comparing recording locations
We compared the anatomical localization of neurons with different response properties by treating the location of each neuron (x, y, z position) as a sample, and testing for differences between sample distributions non-parametrically using a test based on statistical energy (Székely and Rizzo, 2013). P-values were calculated by bootstrap.
Time-frequency analysis
We estimated spectral power as a function of time and frequency using a multi-taper estimation algorithm (Mitra and Bokil, 2007) implemented in the Chronux library (version 2.10, http://chronux.org). For each bipolar local field potential recording, power was calculated in 500 ms windows stepped by 30 ms, using five orthogonal tapers with a time-bandwidth product equal to 3. Spectrograms were normalized to the average baseline spectrum measured −3 to −1 s before visual instruction onset, and transformed to decibels. We quantified changes in the same three time windows we used to analyse spiking data, modelling spectral modulations with a linear mixed model that included fixed effects of recording depth (dorsal, intermediate or ventral), imagination speed (rapid or normal), imagination type (gait or object movement) and recording side (right or left), with an additive random effect for each patient. Resulting model residuals were symmetrically distributed, but had heavier than Gaussian tails. We checked that this did not affect our conclusions by fitting robust versions of the models, and confirmed that our conclusions were unchanged.
Results
Behavioural performance
We trained 14 patients with Parkinson’s disease to perform an imaginary gait task 1 month before and again the day prior to DBS surgery. Each trial consisted of viewing an instruction on a screen and pressing a button to initiate and terminate an epoch of gait imagination with the eyes closed. Patients were instructed to imagine walking down a corridor they were familiar with, and they practiced both real and imaginary gait until they reliably produced shorter imagination times when instructed to imagine walking faster compared to walking at a normal speed. They performed this task when brought out of anaesthesia during DBS surgery while we recorded the spiking activity of neurons in the PPN or STN using microelectrodes (intrasurgical), and in a subset of PPN patients 4 days later while we recorded local field potentials from the implanted DBS macroelectrodes (postsurgical). Patients successfully performed the imagery tasks, producing shorter imagination times when instructed to imagine walking faster compared to normal walking speed, both intrasurgically (7.26 s versus 8.75 s, P < 0.001) and postsurgically (7.55 s versus 8.88 s, P < 0.001). Imagination times did not depend on patient group (PPN versus STN, P > 0.4), and all patients except one in the STN group produced shorter imagination times during rapid compared to normal speed trials. For the postsurgical recordings, patients also imagined object motion at two different speeds, and their performance in these trials was not different from imaginary gait trials (P > 0.8). Thus, both patient groups performed imagery gait similarly, despite differences between patient groups during real gait (Table 2).
PPN and STN neurons respond differently during imaginary gait
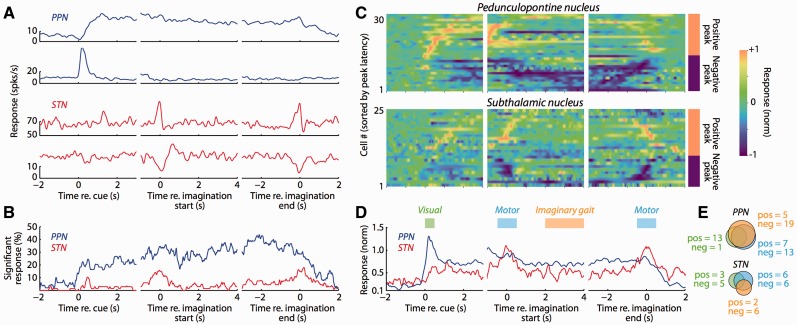
We recorded 49 single- or multi-units in the PPN of five patients and 67 single- or multi-units in the STN of eight patients. Figure 1A illustrates a range of task-related activity that we observed in individual neurons in the PPN and STN, including modulations during imaginary gait, visual instruction, and the physical movements (button presses) initiating and terminating imaginary gait. We summarized the prevalence of task-related changes by comparing activity at each point in time throughout the trial to baseline activity before visual stimulus presentation, pooling significance across neurons within each nucleus (Fig. 1B). There were substantially more modulated neurons in the PPN compared to the STN, which was evident beginning with visual instruction presentation and was maintained until patients terminated imaginary gait. In contrast, there were relatively few modulated neurons in the STN; significant changes from baseline occurred primarily around the time of button presses, and significant changes during imaginary gait were less coherent across the population. For both PPN and STN neurons, we occasionally observed small differences between the rapid and normal speed conditions, but we found fewer than 5% significant differences at any point in time when comparing these conditions, and we therefore pooled the data for all analyses.
Figure 1.
