Heparan sulfate 3-O-sulfotransferase-2 (3OST2) is a heparan sulfate modifying enzyme predominantly expressed in brain. Sepulveda-Diaz, Alavi Naini, Huynh et al. show in vitro and in a zebrafish model of tauopathy that inhibiting 3ost2 expression, or preventing tau interaction with 3-O-sulfated heparan sulfates, blocks abnormal tau phosphorylation of the type seen in Alzheimer’s disease.
Keywords: HS3ST2/3OST2, heparan sulphates, Alzheimer’s disease, tauopathy, 3-O-sulphation, Zebrafish

Heparan sulfate 3-O-sulfotransferase-2 (3OST2) is a heparan sulfate modifying enzyme predominantly expressed in brain. Sepulveda-Diaz, Alavi Naini, Huynh et al. show in vitro and in a zebrafish model of tauopathy that inhibiting 3ost2 expression, or preventing tau interaction with 3-O-sulfated heparan sulfates, blocks abnormal tau phosphorylation of the type seen in Alzheimer’s disease.
Abstract
Heparan sulphate (glucosamine) 3-O-sulphotransferase 2 (HS3ST2, also known as 3OST2) is an enzyme predominantly expressed in neurons wherein it generates rare 3-O-sulphated domains of unknown functions in heparan sulphates. In Alzheimer’s disease, heparan sulphates accumulate at the intracellular level in disease neurons where they co-localize with the neurofibrillary pathology, while they persist at the neuronal cell membrane in normal brain. However, it is unknown whether HS3ST2 and its 3-O-sulphated heparan sulphate products are involved in the mechanisms leading to the abnormal phosphorylation of tau in Alzheimer’s disease and related tauopathies. Here, we first measured the transcript levels of all human heparan sulphate sulphotransferases in hippocampus of Alzheimer’s disease (n = 8; 76.8 ± 3.5 years old) and found increased expression of HS3ST2 (P < 0.001) compared with control brain (n = 8; 67.8 ± 2.9 years old). Then, to investigate whether the membrane-associated 3-O-sulphated heparan sulphates translocate to the intracellular level under pathological conditions, we used two cell models of tauopathy in neuro-differentiated SH-SY5Y cells: a tau mutation-dependent model in cells expressing human tau carrying the P301L mutation hTauP301L, and a tau mutation-independent model in where tau hyperphosphorylation is induced by oxidative stress. Confocal microscopy, fluorescence resonance energy transfer, and western blot analyses showed that 3-O-sulphated heparan sulphates can be internalized into cells where they interact with tau, promoting its abnormal phosphorylation, but not that of p38 or NF-κB p65. We showed, in vitro, that the 3-O-sulphated heparan sulphates bind to tau, but not to GSK3B, protein kinase A or protein phosphatase 2, inducing its abnormal phosphorylation. Finally, we demonstrated in a zebrafish model of tauopathy expressing the hTauP301L, that inhibiting hs3st2 (also known as 3ost2) expression results in a strong inhibition of the abnormally phosphorylated tau epitopes in brain and in spinal cord, leading to a complete recovery of motor neuronal axons length (n = 25; P < 0.005) and of the animal motor response to touching stimuli (n = 150; P < 0.005). Our findings indicate that HS3ST2 centrally participates to the molecular mechanisms leading the abnormal phosphorylation of tau. By interacting with tau at the intracellular level, the 3-O-sulphated heparan sulphates produced by HS3ST2 might act as molecular chaperones allowing the abnormal phosphorylation of tau. We propose HS3ST2 as a novel therapeutic target for Alzheimer’s disease.
Introduction
Heparan sulphate (glucosamine) 3-O-sulphotransferase 2 (HS3ST2, also known as 3OST2) is an enzyme predominantly expressed in brain (Shworak et al., 1999; Yabe et al., 2005), where it generates rare 3-O-sulphated domains of still unrevealed physiological roles in heparan sulphates (Lawrence et al., 2007; Mochizuki et al., 2008; Thacker et al., 2014). During adulthood, HS3ST2 is expressed in brainstem, trigeminal ganglia (Lawrence et al., 2007), cerebral cortex (Mochizuki et al., 2008) and hippocampus (Huynh et al., 2012), brain regions vulnerable to tau pathology in Alzheimer’s disease (Wenk, 2003; Braak and Del Tredici, 2012). In contrast, it is almost absent in cerebellum (Lawrence et al., 2007; Mochizuki et al., 2008; Thacker et al., 2014), a brain region of low disease vulnerability (Wu et al., 2005). Under physiological conditions heparan sulphates are typically located at the cell surface (Kreuger and Kjellén, 2012), whereas in Alzheimer’s disease they accumulate at the intracellular level long before the detection of the tau pathology (Snow et al., 1990) and co-localize with neurofibrillary tangles when the pathology is detected (Snow et al., 1990; Goedert et al., 1996; Hernandez et al., 2002). This suggests the implication of heparan sulphates in the pathophysiological mechanisms leading to tauopathy. The abnormal hyperphosphorylation of the microtubule-associated protein tau is a critical event characterizing both sporadic and inherited tauopathies (Iqbal et al. 2010; Wang et al., 2013), including Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia with parkinsonism linked to chromosome-17 (FTDP-17) and related disorders (Williams, 2006; Spillantini and Goedert, 2013). Interestingly, the abnormal phosphorylation of tau in the disease brain is assumed to be mediated by the same kinases that phosphorylate tau in normal brain (Hashiguchi and Hashiguchi, 2013; Wang et al., 2013); however, in vitro, these kinases can only induce the abnormal phosphorylation of tau at disease-specific sites only if the enzymatic reaction takes place in the presence of polyanions such as heparin (Hasegawa et al., 1997; Zheng-Fischhofer et al., 1998; Paudel and Li, 1999; Sibille et al., 2006). Because heparin is a heparan sulphate analogue carrying high levels of 3-O-sulphation in the sugar backbone (Kreuger and Kjellén, 2012; Shriver et al., 2012), this raises the question whether 3-O-sulphated heparan sulphates, products of neuronal 3-O-sulphotransferases, are involved in the molecular and cellular mechanisms leading to the abnormal phosphorylation of tau. Here, we used biochemical, cellular, and animal strategies to investigate the implication of HS3ST2 in the molecular and cellular mechanisms leading to the abnormal phosphorylation of both wild-type tau and of tau carrying the mutation P301L (hTauP301L) responsible of FTDP-17 (Alonso Adel et al., 2004; Williams, 2006). We show that the chemical inhibition of heparan sulphate sulphation, or the downregulation of HS3ST2 expression in cells, strongly avoids the hyperphosphorylation of tau induced by oxidative stress or by the hTauP301L mutation, and that inhibiting the expression of hs3st2 in a FTDP-17 zebrafish model of tauopathy (Paquet et al., 2009) avoids the abnormal phosphorylation of tau at several Alzheimer’s disease-related epitopes resulting in tauopathy arrest and animal functional recovery.
Materials and methods
Human tissues
Human hippocampus samples were obtained from the Aging and Neurodegenerative Diseases Brain Bank Investigation Laboratory of the Universidade Federal de São Paulo, Brazil. Protocols were approved by the local ethics committee (No.285/04). Two experimental groups were included in the study (Supplementary Table 1), a control group (n = 8) and an Alzheimer’s disease group (n = 8) with subjects’ ages ranging from 60 to 98 years old with a mean age of 73.4 ± 10.7 years. Post-mortem intervals averaged 14 h 59 min ± 5 h 41 min. Neuropathological changes in brains were investigated using Braak and Braak and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) guidelines. Senile plaques and neurofibrillary tangles were determined on Bielschowsky-stained sections of middle frontal gyrus, middle temporal gyrus, inferior parietal lobule, occipital pole, hippocampal CA1 and enthorinal cortex. Senile plaques were counted using a ×10 objective and neurofibrillary tangles were counted with a ×20 objective. An arithmetic mean (mean ± standard error of the mean) was calculated from the counts of six fields for senile plaques by mm2 and neurofibrillary tangles by mm2 for each region. Neuropathological diagnosis was then made using the guidelines proposed by Braak and Braak criteria (Supplementary Table 1). Brain samples were powdered in liquid nitrogen and stored at −80°C until use.
