Responses to L-dopa vary within and between individuals. Moreau et al. reveal that polymorphisms in the dopamine transporter gene (SLC6A3) influence the response to L-dopa and to the dopamine transporter inhibitor methylphenidate. This may help physicians to optimise L-dopa dose and to assess the risk/benefit balance for treatment with methylphenidate.
Keywords: Parkinson’s disease, pharmacogenetics, levodopa, cognitive disorders, methylphenidate
Responses to L-dopa vary within and between individuals. Moreau et al. reveal that polymorphisms in the dopamine transporter gene (SLC6A3) influence the response to L-dopa and to the dopamine transporter inhibitor methylphenidate. This may help physicians to optimise L-dopa dose and to assess the risk/benefit balance for treatment with methylphenidate.
Abstract
After more than 50 years of treating Parkinson’s disease with l-DOPA, there are still no guidelines on setting the optimal dose for a given patient. The dopamine transporter type 1, now known as solute carrier family 6 (neurotransmitter transporter), member 3 (SLC6A3) is the most powerful determinant of dopamine neurotransmission and might therefore influence the treatment response. We recently demonstrated that methylphenidate (a dopamine transporter inhibitor) is effective in patients with Parkinson’s disease with motor and gait disorders. The objective of the present study was to determine whether genetic variants of the dopamine transporter type 1-encoding gene (SLC6A3) are associated with differences in the response to treatment of motor symptoms and gait disorders with l-DOPA and methylphenidate (with respect to the demographic, the disease and the treatment parameters and the other genes involved in the dopaminergic neurotransmission). This analysis was part of a multicentre, parallel-group, double-blind, placebo-controlled, randomized clinical trial of methylphenidate in Parkinson’s disease (Protocol ID:2008-005801-20; ClinicalTrials.gov:NCT00914095). We scored the motor Unified Parkinson's Disease Rating Scale and the Stand-Walk-Sit Test before and after a standardized acute l-DOPA challenge before randomization and then after 3 months of methylphenidate treatment. Patients were screened for variants of genes involved in dopamine metabolism: rs28363170 and rs3836790 polymorphisms in the SLC6A3 gene, rs921451 and rs3837091 in the DDC gene (encoding the aromatic L-amino acid decarboxylase involved in the synthesis of dopamine from l-DOPA), rs1799836 in the MAOB gene (coding for monoamine oxidase B) and rs4680 in the COMT gene (coding for catechol-O-methyltransferase). Investigators and patients were blinded to the genotyping data throughout the study. Eighty-one subjects were genotyped and 61 were analysed for their acute motor response to l-DOPA. The SLC6A3 variants were significantly associated with greater efficacy of l-DOPA for motor symptoms. The SLC6A3 variants were also associated with greater efficacy of methylphenidate for motor symptoms and gait disorders in the ON l-DOPA condition. The difference between motor Unified Parkinson's Disease Rating Scale scores for patients with different SLC6A3 genotypes was statistically significant in a multivariate analysis that took account of other disease-related, treatment-related and pharmacogenetic parameters. Our preliminary results suggest that variants of SLC6A3 are genetic modifiers of the treatment response to l-DOPA and methylphenidate in Parkinson’s disease. Further studies are required to assess the possible value of these genotypes for (i) guiding l-DOPA dose adaptations over the long term; and (ii) establishing the risk/benefit balance associated with methylphenidate treatment for gait disorders.
Introduction
Parkinson’s disease affects >1% of the population over the age of 60 (de Lau and Breteler, 2006). The loss of dopamine production in the striatum (as a result of progressive neuronal degeneration in the substantia nigra pars compacta) is the primary chemical disease marker for Parkinson’s disease (Chaudhuri et al., 2009). Since the introduction of the dopamine precursor l-DOPA in 1969, treatment with this drug has remained the most effective therapeutic strategy in Parkinson’s disease (Nutt et al., 2008). However, the practical value of long-term oral l-DOPA administration is hampered by interindividual variations in the treatment response (i.e. the absolute level of improvement on l-DOPA) (Hauser et al., 2009) and intraindividual variations (i.e. motor fluctuations over time). Moreover, there are still no guidelines for determining the optimal l-DOPA dose in a given patient, which increases the likelihood of incorrect dosage. Better knowledge of pharmacogenetic factors involved in dopamine neurotransmission might enable physicians to determine l-DOPA doses and thus limit l-DOPA-related motor complications.