Neural responses in the human PPN and STN during imaginary gait. (A) Example PPN and STN activity. Each row represents a different neuron, with each column illustrating activity aligned to one of three different trial events. Activity was smoothed with an optimal Gaussian kernel and averaged over trials. We pooled data from normal and rapid gait imagination since we did not observe any differences between these conditions. (B) The PPN is more responsive than the STN during the imaginary gait task. For each neuron, we compared activity at each point in time with the baseline activity of that neuron taken before the presentation of the instruction cue. Significance was pooled across all neural responses after FDR correction, and smoothed with a 50 ms moving average. (C) Individual activity profiles for significantly modulated neural responses. All neurons that were significantly modulated relative to baseline are plotted, aligning to the same events as A and B. The activity for each neural response is smoothed with an optimal Gaussian kernel and normalized to the peak absolute response across the entire trial. In each panel, the neural responses are sorted according to the time of the peak absolute response (separately for positive and negative peak responses). The sign of the peak absolute response is indicated to the right of the last column. (D) The PPN and STN respond differently during imaginary gait. Population activity profile including all neurons that were significantly modulated relative to baseline. Neural activity was z-scored relative to baseline before averaging the absolute response. (E) Neural responses in the PPN more frequently occur to different task epochs than those in the STN. Individual neural responses were tested for significant differences during three different epochs (indicated in D): (i) Visual, corresponding to the presentation of the instruction cue (green); (ii) Motor, corresponding to the overt button presses that indicated the beginning and end of imaginary gait (blue); and (iii) Imaginary gait, when patients had their eyes closed and were imagining walking (orange). ‘Pos’ and ‘neg’ indicate the number of neurons exhibiting positive or negative modulations of activity for each epoch.
Individual neural responses within both the PPN and the STN could exhibit positive or negative modulations relative to baseline (Fig. 1C). There was no significant difference between the proportions of neurons with each sign of response defined by the peak response over the entire trial (P > 0.30 for both PPN and STN). We quantified modulation strength using the absolute response change z-scored relative to baseline (Fig. 1D), and found that across all neurons PPN modulation magnitudes were significantly greater during the visual stimulus (PPN-STN = 0.541, SE = 0.264, P < 0.05) and imaginary gait (PPN-STN = 0.190, SE = 0.080, P < 0.05) epochs. PPN and STN modulation magnitudes did not differ during the button presses initiating and terminating imaginary gait (PPN-STN = 0.085, SE = 0.106, P > 0.4).
Individual neurons could exhibit modulation during one or more epochs of the imaginary gait task. We observed that the proportion of neurons modulated during at least one of three epochs (Fig. 1E; Visual, Motor, Imaginary gait) was significantly greater in the PPN compared to STN [57.1% versus 26.9%, 95% confidence interval (CI) for difference = 11.1–49.5, P < 0.01]. In addition, significantly task-related neurons more frequently exhibited joint modulation in two or three of task epochs in the PPN compared to the STN (62.5% versus 30.2%, 95% CI for difference = 2.12–58.9, P < 0.05).
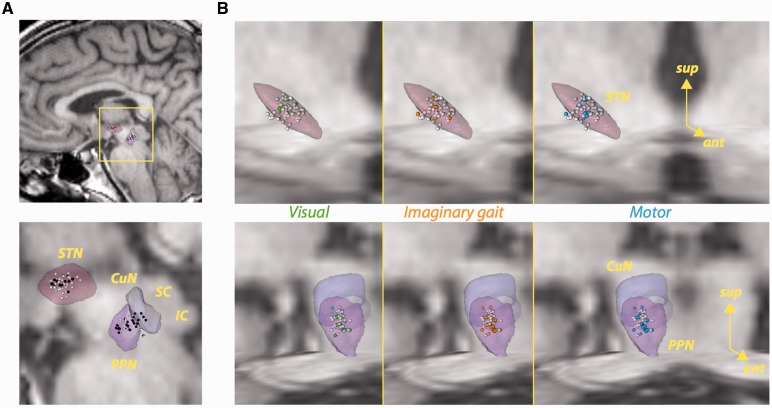
The location of each single- or multi-unit activity is illustrated in Fig. 2. We did not find any difference between the spatial distributions of task-related neurons compared to those without significant modulations (Fig. 2A) in either the PPN or the STN (P-values > 0.10). Nor did we find evidence of spatial clustering when comparing spatial distributions of neurons modulated in the visual, motor or imaginary gait epochs (Fig. 2B) in either the PPN (P-values > 0.09) or the STN (P-values > 0.5). This lack of difference is perhaps unsurprising in the PPN, where many individual neurons were modulated by more than one task event.
Figure 2.
Localization of neurons in the STN and PPN. (A) Sagittal views of task-related (black) and non-task-related (white) neurons. (B) Localization within the STN (top row) or cuneiform nucleus (CuN)/PPN (bottom row) of neurons with significant modulations in activity during visual instruction (first column, green spheres), imaginary gait (second column, orange spheres), and button press (third column, blue spheres). White spheres represent the remaining neurons. The STN and CuN/PPN figures include axial and coronal T1-weighted MRI sections of the histological atlas of Yelnik et al. (2007). IC = inferior colliculus; SC = superior colliculus.