Quantitative polymerase chain reaction
Total RNA was extracted from frozen hippocampus CA1 samples as described (Huynh et al., 2012). The amount of RNA in the extracts was measured spectrophotometrically at 260 and 280 nm. The quality of RNA was confirmed suitable determined by gel electrophoresis. RNA quality was additionally confirmed by RIN (RNA integrity number) determination (Schroeder et al., 2006) obtained by the ‘Standardization of RNA Quality Control Protocol’ in a 2100 Bioanalyzer (Agilent). RIN average was >7.0 for all samples. Extracted RNA was used to synthesize cDNA using reverse transcriptase as previously reported (Huynh et al., 2012). Genes of interest were analysed in template cDNA by quantitative real-time PCR using primers (Eurofins) designed by the Primer3output program. PCR was performed according to the LightCycler® FastStart DNA Master SYBR® Green kit manufacturer’s instructions (Roche). Relative gene expression quantification was performed using the comparative ΔΔCT method (Schefe et al., 2006). Two reference genes (TUBA1A and TBP) were used as endogenous controls. Normalization of gene expression was accomplished with the Genorm program (Vandesompele et al., 2002).
Cells models of tauopathy
SH-SY5Y and SH-SY5Y/hTauP301L (SH/hTauP301L) cells (a gift from J. Gotz, Zürich, Switzerland) were maintained and cultured as previously described (Ferrari et al., 2003). Briefly, SH-SY5Y cells were grown in DMEM-GlutaMAX™ (Invitrogen) supplemented with 10% foetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen). For SH-SY5Y/hTauP301L cells, 300 µg/ml geneticin (Invitrogen) and pyruvate were added to the medium. For all experiments, cells were differentiated 24 h after plating by treatment with 10 µM all-trans-retinoic acid (Sigma) in complete growth medium for 7 days. Media were changed every 2 days. For oxidative stress studies, differentiated SH-SY5Y cells were treated with H2O2 (500 µM final concentration in medium) for 30 min as previously described (Zhang et al., 2013). In assays involving NaClO3 (Keller et al., 2008), the salt was added to cell cultures (25, 50 or 75 mM final concentration) at the third or fifth day of differentiation, as indicated, and maintained until the end of the experiment. For enzymatic treatments, differentiated cells were treated either with a heparitinase mix containing heparitinase I (2 U/ml), heparitinase II (0.2 U/ml), and heparitinase III (0.2 U/ml) (all from Sigma), or with chondroitinase ABC (0.1 U/ml) (Sigma) and incubated at 37°C for 1.5 or 6 h (as indicated). After enzymatic treatment, medium was changed for new fresh medium and cells were subjected to the specified corresponding experimental conditions.
Cell transfections, plasmids and lentivirus
Two lentiviral vectors pTrip-CMV-Egfp-miRNA-HS3ST2 designed to inhibit HS3ST2 expression were purchased from the vectorology platform of the Brain and Spinal Cord Institute (Paris, France). Briefly, four plasmids containing different miRNAs targeting the HS3ST2 gene coding sequence were designed with the following sequences: dsOligo-miRNA1: 5′-TGCTGCCTTCTTCACGCCCACAATGAGTTTTGGCCACTGACTGACTCATTGTGCGTCAAGAAAG-3′, 3′-CGGAAGAAGTGCGGGTGTTACTCAAAACCGGTGACTGACTGAGTAACACGCAGTTCTTTCGTCC-5′; dsOligo-miRNA2: 5′-TGCTGGACTCGCCCCATCTCGCCGGCGTTTTGGCCACTGACTGACGCTGGTGATGGGTCGTATT-3′, 3′-CCTGAGCGGGGTAGAGCGGCCGCAAAACCGGTGACTGACTGCGACCACTACCCAGCATAAGTCC-5′. A sequence of the GFP was also introduced in the vector for monitoring simultaneous expression. The lentiviral vector pTrip-CMV-eGFP-miRneg was used as control. For silencing experiments, SH/hTauP301L cells (300 000) were suspended and transduced with a 1:20 dilution of a concentrated suspension of the two lentiviral vectors mixture containing 0.1 × 109 physical particles/µl. Cells were then plated in 24-well plates (Nunc) and incubated for 72 h under standard conditions. Cell lysates were immuno-blotted as specified above with antibodies directed to HS3ST2, pSer396, Tau-5, and anti-β-actin (Life Technologies).
Immunofluorescence
For immunofluorescence studies, cells were plated in 35-mm Petri dishes (µ-Dish, Ibidi) at a density of 40 000 cells/dish following manufacturer’s instructions. Differentiation, stress and any other treatments were performed as described above. For analysis, cells were washed, fixed in 100% methanol at −20°C and washed again before been incubated with 2% donkey serum in phosphate-buffered saline (PBS) for 20 min at room temperature. Cells were then incubated for 1 h with the corresponding primary antibody at room temperature, washed and incubated with the appropriate secondary antibody for 30 min at room temperature. The antibodies used were HS4C3 (Tem Dam et al., 2006) (TH v Kuppevelt is author of the work; 1:100), tau K9JA (Dako; 1:500) or tau V-20 (Santa Cruz; 1:100). Secondary antibodies were Fluo488 donkey anti-rabbit (Interchim; 1:200), Fluo488 donkey anti-goat (Interchim; 1:200), and Cy3 goat anti-mouse (Sigma; 1:200). The phage display HS4C3 antibody was revealed by an anti-VSV antibody (Sigma; 1:100). Stack images were obtained with the software CellSens from a spinning disk inverted confocal microscope (IX81 DSU Olympus, ×60 N.A. 1.35) coupled to an Orca Hamamatsu RCCD camera. Images were processed with the ImageJ software (W. Rasband, National Institute of Health).
Western blotting
For protein analysis by western blot, powdered frozen tissue or cells were suspended (100 mg of tissue/ml or 1.8 × 105 cells/ml) in an extraction buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton™ X-100, 10 mM NaF, 1 mM Na3VO4, pH 8.0) supplemented with protease and phosphatase inhibitors (Pierce). Tissue homogenates were centrifuged at 12 000 g for 15 min and the protein content in the supernatants was determined using BCA Protein Assay kit (Pierce). Samples containing 20 µg proteins were subjected to SDS-PAGE in 10% acrylamide gel. Primary antibodies for phosphorylated tau were pSer199, pSer199/pSer202, pThr231, pSer262, pSer396 (Life Technologies), AT8, AT270, PHF1, AT180, AT100 (Pierce, Thermo Scientific), and MC1 (a gift from Peter Davies); anti-total tau antibody was Tau-5 (Millipore). Other antibodies used were against HS3ST2, HS3ST4 (Clinisciences), p38, pp38, NF-κB p65, pNF-κB p65 (Cell Signaling technology), and anti-β-actin (Life Technologies). Corresponding secondary antibodies diluted in phosphate-buffered saline containing 1% milk were incubated for 1 h at room temperature. Blots were developed either with Immobilon Western Chemiluminiscent HRP Substrate, Luminata Forte, Luminata Crescendo (Millipore), or SuperSignal West Dura (Thermo Scientific) following manufacturer’s instructions. Densitometric quantification of immunoreactivity was performed by using the ImageJ software. Hyperphosphorylated tau signals were normalized to total tau detected with the anti-Tau-5 antibody. β-Actin was used to standardize total protein load.
Fluorescence resonance energy transfer assay
Fluorescence resonance energy transfer (FRET) assays were performed as in Li et al. (2012) by using a cryptate-d2 system (CISBIO Bioassays). Briefly, cell lysates (10 µl at 2.6 µg/µl of protein) were mixed with HS4C3 antibody in 384-well plates and incubated for 20 min at room temperature. Next, cryptate-conjugated anti-VSV and d2-conjugated anti-tau K9JA were added to the reaction mixture following the manufacturer’s instructions. After 20 min of incubation, energy transfer was measured using a TECAN Infinite 1000 spectrometer, data were processed according to manufacturer’s instructions.
Tau and tauP301L phosphorylation assay
Tau phosphorylation reactions were carried out according to Hasegawa et al. (1997) with some modifications. Briefly, four different solutions containing tau at 200 µg/ml, GSK3B (Promega) at 20 µg/ml, respectively, heparin (Sigma) at 60 µg/ml, and ATP at 250 µM, were prepared in a reaction buffer (40 mM Tris-HCl, 20 mM MgCl2, 0.1 mg/ml BSA, pH 7.5). The four solutions were mixed, and when necessary diluted in reaction buffer, to set reaction mixtures containing 1 µg of the tau substrate, 100 ng of the enzyme, and 50 µM ATP, in the presence or absence of 0.3 µg heparin (20 µl final reaction volume). When indicated, protein kinase A (PKA) (Sigma) was included in the reaction mixture at 3 µg/ml final concentration. Tau was either full-length recombinant human tau (Millipore) or full-length recombinant tauP301L (Interchim). The amount of tau, GSK3B and PKA used in the reactions was selected from dose response experiments with 0.5, 1.0, or 2.0 µg of tau and 50, 100, or 200 ng of enzyme. Reactions were carried out at 30°C for 3 h and stopped by cooling at −20°C. Reaction mixtures were resolved by western blot as described above, with one or several of the following antibodies: pSer396, pSer199/202, AT270, PHF1, AT8, AT100, and MC1.