The occurrence of l-DOPA-related motor complications is related to the progression of neurodegeneration, the absolute dose of l-DOPA and the pulsatile administration of what is a short half-life drug (Fabbrini et al., 1988; de la Fuente-Fernández et al., 2004). In selected patients, the main treatment for l-DOPA-related motor complications is deep brain stimulation of the subthalamic nucleus (Krack et al., 2003). Although subthalamic nucleus stimulation improves a patients’ quality of life, the long-term benefits are often reduced by the development of incapacitating gait disorders (Krack et al., 2003). These disorders include gait hypokinesia (slow walking, with a short step length) and freezing of gait (a brief, episodic absence or notable reduction of forward progression of the feet, despite the intention to walk) (Nutt et al., 2011). We recently demonstrated that chronic, high-dose (1 mg/kg/day) administration of methylphenidate (an inhibitor of SLC6A3) reduces the severity of gait disorders in subthalamic nucleus-stimulated patients with advanced Parkinson’s disease (Moreau et al., 2012). In fact, we observed a greater effect in the OFF l-DOPA condition than in the ON l-DOPA condition (i.e. after an acute l-DOPA challenge). Pharmacogenetic factors (particularly those related to SLC6A3) might thus partly explain the heterogeneity of the response to methylphenidate, especially in the ON condition.
We therefore decided to investigate genetic polymorphisms known to have a functional impact on dopamine metabolism. Most of the presynaptic dopamine reuptake occurs through SLC6A3, which is the most powerful determinant of dopamine neurotransmission (Gainetdinov et al., 1998). The SLC6A3 protein (previously known as DaT1) is encoded by the SLC6A3 gene, which is characterized by repetition alleles [with 3–11 repeats of the 40-bp variable number tandem repeat (VNTR) in the 3'UTR]. The 9- and 10-repeat alleles are the most frequent in patients with Parkinson’s disease (Vandenbergh et al., 1992; Uhl, 2003). However, the functional effect of the SLC6A3 3'UTR VNTR has yet to be established. Hence, we also selected another frequent, functional genetic variant: the SLC6A3 intron 8 VNTR (rs3836790, Int8 VNTR), with two common alleles of five and six repeats. It has been reported that Int8 VNTR is significantly associated with cocaine dependence (Guindalini et al., 2006). At present, there are no literature data to suggest that the distribution of SLC6A3 rs3836790 differs when comparing patients with Parkinson’s disease and control subjects; all of the studies of SLC6A3 rs28363170 have been negative, with the exception of a recent report of a weak, positive association (Supplementary Table 1).
When seeking to analyse the influence of SLC6A3 genotypes, it is essential to control for possible interactions with other genetic factors that are known (or suspected) to influence dopamine metabolism. The major pathway for the synthesis of dopamine from l-DOPA in vivo is decarboxylation by aromatic L-amino acid decarboxylase (encoded by the DDC gene) (Berry et al., 1996). We previously demonstrated that the overall reduction in the severity of motor handicap during an acute l-DOPA challenge [the area under curve of the Unified Parkinson's Disease Rating Scale (UPDRS) score, measured every 15–30 min] was influenced by the DDC gene variants rs921451 and rs3837091 (Devos et al., 2014). The main enzymatic pathways in dopamine metabolism involve monoamine oxidase B (MAOB, encoded by the MAOB gene) and catechol-O-methyltransferase (COMT, encoded by the COMT gene). One of the main variants involved in l-DOPA metabolism is the rs4680 VAL158MET in the COMT gene; this variant modifies the enzyme activities and is known to influence the age at onset of Parkinson’s disease (Klebe et al., 2013). Another frequent gene variant involved in l-DOPA metabolism is rs1799836, in intron 13 of the MAOB gene (Harris et al., 1993). Although the A→G substitution does not change the protein sequence, it is associated with differences in enzyme activity in vitro and in vivo. It has been shown that MAOB activity is higher in G allele carriers than in A allele carriers (Costa-Mallen et al., 2005). The influence of these COMT and MAOB genotypes on the l-DOPA response is subject to debate (Lee et al., 2001; Watanabe et al., 2003; Białecka et al., 2004, 2008; Contin et al., 2005; Corvol et al., 2011).