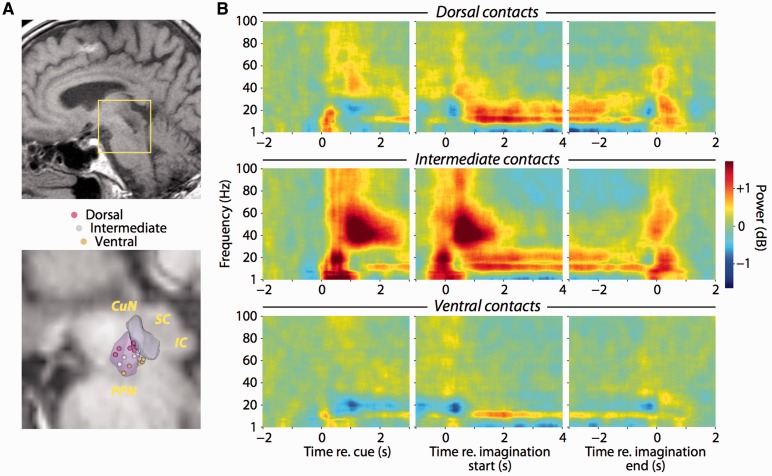
PPN local field potentials exhibit distinct temporally and spatially localized frequency modulations
We further explored PPN activity during imaginary gait by recording local field potentials from the definitive DBS macroelectrodes in a postsurgical session (Fig. 3A). Patients performed the same imaginary gait task as they did intrasurgically, with two additional conditions where they imagined an object moving down the corridor at the two different speeds (normal and rapid). We estimated spectrograms grouping bipolar pairs across patients by relative recording depth (Fig. 3B). PPN local field potentials exhibited robust changes in spectral power that depended on both task events and depth within the nucleus. Following visual instruction onset, we observed transient increases in theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz) and gamma (30–100 Hz) power. These increases were spatially localized, with those in the theta, beta and gamma bands largest at the intermediate dipole, and those in the alpha band equally strong at the dorsal and intermediate dipoles but diminishing significantly at the ventral dipole (P-values < 0.001, see also Supplementary Table 1).
Figure 3.
Task-related local field potential activity is spatially localized within the PPN. (A) Sagittal view of post-surgical localization of PPN electrodes in T1-weighted MRI image from one patient (top). Illustration of dipole centres for all patients in the histological atlas of Yelnik et al. (2007) (bottom). Each sphere represents the midway point between adjacent contacts contributing to each bipolar recording. (B) Spectrograms of local field potentials recorded from definitive DBS contacts. Recordings from each patient were averaged by dorsal-ventral arrangement along the electrode shaft (rows). The panels within each row are aligned to presentation of the visual instruction cue, initiation and termination of imaginary gait. Spectral power is normalized relative to prestimulus power (−3 to −1 s before instruction onset). IC = inferior colliculus; SC = superior colliculus.
Aligning to the button presses that initiated and terminated imagery revealed that power modulations in all frequency bands were also greatest at the intermediate dipole (P-values < 0.001). During imagination, sustained changes in theta, alpha and beta power were also significantly spatially localized; theta power decreased at all dipoles with the smallest decrease at the intermediate dipole, alpha power increased maximally at the intermediate dipole, while beta power increased maximally at the dorsal and intermediate dipoles (P-values < 0.001). Gamma band power increased at the initiation of imagination, but then decreased below baseline in a non-spatially localized manner until imagery ended, although this decrease did not reach significance (P = 0.0674). Thus, while visual instruction and physical movement were associated with spatially and temporally localized broad-band increases in power, mental imagery was associated with spatially localized sustained increases in power specifically in the alpha and beta bands, with suppression elsewhere. Local field potential power changes were similar during both imaginary gait and imaginary object movement, and none of the changes in theta, alpha, beta or gamma band power depended on the type of imagination (gait or object) or speed (normal or rapid) in any of the visual, motor or imagination epochs (P-values > 0.10).
Discussion
We used locomotor imagery to explore the different contributions of the PPN and STN in human gait. Our study has two important strengths. First, we recruited homogeneous populations of patients with idiopathic Parkinson’s disease, with the only notable difference between the two surgical groups being the severity of dopamine-resistant gait and balance disorders. All patients were cognitively normal and extensively trained, ensuring reliable performance of the motor imagery tasks, which required focus, particularly in the operating room. Second, we used an imaginary gait task that has been validated in imaging studies of healthy subjects (Jahn et al., 2008; Karachi et al., 2012) and patients with Parkinson’s disease (Snijders et al., 2011; Crémers et al., 2012). This task has separable phases allowing us to correlate neural activity with distinct events. We found that both the PPN and STN were modulated during motor imagery, but the prevalence of PPN modulations spanning multiple events supports the hypothesis that the PPN, but not the STN, likely integrates multiple sources of information to influence locomotor behaviour in humans.