Kinase and phosphatase activities
The effect of heparin on the kinase and phosphatase enzymatic activities was assessed with kits for GSK3B (Promega), protein phosphatase 2A (PP2A, Promega), and PA (Abcam) by following the manufacturer's instructions. The enzymatic reactions were performed in the absence or presence of heparin at several concentrations (from 0.1 ng/ml to 100 µg/ml).
Enzyme-linked immunosorbent assays
Binding of tau, tauP301L, GSK3B, PKA, or PP2A to heparin was evaluated by ELISA as in Huynh et al. (2012). Briefly, ELISA-type 96-well plates were coated with a 2 µg/ml bovine serum albumin (BSA) heparin conjugate solution. After washing the wells with PBS containing 0.05% Tween-20, wells were saturated with 3% BSA in PBS. Then, the tested protein (full length human tau, tauP301L, GSK3B, PKA or PP2A) was added to the plate at various concentrations and plates were incubated for 12 h at 4°C. After washing, the protein bond to heparin was revealed by the corresponding specific antibody Tau-5, anti-GSK3B (Thermofisher), anti-PKA, or anti-PP2A (Sigma) followed by a peroxidase-labelled secondary antibody. Peroxidase activity was measured by the TMB detection kit (Pierce) following fabricant instructions. For the ELISA competition assays, heparin or heparan sulphate were added to the wells at concentrations ranging from 0.01 ng/ml to 10 µg/ml following addition of the corresponding protein (tau, tauP301L, GSK3B, PKA, or PP2A) to the wells.
Zebrafish
Zebrafish were maintained in a standard zebrafish facility (Aquatic Habitats, Apopka). Developmental stages were determined as hours post fertilization (hpf) or as days post fertilization (dpf), as previously described (Kimmel et al., 1995). Wild-type embryos were from the AB and TL strains. The Tg[HuC::hTauP301L;DsRed] transgenic line has been previously described (Paquet et al., 2009). All procedures involving animal handling complied with the guidelines of the French Animal Ethics Committee and were approved by the same committee under the ethics statement N°: 2012-15/676-0069.
Morpholino oligonucleotides
Morpholinos MO-HS3ST2AUG: 5′-GGCTTGACAGGAACCTATATGCCAT-3′, MO-HS3ST2SPL: 5′-AAAATTAACCTTACCTGTACCAGTC-3′ and mmMO-HS3ST2AUG: 5′-GGCTTGACATGAAGATATATGGTAT-3′, were obtained from Gene Tools. For morpholino-mediated zebrafish hs3st2 transcript inactivation, 2 nl of a 1.5 mM solution, corresponding to 3 pmol of each morpholino were injected in 1- to 2-cell stage embryos using standard protocols. Antibodies directed against pathologic tau epitopes were AT180, MC1 and AT270. AT8 and PHF1 for zebrafish experiments were kindly provided by D. Paquet. Anti-human tau antibody was purchased from DakoCytomation. Primary motor neuron morphology was analysed using the mouse monoclonal anti-synaptotagmin 1 antibody (anti-Syt1; 1/300, Hybridoma Bank).
Analysis of zebrafish behaviour
Touch-induced escape response was assessed by gently touching the tail of embryos with the tip of a fine plastic rod (Paquet et al., 2009). Embryos were then classified as responders or non-responders.
Statistical analyses
All data are expressed as the mean ± standard error of the mean (SEM) or standard deviation (SD) as specified. P-values were generated using non-parametric t-test, Mann Whitney U-test, or ANOVA, as specified. Asterisks indicate significant differences (*P < 0.05, **P < 0.005, ***P < 0.001); n.s. indicates non-significant differences.
Results
HS3ST2 is overexpressed in Alzheimer’s disease brain
In a previous work, we showed that HS3ST1, HS3ST2, HS3ST3A, HS3ST3B, HS3ST4, and HS3ST5 are expressed in human elderly hippocampus (Huynh et al., 2012). However, it is unknown whether the expression of these enzymes is altered in brain of Alzheimer’s disease. To first investigate this possibility, we compared the transcript levels of all human sulphotransferases (Kreuger and Kjellén, 2012) from Alzheimer’s disease hippocampus (CA1) to those from age-matched controls. We used quantitative PCR to determine levels of mRNA encoding for the four heparan sulphate N-deacetylase/N-sulphotransferases (NDST1, NDST2, NDST3 and NDST4) and for the different sulphotransferases including HS2ST, HS3STs (1, 2, 3A, 3B, 4, 5, and 6), and HS6STs (1, 2 mRNA variants 2L and 2S, and 3), as well as C-5 epimerase (GLCE) and HPSE (heparanase), two other enzymes implicated in heparan sulphate metabolism. Among the 18 analysed transcripts, expression levels of most sulphotransferases were slightly (P < 0.05) increased in the Alzheimer’s brains compared to age-matched-individuals. However, only HS3ST2 and HS3ST4 were expressed at higher significant levels (P < 0.001), as analysed by the Mann Whitney U-test (Table 1). Remarkably, western blot analysis of the hippocampal protein extracts showed a higher HS3ST2 expression, compared to HS3ST4, in the Alzheimer’s tissue (Supplementary Fig. 1). This suggests that in Alzheimer’s disease, 3-O-sulphated domains in heparan sulphates are increased in brain regions in where these enzymes are expressed (Lawrence et al., 2007; Mochizuki et al., 2008).
Table 1.
Expression of heparan sulphate metabolic enzyme mRNA in hippocampus from control and Alzheimer’s disease
| Enzymes Isoforms | Control group | AD group (AU ± SD) |
|---|---|---|
| NDST1 | 0.33 ± 0.06 | 0.38 ± 0.06 |
| NDST2 | 0.59 ± 0.38 | 1.38 ± 0.42* |
| NDST3 | n.d. | n.d. |
| NDST4 | n.d. | n.d. |
| HS2ST | 0.89 ± 0.07 | 1.03 ± 0.13* |
| HS3ST1 | 0.75 ± 0.09 | 0.96 ± 0.20 |
| HS3ST2 | 0.37 ± 0.07 | 2.06 ± 0.39*** |
| HS3ST3A1 | 0.35 ± 0.08 | 2.17 ± 1.69* |
| HS3ST3B1 | 0.58 ± 0.21 | 1.76 ± 0.90* |
| HS3ST4 | 1.33 ± 0.35 | 5.04 ± 0.34*** |
| HS3ST5 | 0.10 ± 0.04 | 0.08 ± 0.02 |
| HS3ST6 | n.d. | n.d. |
| HS6ST1 | 0.45 ± 0.57 | 1.35 ± 0.77* |
| HS6ST2(var L) | 0.35 ± 0.30 | 1.06 ± 0.55* |
| HS6ST2(var S) | 0.47 ± 0.48 | 0.64 ± 0.22 |
| HS6ST3 | 2.41 ± 0.77 | 2.96 ± 0.82 |
| GLCE | 0.35 ± 0.21 | 1.13 ± 0.62* |
| HPSE | 0.23 ± 0.03 | 0.38 ± 0.06* |
| GLUL | 0.49 ± 0.08 | 1.23 ± 0.18** |
| CXCR4 | 0.73 ± 0.26 | 0.68 ± 0.14 |
*Significant changes in transcript expression as defined by the Mann Whitney U-test (n = 8).
n.d. = the transcript was not detected.
AU ± SD = arbitrary units ± SD.