We hypothesized that it would be easier to study the association between genotypes and the motor response to l-DOPA during a very standardized, acute l-DOPA challenge than during chronic l-DOPA administration (as the latter is subject to many sources of bias). This assessment might therefore constitute the first step in the investigation of using pharmacogenetic information to guide l-DOPA dose adjustments in clinical practice. Although methylphenidate can reduce the severity of severe gait disorders and the frequency of freezing of gait in Parkinson’s disease (Moreau et al., 2012), the drug’s potential cardiac risk and its variable effects in patients (Devos et al., 2013) make it a good candidate for personalized, pharmacogenetics-based treatment in Parkinson’s disease. To the best of our knowledge, there are no published reports on the pharmacogenetic modulation of the motor response to l-DOPA and methylphenidate.
Materials and methods
The pharmacogenetic analysis was planned and performed as part of a multicentre, parallel-group, double-blind, placebo-controlled, randomized clinical trial of methylphenidate in Parkinson’s disease (Protocol ID:2008-005801-20; ClinicalTrials.gov:NCT00914095) (Moreau et al., 2012), which was reported in line with the 2010 CONSORT guidelines. The main aspects of the trial are summarized below.
Participants
Patients were prospectively enrolled by 13 movement disorders departments throughout France. We included under-80 adults with Parkinson’s disease [diagnosed according to Gibb’s criteria (1998)], subthalamic nucleus stimulation, and mild-to-severe gait disorders (for details, see Moreau et al., 2012). Briefly, we defined gait disorders as gait hypokinesia (subscore ≥2 for UPDRS part II item 15) and freezing of gait (subscore ≥2 for UPDRS part II item 14) in the OFF condition, and score of 2 or more for UPDRS part III item 30 on gait in the ON condition. The main exclusion criteria were gait disorders possibly induced by subthalamic nucleus stimulation, any change in subthalamic nucleus stimulation variables or dopaminergic therapy 90 days before the study or during the study, inability to walk without continuous ambulatory assistance while on treatment, dementia (diagnosed in accordance with the Movement Disorders Society criteria; Emre et al., 2007), progressing axis I psychiatric disorders (as assessed in a semi-structured interview with a trained psychiatrist), serious or unstable medical disorders, and ongoing treatment with sympathomimetics, monoamine oxidase inhibitors, or opiates. All patients provided written, informed consent to participation before the initiation of any study procedures. The study protocol was approved by the local independent ethics committee of CHRU of Lille approved in 2008 (Protocol ID: 2008-005801-20; ClinicalTrials.gov reference: NCT00914095).
Procedures
The acute levodopa challenge
We performed a standardized, acute l-DOPA challenge in the fasting state and in both OFF and ON l-DOPA conditions. To avoid bias, none of the subthalamic nucleus stimulation parameters were modified, and stimulation was continued throughout the study (including the two acute levodopa challenges). The OFF condition was performed first (at 8.30 am), after overnight withdrawal of l-DOPA and 24 h of withdrawal of dopaminergic agonists. The ON condition was then assessed at 9.30 am. The l-DOPA was administered at 9.00 am and corresponded to 150% of the usual morning l-DOPA equivalent dose used by patients to relieve their symptoms (i.e. the first morning dose of l-DOPA, plus the l-DOPA equivalent dose of the first morning dose of dopamine agonist, plus an additional 50 mg of l-DOPA). The l-DOPA used was immediate-release l-DOPA with 25% benserazide (Roche).
The randomized clinical trial
Patients received placebo or a dose of 1 mg/kg/day methylphenidate (four to eight 10 mg tablets) divided into three doses (at 8.00 am, noon and 4.00 pm). An initial 4-week titration period was used, with an increment of 0.25 mg/kg per week. Tolerability and compliance (assessed in an interview and via pill counts) were checked every 2 weeks. In the event of poor tolerance, the titration phase was extended by 1 week, the dosage was reduced to 0.8 mg/kg/day and study centres were asked to achieve and maintain the highest possible tolerated dose (at least 0.8 mg/kg/day). The last dose of study treatment was administered at 7.00 am on Day 90.