It is important to consider the limitations of comparing the brain activity of two different patient groups. At our centre, STN-DBS is contraindicated by severe DOPA-resistant gait disorders. We note that patients in both the PPN-DBS and STN-DBS groups exhibit gait and balance disorders. These disorders are alleviated by DOPA-therapy in both groups, although the PPN group exhibited significantly more severe DOPA-resistant gait symptoms. It is ours and others experience that most patients with Parkinson’s disease eventually develop DOPA-resistant gait and balance disorders, with these disorders signifying the evolution of the same disease (Grabli et al., 2012). This suggests that the STN-DBS group is a reasonable comparison group for the PPN-DBS patients. The differences in real gait led us to use a locomotor imagery task, a useful paradigm in part because patients with Parkinson’s disease have preserved locomotor imagery (Snijders et al., 2011; Crémers et al., 2012), despite being impaired during actual movements. Thus we are not comparing activity related to abnormal movements, but rather to processes related to locomotor imagery that are preserved in both patient groups. Indeed, despite differences in real gait between the PPN and STN cohorts, there was no difference in the duration of imaginary gait between PPN and STN groups, nor was there any correlation between step length during real gait and the duration of imaginary gait. This suggests that while the cohorts differed in terms of real gait, they performed locomotor imagery similarly, suggesting our conclusions are reasonable in the context of previous work in healthy humans and animal models discussed in detail below.
We found that the majority of PPN neurons were modulated during imaginary gait. Our post-surgical recordings further revealed that changes in spiking activity were paralleled by sustained increases in alpha- and beta-band power in the PPN during imaginary gait. These changes in spiking and local field potential activity may be relevant for real gait, strengthening observations that mimicking stepping with alternating movements of the legs (passive or active) during DBS surgery (Piallat et al., 2009; Tattersall et al., 2014) and locomotion in animals (Garcia-Rill et al., 1983; Lee et al., 2014) can elicit activity changes in PPN neurons. Furthermore, in patients with Parkinson’s disease, prominent alpha-band oscillations within the PPN (Androulidakis et al., 2008) are enhanced during stepping in place (Fraix et al., 2013) and correlate with speed during actual walking (Thevathasan et al., 2012). Our results agree on certain points with the recent report by Tattersall et al. (2014), who also observed that the majority of PPN neurons are modulated during imaginary gait. However, they did not find that imagery modulated alpha- or beta-band oscillations, which contrasts with our observations of sustained power increases in these frequency bands. This difference may be due to targeting differences, as we showed that task-related modulations are spatially restricted within the PPN. Taken together, these results suggest that the PPN area is normally active during locomotion in humans. The specific roles remain unclear, although functions including motor planning and attention that are common to both real and imaginary gait are likely possibilities.
Our data support the hypothesis that the PPN may be an appropriate DBS target for treating DOPA-resistant gait disorders. We demonstrated a marked spatial specificity of task-related responses during locomotor imagery within the PPN, which may be useful for targeting this structure during surgery as it has poorly defined boundaries that are not visible using current imaging techniques. Moreover, the diversity of responses in the PPN suggests that DBS of this area likely influences a wide range of functions, which may account for the heterogeneity of clinical results from PPN-DBS obtained thus far. Currently, the best strategy for selecting patients that could benefit from PPN-DBS remains unknown. Low-frequency stimulation of the PPN is thought to activate neurons, and one possibility is that PPN-DBS is effective in those patients with less severe degeneration of the PPN cholinergic neurons. It will be important in the future to understand the relationship between preserved cholinergic function and PPN-DBS effectiveness.
The PPN modulations we observed are potentially related to processes common to both gait and visuomotor imagery. Indeed, we did not observe significant differences in PPN spiking activity according to changes in imaginary gait speed, nor did we observe significant differences in local field potential modulations between imaginary gait and object motion. This raises the intriguing possibility that PPN activity changes signify processes including visual or kinaesthetic imagery, or sustained attention (Kinomura et al., 1996; Paus et al., 1997; Androulidakis et al., 2008) involved in imagining movement. This is in line with evidence implicating the PPN in a number of general functions, including regulating arousal and sleep (Steriade and McCarley, 1990; Urbano et al., 2012) and linking appetitive and aversive outcomes with behavioural responses (Bechara and van der Kooy, 1989; Winn, 2008; Okada and Kobayashi, 2013; Hong and Hikosaka, 2014), which may be mediated via rich connectivity with non-motor brain areas (Chiba et al., 2001). For example, manipulating mesencephalic locomotor region activity can induce startle and escape responses in addition to locomotion (Mori et al., 1989; Condé et al., 1998), and PPN lesions can disrupt performance in certain associative learning tasks despite leaving abilities such as feeding and basic movement intact (Condé et al., 1998; Winn, 2008). These results indicate that PPN functions extend beyond driving locomotor pattern generation or regulating postural tonus, and suggest that the PPN supports processes that are necessary for adaptive locomotion but that may not be gait-specific per se.