3-O-sulphated heparan sulphates interact with tau in cell models of tauopathy
In Alzheimer’s disease, heparan sulphates accumulate in the intracellular space of neurons before the apparition of the tau pathology (Snow et al., 1990) and strongly co-stain with tau in intracellular neurofibrillary tangles during disease (Snow et al., 1990; Goedert et al., 1996; Hernandez et al., 2002). However, under physiological conditions, these sulphated polysaccharides are located at the extracellular space, in where they exert their known biological functions (Kreuger and Kjellén, 2012; Thacker et al., 2014). Thus, we first thought to investigate whether 3-O-sulphated heparan sulphate internalization and interaction with tau can be provoked under tauopathy-associated conditions, such as oxidative stress or expression of a pathologic tau mutation. We used two cell models: (i) an oxidative stress (SH/ox) model in wild-type SH-SY5Y cells (Zhang et al., 2013); and (ii) a tau-mutation-dependent model in SH-SY5Y cells overexpressing the hTauP301L mutant (SH/hTauP301L) (Ferrari et al., 2003), which underlies FTDP-17 (Alonso Adel et al., 2004). By using a 3-O-sulphated heparan sulphate specific antibody (HS4C3) (Ten Dam et al., 2006), we confirmed by confocal microscopy that the sulphated polysaccharide accumulates at the cell surface in wild-type and control cells (Fig. 1A and C, and Supplementary Fig. 2), whereas a strong intracellular immunostaining was observed in both SH/ox (Fig. 1B and Supplementary Fig. 3) and SH/hTauP301L cells (Fig. 1D and Supplementary Fig. 4A). In SH/ox cells we observed a strong 3-O-sulphated heparan sulphate staining near the cell membrane at 10 min after the stress pulse (Supplementary Fig. 3A), whereas at 6 h after stress the membrane staining was strongly decreased and highly pronounced at the intracellular space (Supplementary Fig. 3B), suggesting a stress-driven time course internalization of the membrane-associated polysaccharides. In contrast, in SH/hTauP301L cells, the 3-O-sulphated polysaccharide was permanently and highly accumulated in the form of intracellular inclusions showing different levels of overlap with tau (Supplementary Fig. 4A), but nearly absent at the cell membrane. No differences in the level or distribution of chondroitin sulphates were observed in either of the cell models (not shown).
Figure 1.
Intracellular 3-O-sulphated heparan sulphates and tau co-staining in two cell models of tauopathy. (A) Immunostaining of cell membrane-associated 3-O-sulphated heparan sulphates (red) and intracellular tau (green) in control cells (optical section). (B) 3-O-sulphated heparan sulphate immunostaining increases in cell membranes early after stress to then decrease in a time dependent manner with concomitant increase of intracellular staining. (C) Empty vector (EV) transfected cells. (D) Intracellular 3-O-sulphated heparan sulphate inclusions accumulate and co-stain with tau at different extents in SH/hTauP301L cells. Pictures represent observations on cells from three independent experiments. Scale bars = 10 µm.
The accumulation of intracellular 3-O-sulphated heparan sulphates in both SH/ox and SH/hTauP301L cells raised the question whether these sulphated polysaccharides were of membrane origin. To address this issue, we cultured SH/ox and SH/hTauP301L cells in the presence of a mixture of heparitinase I, II and III, an enzymatic cocktail that digests the heparan sulphate glycanic chains of membrane-bound heparan sulphate proteoglycan (HSPG). As expected, the heparitinases treatment strongly decreased the staining of membrane-associated heparan sulphate in control cells (Fig. 2A), and, surprisingly, nearly suppressed the staining of the intracellular heparan sulphate inclusions in the two cell models (Fig. 2B–D and Supplementary Fig. 4B), suggesting that the intracellular 3-O-sulphated heparan sulphates observed in both SH/ox and SH/TauP301L cells existed as HSPG in cell membranes before being internalized within the cells. As confocal microscopy co-localization cannot be considered as an evidence of molecular interactions, we further investigated whether the 3-O-sulphated heparan sulphates interact with tau at the molecular level by performing FRET experiments (Li et al., 2012) in SH/ox cells. We incubated cell lysates with a europium-labelled secondary antibody directed against the HS4C3 antibody and a d2-labelled anti-tau primary antibody. The FRET signal observed in the stressed cells as early as 10 min and at 6 h after the stress pulse was nearly abolished when cells were cultured in the presence of heparitinase (Fig. 2E), but not of chondroitinase (not shown), indicating a close interaction of 3-O-sulphated heparan sulphates with tau.
Figure 2.
3-O-sulphated heparan sulphates interact with tau under pathological conditions. Heparitinase (Hep-ase) treatment decreases membrane-associated and intracellular 3-O-sulphated heparan sulphates (red) staining and co-localization with tau. Z-projections are depicted for (A) control cells; (B) SH/ox cells treated with heparitinase before the oxidative stress pulse; and (C) SH/hTauP301L cells. (D) Quantification of heparan sulphates (HS) intracellular inclusions in SH/hTauP301L cells treated with hep-ase during 1.5 or 6 h. (E) FRET-based measurement of the molecular interaction between 3-O-sulphated heparan sulphates and tau in SH/ox cell lysates at different time points after the stress pulse. Calculated delta F (ΔF) shows a time-dependent molecular interaction abolished when cells were cultured in the presence of heparitinase I, II, III cocktail (Hep-ase). (F) ELISA competition assay showing the higher capacity of heparin, used as a prototype of 3-O-sulphate heparan sulphates, to inhibit the binding of tau to immobilized heparin compared to heparan sulphates. Data represents the mean of three different experiments performed in triplicate each time; error bars show SEM.
To investigate the importance of 3-O-sulphates in the interaction of heparan sulphates with tau, we performed an ELISA-based competition essay to compare the capacity of tau to bind to highly 3-O-sulphated heparan sulphates, here represented by heparin, compared to less sulphated heparan sulphates (Shriver et al., 2012). As expected, we observed that tau binds to heparin better than to heparan sulphates (Fig. 2F), suggesting that increasing 3-O-sulphation levels in heparan sulphate could result in heparan sulphate increased capacity to interact with tau. Together, these results suggest that under pathological conditions, 3-O-sulphated heparan sulphates can interact with tau inside cells in where they could participate to the cellular mechanisms inducing the pathological phosphorylation of the protein.
Pathological phosphorylation of tau requires 3-O-sulphated heparan sulphates
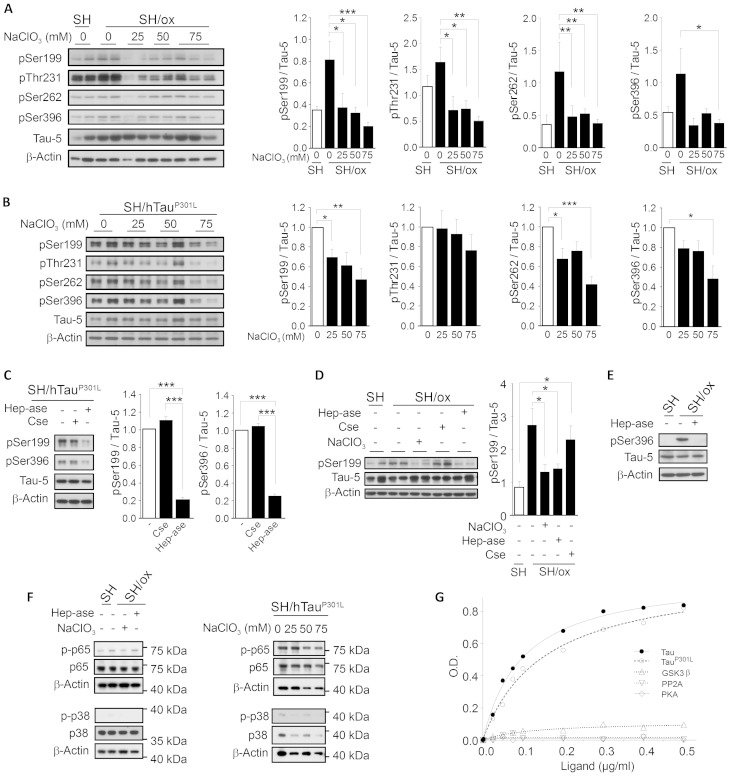
In an attempt to understand the role of the 3-O-sulphated heparan sulphates in the abnormal hyperphosphorylation of tau, we sought to investigate whether these polysaccharides are required for tau hyperphosphorylation in both SH/ox and SH/hTauP301L cells. As western blot analysis using the Tau-5 antibody showed a higher level of total tau in the SH/hTauP301L cells compared to SH/ox cells (Supplementary Fig. 5A), all phosphorylated tau levels were further normalized against total tau (Tau-5). First, we confirmed that, compared to control cells, SH/ox and SH/hTauP301L cells accumulate higher levels of tau phosphorylated at Ser199 (pSer199), Thr231 (pThr231), Ser262 (pSer262), and Ser396 (pSer396), characteristic hyperphosphorylated residues in Alzheimer’s disease (Steinhilb et al., 2007; Duka et al., 2013). In the SH/ox cells, maximum pSer199, pThr231, and pSer262 levels were observed 10 min after the stress pulse, while maximum pSer396 was detected 6 h later (Supplementary Fig. 5B). To investigate whether heparan sulphates are required for tau hyperphosphorylation at these sites, we treated cells with sodium chlorate (NaClO3), an inhibitor of the synthesis of PAPS (3′-phosphoadenosine 5′-phosphosulphate), the sulphate donor in glycosaminoglycan biosynthesis (Venkatachalam, 2003). High NaClO3 concentrations have shown to inhibit the formation of highly sulphated heparan sulphates under classic cell culture conditions (Safaiyan et al., 1999; Keller et al., 2008). Here, following treatment with NaClO3, we observed a significant decrease in hyperphosphorylated tau accumulation, notably in SH/ox cells (Fig. 3A). Although this decrease was less prominently in SH/hTauP301L cells (Fig. 3B), which required higher NaClO3 concentrations, this suggests the involvement of sulphated glycosaminoglycans in the hyperphosphorylation event. To determine which glycosaminoglycan species are involved in this process, we incubated SH/ox and SH/TauP301L cells with either chondroitinase ABC or heparitinase. While heparitinase digestion significantly decreased the accumulation of hyperphosphorylated tau in both SH/ox and SH/TauP301L cells (Fig. 3C–E), chondroitinase treatment showed no effect (Fig. 3C and D), indicating that highly sulphated heparan sulphates, but not chondroitin sulphates, are required for tau hyperphosphorylation in both cell models. To investigate whether inhibiting the cellular heparan sulphates can affect the phosphorylation of other cytosolic proteins, we evaluated the phosphorylation of the subunit p65 of NF-κB and of p38 in both models. No differences in the phosphorylation of these proteins were observed (Fig. 3F). This result, as well as the 3-O-sulphated heparan sulphates interaction with tau observed by FRET, suggests a specific effect of 3-O-sulphated heparan sulphates on tau. However, this effect could also be driven by altered kinases or phosphatases activities through indirect mechanisms that should be further studied. Thus, by using an ELISA assay, we compared the capacities of tau, tauP301L, GSK3B, PKA and PP2A, to bind to immobilized heparin as a prototype of 3-O-sulphated heparan sulphates. Our results show that tau and tauP301L, but not GSK3B, PKA, and PP2A, could efficiently bind to the sulphated polysaccharide (Fig. 3G).