Randomization was balanced by centre. The 1:1 assignment sequence (based on a block size of four and the use of a computer random-number generator) was produced by the Statistics Department at Lille University Hospital. The randomization list was sent to an independent contract research organization (LC2) for preparation and distribution of identical capsules of methylphenidate and placebo. Patients, carers, study staff and investigators were blinded to the group assignments.
Efficacy criteria
We investigated the associations between SLC6A3 rs3836790 (the VNTR in intron 8), SLC6A3 rs28363170 (the VNTR in the 3'UTR), DDC rs921451 (T>C), DDC rs3837091 (insdel AGAG), COMT rs4680 (Val158Met), and MAOB rs1799836 gene variants on one hand and the motor and gait responses to l-DOPA on the other (defined as the difference between the OFF and ON scores during the acute l-DOPA challenge at baseline, i.e. before randomization). The efficacy criteria were the motor UPDRS score (part III), and the number of steps (the trial’s primary efficacy criterion) and the completion time in the Stand-Walk-Sit Test (which involves standing up, making a 14 m round trip, and sitting down as quickly as possible; Martínez-Martín et al., 1997). We also rated the number of episodes of freezing of gait during a sensitive trajectory (Snijders et al., 2012). The participants performed a standardized gait trajectory with the usual triggers of freezing of gait, composed by gait initiation and termination (self-triggered, cued), narrow turning (360° and 540°turns in different directions, at preferred and maximal speed), walking through narrow space and dual tasking (walking and subtracting serials of three). We took into account freezing of gait severity evaluated by the number and duration of freezing of gait episodes (short episodes of freezing of gait <10 s were rated 1, medium episodes between 10 and 30 s were rated 2, episodes >30 s were rated 3) during the freezing of gait trajectory. The freezing episodes were rated by each investigator in each centre and controlled on the video recording.
The associations between SLC6A3, DCC, MAOB, and COMT genotypes on one hand and the response to methylphenidate on the other was analysed in terms of the changes in motor and gait criteria after 90 days of methylphenidate treatment, as defined by the difference in the above mentioned efficacy criteria between inclusion (Day 0) and Day 90 in both OFF and ON conditions, respectively (to control for the confounding effects of l-DOPA) and with respect to the placebo group. We also tested patients for the association between genotypes and the methylphenidate’s effect on gait, as the SLC6A3 variants and COMT Val158Met are reportedly correlated with basal dopaminergic prefrontal activity and thus might interact with the l-DOPA response (Frank et al., 2009; Hoogland et al., 2010).
All measurements of efficacy criteria were reported on a study-specific case report form in each centre and were subsequently double-checked in a blinded video assessment by two neurologists (C.M. and A.D.). If the video data differed from the case report form data, the mean offline video rating was used in the analysis.