We also showed, for the first time in humans to our knowledge, that PPN neurons can be strongly activated by visual stimuli, which in our case were task instructions. In postsurgical recordings, these visual stimuli induced transient broad-band changes in local field potential power that extended well into the gamma band. Similarly sharp responses have been observed in the PPN of monkeys performing visually guided saccades (Okada and Kobayashi, 2009; Hong and Hikosaka, 2014), and may be mediated by direct projections from cortical areas such as the frontal or supplementary eye fields (Matsumura et al., 2000), or from subcortical structures like the superior colliculus (Redgrave et al., 1988). These visual responses appeared distinct from overt motor modulations, which we also observed in spiking activity and local field potentials during button pressing, in agreement with observations that PPN neurons can be modulated during movements outside of any locomotor context (Matsumura et al., 1997; Weinberger et al., 2008; Okada and Kobayashi, 2009; Shimamoto et al., 2010; Tsang et al., 2010; Thompson and Felsen, 2013; Hong and Hikosaka, 2014; Tattersall et al., 2014). PPN visual activity may play a role in increasing alertness or attention to visual stimuli relevant for task performance by influencing cortical processing via ascending projections (Steriade and McCarley, 1990; Lee et al., 2014). These ascending projections may account for reports that PPN-DBS patients experience enhanced alertness during stimulation (Stefani et al., 2013). Such a function likely extends beyond the visual modality, as PPN neurons can also be modulated at short latency by auditory (Reese et al., 1995; Dormont et al., 1998), vestibular (Aravamuthan and Angelaki, 2012), nociceptive (Carlson et al., 2004) and somatosensory (Yeh et al., 2010) stimuli, suggesting that this structure integrates a variety of ongoing sensory information that may be useful for adapting locomotor behaviour to environmental demands (Rossignol et al., 2006). Further experiments are necessary to determine whether individual neurons integrate multimodal sensory information, and how these responses are related to preparatory, associative or cognitive processes linking sensory stimuli and movement.
We used a locomotor imagery task with the eyes closed to isolate the activity changes from potentially confounding visual stimuli. Importantly, the visual responses we observed in the PPN when the eyes were open were transient, and we observed task-related changes that were sustained throughout imaginary gait with the eyes closed, well after the transient visual responses. Moreover, our postsurgical recordings showed that sustained task-related changes were primarily in the alpha and beta bands that were spatially localized within the PPN area. Together with the fact that the patients performed the imaginary gait task correctly, these results argue against the interpretation that the activity changes exclusively from closing the eyes. Supporting this are functional imaging results using imaginary gait with the eyes closed showing blood oxygenation level-dependent changes in the PPN area in healthy volunteers (Jahn et al., 2008; Karachi et al., 2012) and patients with Parkinson’s disease (Snijders et al., 2011; Crémers et al., 2012). In these experiments, all conditions contrasted were performed with the eyes closed, indicating that the PPN was modulated by locomotor imagery.
The involvement of the STN during gait appears different, with most task-related modulations occurring during button pressing. This agrees with observations that STN neurons are modulated during voluntary movements, which reflects regulation of basal ganglia inhibition around movement initiation (Matsumura et al., 1992; Wichmann et al., 1994; Hutchison et al., 1998; Williams et al., 2005; Hanson et al., 2012). Few STN neurons were modulated during the imaginary gait epoch, suggesting that the STN may not be specifically active during gait, consistent with the lack of STN modulation during functional imaging of imaginary (Snijders et al., 2011; Crémers et al., 2012; Karachi et al., 2012) or real gait (Hanakawa et al., 1999; La Fougère et al., 2010). Note that the STN is active during imaginary arm movements in patients with Parkinson’s disease (Kühn et al., 2006), suggesting that we might have observed STN modulation during imaginary gait if this structure is involved during real gait. The fact that we observed little activity in the STN during imaginary gait constitutes indirect evidence that the STN may not be involved in gait per se. However, in the locomotor imagery experiments cited above, gait was relatively automatic, with subjects walking or imagining gait at constant speed without obstacles. Thus, we may have observed relatively few responsive STN neurons as our task did not involve motor or cognitive conflict or a need to switch from automatic behaviour, situations that may specifically engage the STN (Aron and Poldrack, 2006; Frank et al., 2007; Isoda and Hikosaka, 2008). Indeed, the STN is differentially activated in Parkinsons’s disease patients with freezing of gait (Vercruysse et al., 2014) as well as in patients with Parkinson’s disease navigating a virtual environment with cognitive loading that induced motor arrests (Shine et al., 2013). Such complex situations likely arise during natural locomotion, raising the possibility that the PPN and STN can interact during natural locomotor behaviour to adapt automatic gait programs to internal and external needs.