Figure 3.
Sulphated heparan sulphates are required for tau hyperphosphorylation in two cell models of tauopathy. Tau hyperphosphorylated at Ser199, Thr231, Ser262, and Ser396, total tau (Tau-5), and β-actin levels in cells treated with NaClO3 in (A) SH/ox cells, and in (B) SH/hTauP301L cells. (C) Hyperphosphorylated tau at Ser199 and Ser396 in SH/hTauP301L cells treated or not with heparitinases I, II, and III cocktail (Hep-ase) or chondroitinase ABC (Cse). (D) Tau hyperphosphorylated at Ser199 in SH/ox cells after a stress pulse treated or not with heparitinase, Cse or NaClO3. (E) Tau hyperphosphorylated at Ser396 in SH/ox cells treated or not with heparitinase prior to the oxidative stress pulse. Data represent the mean of three different experiments performed in duplicate each time; error bars show SEM. (F) Western blots showing the phosphorylation of NF-kB p65 and p38 proteins in SH/ox (left) and in SH/hTauP301L (right) cells treated or not with the heparitinases cocktail or with NaClO3. Total levels of each protein, as well as that of β-actin, were used as loading controls. (G) ELISA assay showing the binding of heparin to tau (filled circles, solid line), TauP301L (open circles, dashed line), GSK3B (upward triangles, spotted line), PP2A (downward triangles, spotted line), and PKA (diamonds, solid line). Data in histograms were made from the average signal obtained from the densitometric analysis of duplicates carried in each individual western blot, or by the average of two different western blots when duplicates were not performed.
Together, our results suggest that highly sulphated heparan sulphates favour the abnormal phosphorylation of tau in cells in where 3-O-sulphated heparan sulphates interact with tau. To examine if 3-O-sulphated heparan sulphates directly participate to the abnormal phosphorylation of wild-type tau, we used an in vitro GSK3B-dependent tau phosphorylation reaction and examined the occurrence of phosphorylation at residues Thr181 (AT270 antibody), Ser396/Ser404 (PHF1 antibody), Ser202/Thr205 (AT8 antibody), Ser212/Ser214 (AT100), and the conformational anti-tau MC1 antibody, specific of Alzheimer’s disease and other tauopathies (Cripps et al., 2006; Yoshida and Goedert 2006; Steinhilb et al., 2007; Duka et al., 2013). We observed that the GSK3B-dependent phosphorylation at AT270, PHF1, and AT8 epitopes requires the presence of heparin in the reaction mixture (Fig. 4A). Interestingly, the effect of heparin at the AT100 epitope required the introduction of PKA into the GSK3B reaction mixture. Under these conditions, we observed that the abnormal phosphorylation of tau at the AT100 epitope is also heparin dependent (Fig. 4B). However, when we used the MC1 antibody, no differences were observed in western blots of reactions performed in the presence or absence of heparin with and without addition of PKA (not shown). This can be possibly due to a blotting condition-dependent loss of the tau conformation required for recognition of tau by this antibody, most classically used for histological applications. We then analysed the effect of heparin in the GSK3B-dependent phosphorylation of tau at sites Ser396 and Ser199/202, found phosphorylated at some extent in normal brain but hyperphosphorylated in case of tauopathy. We observed an increased level of phosphorylation at these sites in reactions carried out in the presence of heparin (Fig. 4C). We then carried out the phosphorylation reaction using tauP301L instead of the wild-type tau as substrate of the phosphorylation reaction. Heparin favoured the tauP301L abnormal phosphorylation (Fig. 4D), in agreement with the role of heparan sulphates in the abnormal phosphorylation of tau in the HS/hTauP301L model of tauopathy. Interestingly, phosphorylation reaction showed to be more efficient for tauP301L than for wild-type tau, suggesting a higher susceptibility to tau pathology when the mutation is present.
Figure 4.
Phosphorylation of tau and tauP301L at disease-related sites is induced by heparin in vitro and by 3-O-sulphated heparan sulphates in vitro and in cells. (A–C) GSK3B-mediated abnormal phosphorylation of wild-type tau and tauP301L is dependent on the presence of heparin in the reaction mixture. (A) GSK3B-mediated abnormal phosphorylation of tau at AT270, PHF1 and AT8. (B) GSK3B/PKA-mediated abnormal phosphorylation of tau at the AT100 site. (C) GSK3B-mediated tau hyperphosphorylation at sites pSer396 and pSer199/202. (D) GSK3B-mediated abnormal phosphorylation of tau and tauP301L at epitopes AT270 and PHF1. (E) Lentiviral vector miR-HS3ST2-mediated down regulation of HS3ST2 shows a decreased accumulation of hyperphosphorylated tau in SH/hTauP301L cells. All experiments were performed at least three times.
Our results suggest that implication of heparan sulphates in the abnormal phosphorylation reaction is mediated by their direct interaction with tau, and less probably by their direct interaction with kinases or phosphatases (Fig. 3F and G). To reinforce this hypothesis, we investigated if heparin can modulate the enzymatic activity of GSK3B, PP2A, and AP in vitro. By using commercial kits we measured the activity of these enzymes and found that the addition of heparin at various concentrations has no modulatory effect in any of the tested enzymes (not shown), weakening the assumption of a putative role of the sulphated polysaccharides as kinase activators or phosphatase inhibitors. Nevertheless, it remains to be explored if the activities of kinases and phosphatases are altered in cells by mechanisms independent of their direct interaction with the sulphated sugars.
Together, the in vitro and the intracellular interaction of tau with heparin or heparan sulphates, as well as the effect of these interactions on the abnormal phosphorylation of tau, support the idea that intracellular 3-O-sulphated heparan sulphates could display a tau-chaperone activity critical for the abnormal phosphorylation of tau observed under pathological conditions. However, the implication of the specific 3-O-sulphation of heparan sulphates remains unclear. To explore this possibility, we investigated the effect of specifically silencing HS3ST2 expression in pathological tau phosphorylation in SH/hTauP301L cells. We used lentiviral vectors carrying miRNA designed to suppress HS3ST2 expression. Cells were transfected and monitored for pSer396 as a marker of tau hyperphosphorylation. Inhibition of HS3ST2 expression resulted in a strong decrease of pSer396 accumulation (Fig. 4E), supporting the implication of 3-O-sulphated heparan sulphates in the tau phosphorylation event.