There is strong evidence that the PPN and STN interact. In Parkinson’s disease, high frequency STN stimulation (>100 Hz) alleviates dopamine-sensitive motor symptoms (tremor, rigidity and akinesia), likely through a combination of effects local to the STN as well as effects distributed across brain networks (Kringelbach et al., 2007; Lozano and Lipsman, 2013). Curiously, at these frequencies dopamine-resistant gait disorders can be aggravated, whereas reducing the STN stimulation frequency (<100 Hz) can mildly improve these gait disorders while worsening dopamine-sensitive motor symptoms in certain patients (Moreau et al., 2008). The mechanisms underlying this reversal are not understood, but one possibility is that high frequency STN stimulation worsens dopamine-resistant gait disorders in patients with Parkinson’s disease by aggravating PPN dysfunction, possibly through direct projections (Lavoie and Parent, 1994; Neagu et al., 2013). Moreover, PPN lesions in parkinsonian monkeys mitigate dopamine-sensitive motor symptoms while at the same time inducing gait disorders (Grabli et al., 2013). These results highlight a close relationship between these two nuclei in Parkinson’s disease, and suggest that the PPN and STN likely interact during natural locomotion, perhaps providing a route for the state of the locomotor system to influence the basal ganglia.
Acknowledgements
We are grateful to the patients for their participation. We thank Dr S. Gallais for managing the anaesthesiology; A. Buot, A. Demain, A. El Helou, C. Ewenczyk, V. Marchal and J. Sellers for training the patients and help collecting the data; and Drs C. François and J. Yelnik for feedback and help in targeting the PPN.
Glossary
Abbreviations
- DBS
deep brain stimulation
- PPN
pedunculopontine nucleus
- STN
subthalamic nucleus
Funding
This study was supported by the Institut National de la Santé et de la Recherche Médicale et Direction Générale de l’Organisation des Soins (INSERM-DGOS), a grant from the Thierry and Annick Desmarest Foundation to C.K. and B.L., an ATIP-Avenir grant and a grant from the Mairie de Paris to B.L., and a grant from the Régie Autonome des Transports Parisiens (RATP) to M.L.W.
Supplementary material
Supplementary material is available at Brain online.
References
- Androulidakis AG, Mazzone P, Litvak V, Penny W, Dileone M, Gaynor LMFD, et al. Oscillatory activity in the pedunculopontine area of patients with Parkinson’s disease. Exp Neurol. 2008;211:59–66. doi: 10.1016/j.expneurol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, Angelaki DE. Vestibular responses in the macaque pedunculopontine nucleus and central mesencephalic reticular formation. Neuroscience. 2012;223:183–99. doi: 10.1016/j.neuroscience.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M, de Lange FP, Stevens JA, Toni I, Bloem BR. Motor imagery of gait: a quantitative approach. Exp Brain Res. 2007;179:497–504. doi: 10.1007/s00221-006-0807-x. [DOI] [PubMed] [Google Scholar]
- Bardinet E, Bhattacharjee M, Dormont D, Pidoux B, Malandain G, Schüpbach M, et al. A three-dimensional histological atlas of the human basal ganglia. II. Atlas deformation strategy and evaluation in deep brain stimulation for Parkinson disease. J Neurosurg. 2009;110:208–19. doi: 10.3171/2008.3.17469. [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci. 1989;9:3400–9. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, et al. Bilateral subthalamic stimulation for Parkinson’s disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000;92:615–25. doi: 10.3171/jns.2000.92.4.0615. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bohnen NI, Müller MLTM, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–6. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JD, Iacono RP, Maeda G. Nociceptive excited and inhibited neurons within the pedunculopontine tegmental nucleus and cuneiform nucleus. Brain Res. 2004;1013:182–7. doi: 10.1016/j.brainres.2004.03.069. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Condé H, Dormont JF, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. II. Effects of reversible inactivation by intracerebral microinjections. Exp Brain Res. 1998;121:411–8. doi: 10.1007/s002210050475. [DOI] [PubMed] [Google Scholar]
- Crémers J, D’Ostilio K, Stamatakis J, Delvaux V, Garraux G. Brain activation pattern related to gait disturbances in Parkinson’s disease. Mov Disord. 2012;27:1498–505. doi: 10.1002/mds.25139. [DOI] [PubMed] [Google Scholar]
- Demain A, Westby GWM, Fernandez-Vidal S, Karachi C, Bonneville F, Do MC, et al. High-level gait and balance disorders in the elderly: a midbrain disease? J Neurol. 2014;261:196–206. doi: 10.1007/s00415-013-7174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormont JF, Condé H, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat. I. Context-dependent and reinforcement-related single unit activity. Exp Brain Res. 1998;121:401–10. doi: 10.1007/s002210050474. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Walden JG, Nguyen LH. Locomotor control in macaque monkeys. Brain. 1981;104:647–63. doi: 10.1093/brain/104.4.647-a. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- La Fougère C, Zwergal A, Rominger A, Förster S, Fesl G, Dieterich M, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–98. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, Benabid A-L, et al. Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson’s disease. PLoS One. 2013;8:e83919. doi: 10.1371/journal.pone.0083919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–12. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Activity in the mesencephalic locomotor region during locomotion. Exp Neurol. 1983;82:609–22. doi: 10.1016/0014-4886(83)90084-5. [DOI] [PubMed] [Google Scholar]
- Grabli D, Karachi C, Folgoas E, Monfort M, Tande D, Clark S, et al. Gait disorders in parkinsonian monkeys with pedunculopontine nucleus lesions: a tale of two systems. J Neurosci. 2013;33:11986–93. doi: 10.1523/JNEUROSCI.1568-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabli D, Karachi C, Welter M-L, Lau B, Hirsch EC, Vidailhet M, et al. Normal and pathological gait: what we learn from Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:979–85. doi: 10.1136/jnnp-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, et al. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999;122:1271–82. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- Hanson TL, Fuller AM, Lebedev MA, Turner DA, Nicolelis MAL. Subcortical neuronal ensembles: an analysis of motor task association, tremor, oscillations, and synchrony in human patients. J Neurosci. 2012;32:8620–32. doi: 10.1523/JNEUROSCI.0750-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA. 1987;84:5976–80. doi: 10.1073/pnas.84.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience. 2014;282:139–55. doi: 10.1016/j.neuroscience.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol. 1998;44:622–8. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–18. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–92. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Jellinger K. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1988;51:540–3. doi: 10.1136/jnnp.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, André A, Bertasi E, Bardinet E, Lehéricy S, Bernard FA. Functional parcellation of the lateral mesencephalus. J Neurosci. 2012;32:9396–401. doi: 10.1523/JNEUROSCI.0509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Bernard FA, Tandé D, Wattiez N, Belaid H, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest. 2010;120:2745–54. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyás B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–5. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–35. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Doyle L, Pogosyan A, Yarrow K, Kupsch A, Schneider G-H, et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson’s disease. Brain. 2006;129:695–706. doi: 10.1093/brain/awh715. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol. 1994;344:210–31. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83:455–66. doi: 10.1016/j.neuron.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffman D, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–11. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–24. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Maillet A, Thobois S, Fraix V, Redouté J, Le Bars D, Lavenne F, et al. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson’s disease: a kinesthetic imagery approach. Hum Brain Mapp. 2015;36:959–80. doi: 10.1002/hbm.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol. 1992;67:1615–32. doi: 10.1152/jn.1992.67.6.1615. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Nambu A, Yamaji Y, Watanabe K, Imai H, Inase M, et al. Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience. 2000;98:97–110. doi: 10.1016/s0306-4522(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res. 1997;28:155–65. doi: 10.1016/s0168-0102(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16:1877–81. doi: 10.1097/01.wnr.0000187629.38010.12. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed brain dynamics. New York: Oxford University Press; 2007. [Google Scholar]
- Moreau C, Defebvre L, Destée a, Bleuse S, Clement F, Blatt JL, et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008;71:80–4. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K. Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res. 1989;505:66–74. doi: 10.1016/0006-8993(89)90116-9. [DOI] [PubMed] [Google Scholar]
- Moro E, Hamani C, Poon Y-Y, Al-Khairallah T, Dostrovsky JO, Hutchison WD, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson’s disease. Brain. 2010;133:215–24. doi: 10.1093/brain/awp261. [DOI] [PubMed] [Google Scholar]
- Neagu B, Tsang E, Mazzella F, Hamani C, Moro E, Hodaie M, et al. Pedunculopontine nucleus evoked potentials from subthalamic nucleus stimulation in Parkinson’s disease. Exp Neurol. 2013;250:221–7. doi: 10.1016/j.expneurol.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Okada KI, Kobayashi Y. Characterization of oculomotor and visual activities in the primate pedunculopontine tegmental nucleus during visually guided saccade tasks. Eur J Neurosci. 2009;30:2211–23. doi: 10.1111/j.1460-9568.2009.07009.x. [DOI] [PubMed] [Google Scholar]
- Okada KI, Kobayashi Y. Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci. 2013;7:36. doi: 10.3389/fnint.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J, Baxter D. Cytoarchitecture of the human brain stem. Philadelphia: Lippincott; 1954. [Google Scholar]