HS3ST2 is critical for the abnormal phosphorylation of tau in vivo
We then investigated whether HS3ST2 is critical for abnormal phosphorylation of tau in vivo. To address this issue, we used the Tg[HuC::hTauP301L;DsRed] zebrafish transgenic line (Paquet et al., 2009) and morpholino-mediated inactivation of the zebrafish hs3st2. Tg[HuC::hTauP301L;DsRed] transgenic embryos express the human mutant TauP301L protein under the control of the pan-neuronal HuC promoter and reproduce several characteristic features of tauopathies, including an increased accumulation of hyperphosphorylated tau in brain and spinal cord neurons, neuronal abnormalities, neuronal death and impaired motility (Paquet et al., 2009). As expected, we detected high levels of abnormally phosphorylated tau in a large number of neurons scattered in several brain regions of 5 dpf Tg[HuC::hTauP301L;DsRed] larvae (n = 100) (Supplementary Fig. 6). By BLAST analysis of the Zv9 version of the zebrafish genome sequence, we identified a single hs3st2 gene in this species (ENSDARG000000;?>59616). We confirmed that zebrafish hs3st2 is composed of two exons separated by a >52 kb intron, allowing the synthesis of both translation- and splice-blocking morpholinos, which are referred to below as MO-hs3st2AUG and MO-hs3st2SLP, respectively (Supplementary Fig. 7A). RT-PCR analysis demonstrated efficient zebrafish hs3st2 splicing inhibition in Tg[HuC::hTauP301L;DsRed] embryos after injection of 1.5 mM MO-hs3st2SPL (Supplementary Fig. 7B). As control, we used a five mismatch-containing derivative of MO-hs3st2AUG (mmMO-hs3st2AUG). Tg[HuC::hTauP301L;DsRed] embryos injected with 1.5 mM MO-hs3st2SPL (n = 250) or MO-hs3st2AUG (n = 250) did not show increased lethality or any visible phenotype including developmental delay when compared to non-injected controls or embryos that received 1.5 mM mmMO-hs3st2AUG (n = 250) (Supplementary Fig. 7C and D). We then quantified abnormally phosphorylated tau at Thr181 (pThr181) and observed a nearly 60% decrease in the pThr181 accumulation in Tg[HuC::hTauP301L;DsRed] embryos (n = 300) following either morpholino-mediated zebrafish hs3st2 inactivation, or treatment with LiCl, an inhibitor of tau phosphorylation used as control treatment (Supplementary Fig. 7E). Using fluorescence microscopy we quantified the accumulation of abnormally phosphorylated tau with antibodies AT270, AT180 (pThr231/pSer235), and AT8 in brains of 5 dpf Tg[HuC::hTauP301L;DsRed] larvae injected or not with MO-hs3st2SPL (n = 150). We observed a significant fluorescence decrease in all the assayed sites in the brain in zebrafish hs3st2 depleted embryos (n = 150) (Fig. 5). Accordingly, we observed a strong decreased accumulation of AT270, AT180, PHF1, and AT8 epitopes (Fig. 6) and of the conformational antibody MC1 (Supplementary Fig. 8) in the spinal cord neurons of the 2 dpf zebrafish hs3st2-depleted Tg[HuC::hTauP301L;DsRed] larvae injected with MO-sh3st2SPL (n = 150). These results confirm that 3-O-sulphated heparan sulphates play a key role in abnormal phosphorylation of tau in tauopathy in vivo.
Figure 5.
Hs3st2-mediated HSPG sulphation is required for abnormal phosphorylation of tau in zebrafish brain. Double immunostaining for total tau (red) and phosphorylated tau (green) at residues AT270 (A1-A2”), AT180 (B1-B2”), and AT8 (C1-C2”) in 5 dpf dissected brains from Tg[HuC::hTauP301L] (Hu::Tau) embryos following morpholino-mediated depletion of zebrafish hs3st2 (Hu::Tau + MO-hs3st2) (n = 150). Scale bar = 50 µm. The percentage of cells expressing phospho-tau epitopes recognized by antibodies AT270 (left), AT180 (middle) and AT8 (right) compared to cells immunolabelled for total tau is shown (n = 150).
Figure 6.
HS3ST2-mediated sulphation of heparan sulphates is required for the pathological phosphorylation of tau in spinal cord neurons in zebra fish. (A) Double immunostaining for total tau (red) and abnormally phosphorylated tau (green) with anti-tau antibodies AT180, AT270, PHF1, and AT8 in spinal cord neurons of Tg[HuC::hTauP301L] (Hu::Tau) embryos following morpholino-mediated depletion of zebrafish hs3st2 (Hu::Tau + MO-hs3st2). (B) Immunostaining quantification of antibodies AT180, AT270, PHF1, and AT8 signals in spinal cord neurons. Panels in A represent observations (n = 150) of 5 dpf larvae from two independent experiments. Panels in B represents means of fluorescence analyses on 150 larvae; error bars show SEM. Scale bar = 50 µm.
Hs3st2 depletion rescues TauP301L induced functional defects
Because 2 dpf Tg[HuC::hTauP301L;DsRed] larvae display reduced motor neuronal axon branching and elongation and, as a likely consequence, an impaired escape response to touch stimuli (Paquet et al., 2009), this line provides us with a tool to further investigate in vivo the relationship between 3-O-sulphated heparan sulphates, tau abnormal phosphorylation and neuronal pathophysiology. First, we analysed motor neuronal axon morphology using the anti-syt1, antibody that specifically recognizes synaptotagmin 1, a synaptic protein expressed in primary motor neurons (Trevarrow et al., 1990; Fox and Sanes, 2007). As previously described (Paquet et al., 2009), we observed that motor neuronal axons were morphologically altered in the Tg[HuC::hTauP301L;DsRed] embryos, whereas morphology was partially recovered after zebrafish hs3st2 depletion (Fig. 7A). We then measured the length of outgrowing axons of the four caudal primary motor neurons immediately anterior to the end of the yolk extension. As expected, the mean length of motor neuronal axons was markedly reduced in 48 hpf Tg[HuC::hTauP301L;DsRed] embryos when compared to that observed in their non-transgenic siblings (Paquet et al., 2009) However, after zebrafish hs3st2 depletion, the mean length of motor neuronal axons was significantly increased in Tg[HuC::hTauP301L;DsRed] embryos to nearly normal length (n = 25; P < 0.005) (Fig. 7B). We next sought to investigate whether zebrafish hs3st2 depletion can rescue, at least partially, the motility defects observed in Tg[HuC::hTauP301L;DsRed] zebrafish larvae (Paquet et al., 2009). Following injection of 1.5 mM MO-hs3st2SPL, the escape response to touch stimulus in Tg[HuC::hTauP301L;DsRed] larvae was significantly increased to near levels measured in non-transgenic siblings (n = 150; P < 0.005) (Fig. 7C). Collectively, these results provide functional evidence supporting the essential involvement of 3-O-sulphated heparan sulphates in tau neuropathology in vivo.
Figure 7.
Partial depletion of zebrafish hs3st2 rescues both motor neuron axon outgrowth defects and behavioural abnormalities in Tg[HuC::hTauP301L] embryos. (A) Spinal cord neurons lateral views in yolk extensions, visualized by anti-Syt1, in wild-type embryos (Control), in Tg[HuC::hTauP301L] embryos that express the hTauP301L mutation (Hu::tau), and in Tg[HuC::hTauP301L] embryos injected with MO-hs3st2 (Hu::tau + MO-hs3st2). (B) Motor neuronal axons length mean (n = 25) in control wild-type embryos (WT), in Tg[HuC::hTauP301L] embryos (Hu::Tau), and in Tg[HuC::hTauP301L] embryos following depletion of hs3st2 (Hu::Tau + MO-hs3st2). (C) Motor response to touching stimuli determined by the percentage of responding larvae in Tg[HuC::hTauP301L] embryos (Hu::Tau) and in Tg[HuC::hTauP301L] embryos following depletion of hs3st2 (Hu::Tau + MO-hs3st2) (n = 150); error bars show SEM.