- Orlovsky G, Deliagina T, Grillner S. Neural Control of Locomotion: from Mollusc to Man. London: Oxford University Press; 1999. [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123 (Pt 9):1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Piallat B, Chabardès S, Torres N, Fraix V, Goetz L, Seigneuret E, et al. Gait is associated with an increase in tonic firing of the sub-cuneiform nucleus neurons. Neuroscience. 2009;158:1201–5. doi: 10.1016/j.neuroscience.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport. 2005;16:1883–7. doi: 10.1097/01.wnr.0000187637.20771.a0. [DOI] [PubMed] [Google Scholar]
- Le Ray D, Juvin L, Ryczko D, Dubuc R. Supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res. 2011;188:51–70. doi: 10.1016/B978-0-444-53825-3.00009-7. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Dean P, Mitchell IJ, Odekunle A, Clark A. The projection from superior colliculus to cuneiform area in the rat. I. Anatomical studies. Exp Brain Res. 1988;72:611–25. doi: 10.1007/BF00250606. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain Res Bull. 1995;37:265–73. doi: 10.1016/0361-9230(95)00001-u. [DOI] [PubMed] [Google Scholar]
- Rolland AS, Karachi C, Muriel MP, Hirsch EC, François C. Internal pallidum and substantia nigra control different parts of the mesopontine reticular formation in primate. Mov Disord. 2011;26:1648–56. doi: 10.1002/mds.23705. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Shik M, Severin F, Orlovsky G. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–66. [PubMed] [Google Scholar]
- Shimamoto SA, Larson PS, Ostrem JL, Glass GA, Turner RS, Starr PA. Physiological identification of the human pedunculopontine nucleus. J Neurol Neurosurg Psychiatry. 2010;81:80–6. doi: 10.1136/jnnp.2009.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki H, Shinomoto S. Kernel bandwidth optimization in spike rate estimation. J Comput Neurosci. 2010;29:171–82. doi: 10.1007/s10827-009-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain. 2013;136:1204–15. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–9. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6:63–74. doi: 10.1016/S1474-4422(06)70678-0. [DOI] [PubMed] [Google Scholar]
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain. 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- Stefani A, Peppe A, Galati S, Bassi MS, D’Angelo V, Pierantozzi M. The serendipity case of the pedunculopontine nucleus low-frequency brain stimulation: chasing a gait response, finding sleep, and cognition improvement. Front Neurol. 2013;4:68. doi: 10.3389/fneur.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley R. New York: Plenum Press; 1990. Brainstem control of wakefulness and sleep. [Google Scholar]
- Székely GJ, Rizzo ML. Energy statistics: A class of statistics based on distances. J Stat Plan Inference. 2013;143:1249–72. [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119:293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, et al. Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci. 2014;17:449–54. doi: 10.1038/nn.3642. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain. 2012;135:148–60. doi: 10.1093/brain/awr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol. 2013;110:2817–29. doi: 10.1152/jn.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, Lozano A M, et al. Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology. 2010;75:950–9. doi: 10.1212/WNL.0b013e3181f25b35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, Garcia-Rill E. Gamma band activity in the reticular activating system. Front Neurol. 2012;3:6. doi: 10.3389/fneur.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse S, Spildooren J, Heremans E, Wenderoth N, Swinnen SP, Vandenberghe W, et al. The neural correlates of upper limb motor blocks in parkinson’s disease and their relation to freezing of gait. Cereb Cortex. 2014;24:3154–66. doi: 10.1093/cercor/bht170. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res. 2008;188:165–74. doi: 10.1007/s00221-008-1349-1. [DOI] [PubMed] [Google Scholar]
- Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain. 2002;125:575–83. doi: 10.1093/brain/awf050. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. J Neurophysiol. 1994;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Neimat JS, Cosgrove GR, Eskandar EN. Timing and direction selectivity of subthalamic and pallidal neurons in patients with Parkinson disease. Exp Brain Res. 2005;162:407–16. doi: 10.1007/s00221-004-2035-6. [DOI] [PubMed] [Google Scholar]
- Winn P. Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord. 2008;14(Suppl 2):S194–8. doi: 10.1016/j.parkreldis.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Yeh IJ, Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, et al. Somatosensory evoked potentials recorded from the human pedunculopontine nucleus region. Mov Disord. 2010;25:2076–83. doi: 10.1002/mds.23233. [DOI] [PubMed] [Google Scholar]
- Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tandé D, et al. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. Neuroimage. 2007;34:618–38. doi: 10.1016/j.neuroimage.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Pack CC. Removal of spurious correlations between spikes and local field potentials. J Neurophysiol. 2011;105:474–86. doi: 10.1152/jn.00642.2010. [DOI] [PubMed] [Google Scholar]
- Zrinzo L, Zrinzo L V, Tisch S, Limousin PD, Yousry TA, Afshar F, et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131:1588–98. doi: 10.1093/brain/awn075. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol. 1989;26:41–6. doi: 10.1002/ana.410260106. [DOI] [PubMed] [Google Scholar]