Discussion
We show that HS3ST2 is overexpressed in the hippocampus of patients with Alzheimer’s disease and that intracellular 3-O-sulphated heparan sulphates are critical for the abnormal phosphorylation of both wild-type tau and tau carrying the P301L mutation in cells and in a zebrafish model of tauopathy. Although the occurrence of the intracellular accumulation of heparan sulphates long before the development of the tau pathology was known in neurons of patients with Alzheimer’s disease and those with Down’s syndrome for several years (Snow et al., 1990; Goedert et al., 1996), the mechanisms, biological significance, and pathophysiological consequences of this event remain unknown. Similarly, although HS3ST2 expression occurs in neurons of brain regions vulnerable to Alzheimer’s disease, the involvement of neuronal 3-O-sulphated heparan sulphates and of their 3-O-sulphotransferase synthetizing enzymes in the mechanisms leading to tau pathology had never been investigated. Here, we show that under pathology-associated conditions, such as hTauP301L expression or oxidative stress, membrane-associated heparan sulphate uptake occurs concomitantly with tau hyperphosphorylation, delineating a link between membrane biology, heparan sulphate biology, and tau pathology. This link is supported by several observations associating HSPG with altered-membrane biology in Alzheimer’s disease, for instance: (i) the intracellular co-staining of membrane-associated HSPG, but not of secreted HSPGs, with neurofibrillary tangles in Alzheimer’s disease (van Horssen et al., 2003); (ii) the heparan sulphate-dependent endocytosis of amyloid-β oligomers (Sandwall et al., 2010), tau fibrils (Holmes et al., 2013), apolipoprotein-E (Wilsie et al., 2006), and other proteins for which heparan sulphates are cell-surface endocytic receptors (Christianson and Belting, 2014); (iii) the relevance in lipid rafts of HSPG, which act as molecular mediators of raft-dependent endocytosis (Chen and Williams, 2013); (iv) the heparan sulphates capacity to interact and regulate the activity of membrane-associated enzymes involved in Alzheimer’s disease such as the raftophilic β-secretase (Patey et al., 2006). Moreover, the 3-O-sulphated heparan sulphates requirement for HSV1 uptake by neuronal cells (O'Donnell et al., 2006; Antoine et al., 2014) supports the existence of the proposed synergy between HSV1 infection and Alzheimer’s disease pathology (Carter, 2008). Finally, the central importance of an altered neuronal membrane biology in Alzheimer’s disease (Williamson and Sutherland, 2011) is strongly supported by the striking observation that most, if not all, Alzheimer’s disease associated loci identified by genome-wide association studies (GWAS) including ABCA7, MS4A6A/MS4A4E, EPHA1, CD33, CD2AP, CLU, PICALM, CR1, BIN1, and SNORA7A (Hollingworth et al., 2011), are most of them, as APP, PSEN1, and PSEN2, genetic variants of membrane-associated proteins. Whether these genetic variants, as well as an altered tau biology (Stamer et al. 2002; Ke et al. 2012), can provoke cell-membrane alterations allowing increased heparan sulphate internalization, are important points that remain to be explored.
Concerning the implication of intracellular heparan sulphates in the molecular mechanisms leading to the abnormal phosphorylation of tau, our data suggest that the 3-O-sulphated glycosaminoglycans bind to tau in a molecular chaperon-like manner allowing the tau abnormal phosphorylation. This hypothesis is supported by previous 1H NMR (proton nuclear magnetic resonance) and biochemical cross-linking studies showing that heparin readily induces conformational changes in tau allowing the access of different kinases to epitopes otherwise inaccessible for phosphorylation (Paudel and Li, 1999; Sibille et al., 2006), and further supported by our immunostaining, FRET and phosphorylation data, as well as by the intracellular accumulation of heparan sulphates observed previous to the apparition of the tau pathology in neuronal cells of Alzheimer’s disease and Down’s syndrome (Snow el al., 1990; Goedert et al., 1996). Furthermore, as the 3-O-sulphated heparan sulphates prompt the abnormal phosphorylation of both tau and tauP301L, the enhanced effect observed on tauP301L could be associated with the presence of the tau mutation (Alonso Adel et al., 2004). Thus, the higher susceptibility of tauP301L to undergo heparin-dependent abnormal phosphorylation can explain the effect of inhibiting HS3ST2 expression in SH/hTauP301L cells and of hs3st2 in Tg[HuC::hTauP301L; DsRed] zebrafish embryos, in which the levels of hs3st2 transcripts were found unchanged compared to those observed in control embryo (not shown).
Whether the central role of the abnormal phosphorylation of tau in the Alzheimer’s disease neurodegenerative process is still controverted, the possibility of a correlation between genetic variability in membrane associated-proteins, or between altered tau biology, with heparan sulphate internalization and interaction with tau, requires to be considered. This additionally raises questions concerning the intracellular association of different species of glycosaminoglycans with other intracellular heparin-binding proteins, such as α-synuclein and superoxide dismutase, proteins involved in important neurodegenerative diseases. Indeed, as for neurofibrillary tangles, sulphated glycosaminoglycans generally co-localize with amyloidosis lesions, including α-synucleinopathies and prion diseases (Zhang and Li, 2010), suggesting the existence of common events in the mechanisms involving the sulphated sugars in proteinopathies. In conclusion, our results position intracellular 3-O-sulphated heparan sulphates, and the enzymes responsible of their high sulphation in neurons, as central modulators of tau abnormal phosphorylation before it occurs. This opens a wide area of research in the field of glyco-neurobiology, positioning HS3ST2 and its products as potential key players in the development and evolution of the tau pathology, with the therapeutic consequences that this implies.
Acknowledgements
We thank to C. Haass, B. Schmid, and D. Paquet (DZNE, Munich, Germany) for providing the Tg[HuC::hTauP301L;DsRed] transgenic line, to A. Eckert (University of Basel, Switzerland) and J. Gotz (University of Queensland-Brisbane, Australia) for providing the initial vials of SH-SY5Y cells overexpressing mutant hTauP301L, and to D. Biard (CEA, Fontenay-aux-Roses, France) for his help in gene silencing experiments. We also thank to G. Carpentier for assistance in microscopy experiments, to A. Boiret, S. Christiaanse (UPEC), and P. Claus (INSERM U676) for technical assistance and to C. Romain for help mantaining zebrafish facility. We thank to B. Jacquet, O. Stettler (UPEC), and C. Duyckaerts (P. Salpetrière Hospital Brain Bank) for interesting discussions.
Glossary
Abbreviations
- dpf
days post fertilization
- FRET
fluorescence resonance energy transfer
- HSPG
heparan sulphate proteoglycan
- PKA
protein kinase A
- PP2A
protein phosphatase 2A
Funding
Authors thank to the Association France Alzheimer & Maladies Apparentées and to the SATT Idf Innov for supporting this work. J.E. Sepulveda-Diaz received the scholarship No. 308978 from CONACyT, Mexico. M.B. Huynh received scholarship from the French Ministry of Higher Education and Research. S.M. Alavi Naini received a grant from « Institute de Recherche Servier » independently of the work in this article.
Supplementary material
Supplementary material is available at Brain online.
References
- Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–81. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- Antoine TE, Yakoub A, Maus E, Shukla D, Tiwari V. Zebrafish 3-O-sulfotransferase-4 generated heparan sulfate mediates HSV1 entry and spread. PLoS One. 2014;9:e87302. doi: 10.1371/journal.pone.0087302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer's disease begin? [Review] Curr Opin Neurol. 2012;25:708–14. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- Carter CJ. Interactions between the products of the Herpes simplex genome and Alzheimer's disease susceptibility genes: relevance to pathological-signalling cascades [Review] Neurochem Int. 2008;52:920–34. doi: 10.1016/j.neuint.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Chen K, Williams KJ. Molecular mediators for raft-dependent endocytosis of syndecan-1, a highly conserved, multifunctional receptor. J Biol Chem. 2013;288:13988–99. doi: 10.1074/jbc.M112.444737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor [Review] Matrix Biol. 2014;35:51–5. doi: 10.1016/j.matbio.2013.10.004. 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Cripps D, Thomas SN, Jeng Y, Yang F, Davies P, Yang AJ. Alzheimer disease-specific conformation of hyperphosphorylated paired helical filament-Tau is polyubiquitinated through Lys-48, Lys-11, and Lys-6 ubiquitin conjugation. J Biol Chem. 2006;281:10825–38. doi: 10.1074/jbc.M512786200. [DOI] [PubMed] [Google Scholar]
- Duka V, Lee JH, Credle J, Wills J, Oaks A, Smolinsky C, et al. Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer's diseases. PloS One. 2013;8:e75025. doi: 10.1371/journal.pone.0075025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Gotz J. beta-Amyloid induces paired helical filament-like tau filaments in tissue culture. J Biol Chem. 2003;278:40162–8. doi: 10.1074/jbc.M308243200. [DOI] [PubMed] [Google Scholar]
- Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–96. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–3. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Crowther RA, Jakes R, Goedert M. Alzheimer-like changes in microtubule-associated protein Tau induced by sulfated glycosaminoglycans. Inhibition of microtubule binding, stimulation of phosphorylation, and filament assembly depend on the degree of sulfation. J Biol Chem. 1997;272:33118–24. doi: 10.1074/jbc.272.52.33118. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Hashiguchi T. Kinase-kinase interaction and modulation of tau phosphorylation [Review] Int Rev Cell Mol Biol. 2013;300:121–60. doi: 10.1016/B978-0-12-405210-9.00004-7. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Perez M, Lucas JJ, Avila J. Sulfo-glycosaminoglycan content affects PHF-tau solubility and allows the identification of different types of PHFs. Brain Res. 2002;935:65–72. doi: 10.1016/s0006-8993(02)02455-1. [DOI] [PubMed] [Google Scholar]
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA. 2013;110:E3138–47. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–35. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh MB, Villares J, Díaz JE, Christiaans S, Carpentier G, Ouidja MO, et al. Glycosaminoglycans from aged human hippocampus have altered capacities to regulate trophic factors activities but not Abeta42 peptide toxicity. Neurobiol Aging. 2012;33:1005, e11–22. doi: 10.1016/j.neurobiolaging.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies [Review] Curr Alzheimer Res. 2010;7:656–64. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, et al. [Review]. Lessons from tau-deficient mice. Int J Alzheimers Dis. 2012;2012:873270. doi: 10.1155/2012/873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Kelley MJ, Acott TS. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49:2495–505. doi: 10.1167/iovs.07-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kreuger J, Kjellén L. Heparan sulfate biosynthesis: regulation and variability. [Review] J Histochem Cytochem. 2012;60:898–907. doi: 10.1369/0022155412464972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R, Yabe T, Hajmohammadi S, Rhodes J, McNeely M, Liu J, et al. The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues. Matrix Biol. 2007;26:442–55. doi: 10.1016/j.matbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen X, Ye QZ, Vogt A, Yin XM. A high-throughput FRET-based assay for determination of Atg4 activity. Autophagy. 2012;8:401–12. doi: 10.4161/auto.18777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Yoshida K, Shibata Y, Kimata K. Tetrasulfated disaccharide unit in heparan sulfate: enzymatic formation and tissue distribution. J Biol Chem. 2008;283:31237–45. doi: 10.1074/jbc.M801586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell CD, Tiwari V, Oh MJ, Shukla D. A role for heparan sulfate 3-O-sulfotransferase isoform 2 in herpes simplex virus type 1 entry and spread. Virology. 2006;346:452–9. doi: 10.1016/j.virol.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Paquet D, Bhat R, Sydow A, Mandelkow EM, Berg S, Hellberg S, et al. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest. 2009;119:1382–95. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patey SJ, Edwards EA, Yates EA, Turnbull JE. Heparin derivatives as inhibitors of BACE-1, the Alzheimer's beta-secretase, with reduced activity against factor Xa and other proteases. J Med Chem. 2006;49:6129–32. doi: 10.1021/jm051221o. [DOI] [PubMed] [Google Scholar]
- Paudel HK, Li W. Heparin-induced conformational change in microtubule-associated protein Tau as detected by chemical cross-linking and phosphopeptide mapping. J Biol Chem. 1999;274:8029–38. doi: 10.1074/jbc.274.12.8029. [DOI] [PubMed] [Google Scholar]
- Safaiyan F, Kolset SO, Prydz K, Gottfridsson E, Lindahl U, Salmivirta M. Selective effects of sodium chlorate treatment on the sulfation of heparan sulfate. J Biol Chem. 1999;274:36267–73. doi: 10.1074/jbc.274.51.36267. [DOI] [PubMed] [Google Scholar]
- Sandwall E, O'Callaghan P, Zhang X, Lindahl U, Lannfelt L, Li JP. Heparan sulfate mediates amyloid-beta internalization and cytotoxicity. Glycobiology. 2010;20:533–41. doi: 10.1093/glycob/cwp205. [DOI] [PubMed] [Google Scholar]
- Shriver Z, Capila I, Venkataraman G, Sasisekharan R. Heparin and heparan sulfate: analyzing structure and microheterogeneity [Review] Handb Exp Pharmacol. 2012;207:159–76. doi: 10.1007/978-3-642-23056-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula [Review] J Mol Med. 2006;84:901–10. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shworak NW, Liu J, Petros LM, Zhang L, Kobayashi M, Copeland NG, et al. Multiple isoforms of heparan sulphate D-glucosaminyl 3-O-sulfotransferase. Isolation, characterization, and expression of human cDNAs and identification of distinct genomic loci. J Biol Chem. 1999;274:5170–84. doi: 10.1074/jbc.274.8.5170. [DOI] [PubMed] [Google Scholar]
- Sibille N, Sillen A, Leroy A, Wieruszeski JM, Mulloy B, Landrieu I, et al. Structural impact of heparin binding to full-length Tau as studied by NMR spectroscopy. Biochemistry. 2006;45:12560–72. doi: 10.1021/bi060964o. [DOI] [PubMed] [Google Scholar]
- Snow AD, Mar H, Nochlin D, Sekiguchi RT, Kimata K, Koike Y, et al. Early accumulation of heparan sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer's disease and Down's syndrome. Am J Pathol. 1990;137:1253–70. [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Goedert M. Tau pathology and neurodegeneration [Review] Lancet Neurol. 2013;12:609–22. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156:1051–63. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilb ML, Dias-Santagata D, Fulga TA, Felch DL, Feany MB. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol Biol Cell. 2007;18:5060–8. doi: 10.1091/mbc.E07-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Dam GB, Kurup S, van de Westerlo EM, Versteeg EM, Lindahl U, Spillmann D, et al. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem. 2006;281:4654–62. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
- Thacker BE, Xu D, Lawrence R, Esko JD. Heparan sulfate 3-O-sulfation: a rare modification in search of a function [Review] Matrix Biol. 2014;35:60–72. doi: 10.1016/j.matbio.2013.12.001. 10.1016/j.matbio.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–79. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Wesseling P, van den Heuvel LP, de Waal RM, Verbeek MM. Heparan sulphate proteoglycans in Alzheimer's disease and amyloid-related disorders [Review] Lancet Neurol. 2003;2:482–92. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam KV. Human 3'-phosphoadenosine 5'-phosphosulfate (PAPS) synthase: biochemistry, molecular biology and genetic deficiency [Review] IUBMB Life. 2003;55:1–11. doi: 10.1080/1521654031000072148. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. [Review] J Alzheimers Dis. 2013;33(Suppl 1):S123–39. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Neuropathologic changes in Alzheimer's disease [Review] J Clin Psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- Wilsie LC, Gonzales AM, Orlando RA. Syndecan-1 mediates internalization of apoE-VLDL through a low density lipoprotein receptor-related protein (LRP)-independent, non-clathrin-mediated pathway. Lipids Health Dis. 2006;5:23. doi: 10.1186/1476-511X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau [Review] Intern Med J. 2006;36:652–60. doi: 10.1111/j.1445-5994.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- Williamson R, Sutherland C. Neuronal membranes are key to the pathogenesis of Alzheimer's disease: the role of both raft and non-raft membrane domains [Review] Curr Alzheimer Res. 2011;8:213–21. doi: 10.2174/156720511795256008. [DOI] [PubMed] [Google Scholar]
- Wu X, Jiang X, Marini AM, Lipsky RH. Delineating and understanding cerebellar neuroprotective pathways: potential implication for protecting the cortex [Review] Ann N Y Acad Sci. 2005;1053:39–47. doi: 10.1196/annals.1344.004. [DOI] [PubMed] [Google Scholar]
- Yabe T, Hata T, He J, Maeda N. Developmental and regional expression of heparan sulphate sulfotransferase genes in the mouse brain. Glycobiology. 2005;15:982–993. doi: 10.1093/glycob/cwi090. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38delta or JNK2 in the presence of heparin generates the AT100 epitope. J Neurochem. 2006;99:154–64. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhöfer Q, Biernat J, Mandelkow EM, Illenberger S, Godemann R, Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li JP. Heparan sulfate proteoglycans in amyloidosis [Review] Progr Mol Biol Transl Sci. 2010;93:309–34. doi: 10.1016/S1877-1173(10)93013-5. [DOI] [PubMed] [Google Scholar]
- Zhang G, Morin C, Zhu X, Bao Huynh M, Ouidir Ouidja M, Sepulveda-Diaz JE, et al. Self-evolving oxidative stress with identifiable pre- and postmitochondrial phases in PC12 cells. J Neurosci Res. 2013;91:273–84. doi: 10.1002/jnr.23146. [DOI] [PubMed] [Google Scholar]