Genotyping
Genomic DNA was extracted from venous blood samples using standard procedures. The SLC6A3 rs28363170 variant was genotyped by PCR in a total volume of 25 µl of AmpliTaq® PCR buffer, containing 50 ng genomic DNA, one unit of AmpliTaq® polymerase (Applied Biosystems), 1.5 mM MgCl2, 250 µM dNTPs, and 15 pmol of the forward primer (5′GGTGTAGGGAACGGCCTGAGAG3′) and the reverse primer (5′-CTTCCTGGACACGGCTCAAGG-3′). The cycling conditions for amplification were as follows: 5 min at 96°C, 35 cycles of 1 min at 94°C, 30 s at 65°C and 1 min at 72°C, and then an extra 5 min at 72°C. Amplification products were resolved in 2% agarose gels. The SLC6A3 rs3836790 was genotyped under the same PCR conditions, with 15 pmol of forward primer (5′-GCACAAATGAGTGTTCGTGCATGTG-3′) and reverse primer (5′-AGCAGGAGGGGCTTCCAGGC-3′). We then used 5% polyacrylamide gel electrophoresis to separate the amplification products. The DDC rs921451 was analysed by Sanger sequencing following PCR amplification of a 216 bp DNA fragment, using a 3130XL DNA analyser (Applied Biosystems). The DDC rs3837091 was characterized by measuring the size of the products following DNA amplification by PCR in a total volume of 25 µl of AmpliTaq PCR buffer, containing 50 ng genomic DNA, one unit of AmpliTaq polymerase, 1.5 mM MgCl2, 250 µM dNTPs, and 15 pmol of forward primer (5′-GGCAATCACATCTTCTGTGC-3′) and reverse primer (5′-GAGCCATGAGGACAAAGAGC-3′). The cycling conditions for amplification were as follows: 5 min at 96°C, 30 cycles of 30 s at 94°C, 1 min at 57°C and 2 min at 72°C and then an extra 5 min at 72°C. On 2% agarose gels, amplification of the two alleles yielded 112 and 108 bp bands, respectively. For each patient, two control experiments were analysed concomitantly for each genotype. The catechol-O-methyltransferase (COMT) VAL158MET variant (rs4608) was analysed using a polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay, as described previously (Kunugi et al., 1997) The MAOB rs1799836 was analysed using a PCR-RFLP assay in a total volume of 25 µl of AmpliTaq PCR buffer containing 50 ng genomic DNA, one unit of AmpliTaq polymerase (Applied Biosystems), 1.50 mM MgCl2, 250 µM dNTPs, and 15 pmol of forward primer (5′-TTCTGGCCTTTACCTTGGTG-3′) and reverse primer (5′-GCCAGATTTCATCCTCTGGA-3′). The cycling conditions involved 5 min at 96°C, followed by 30 cycles of 30 s at 94°C, 2 min at 59°C and 1 min at 72°C and an extra 5 min at 72°C. Amplification products were resolved in 5% acrylamide gels. Tsp45I endonuclease was used to characterize the A and G alleles of MAOB rs1799836.
SLC6A3 density analysis
At the study centre in Lille, we used 123IFP-CIT (Ioflupane) single photon emission computed tomography (SPECT) to examine whether or not methylphenidate reduced SLC6A3 binding in the striatum of patients with Parkinson’s disease—a phenomenon that would be suggestive of a dopaminergic, pharmacodynamic action in Parkinson’s disease—and the putative effect of SLC6A3 genotypes on this reduction. The SLC6A3 density analysis was performed in the morning on non-fasted patients taking their usual dopaminergic treatment, once before treatment initiation and then on Day 91 of the treatment period. The mean presynaptic SLC6A3 density 4 h after intravenous injection of 92 MBq of 123IFP-CIT was measured on a SPECT system (DaTSCAN™, GE Healthcare) equipped with a Symbia S gamma camera (Siemens). Data acquisition and analysis were performed according to the European Association of Nuclear Medicine guidelines. A total of 120 40-s projections were acquired for each patient. Images were analysed using the Flash 3D iterative reconstruction algorithm (Siemens) with 10 iterations, eight subsets and a Gaussian pre-filter (6 mm full-width at half-maximum, yielding a pixel size of 3.3 mm). The SPECT results were compared after data registration using a mutual information algorithm, with masking of non-brain uptake and normalization against the maximum uptake value. The data were evaluated visually by two nuclear medicine physicians (G.P. and C.H.F., who were blinded to the clinical observations). A quantitative analysis was performed on the binding potential of each striatum (computed from rectangular regions of interest and after series registration).
Statistical analysis
In view of the sample size and the skewed distributions with extreme values for most of the efficacy criteria, all quantitative variables are expressed as the median (interquartile range). Qualitative variables are expressed as the number (percentage). Comparisons in efficacy criteria were performed using a non-parametric Kruskal-Wallis or Wilcoxon tests. Associations between genotypes and efficacy criteria were first studied with a co-dominant model. The Tukey-Kramer method was used to test differences between genotypes. Linkage disequilibrium and haplotype structures were determined using Haploview software (Broad Institute: www.broadinstitute.org). Hardy-Weinberg equilibrium was tested with both Haploview software and a chi-squared test with Yates’ correction. Associations between haplotypes and continuous traits were assessed with Haplotype Trend Regression software (Zaykin et al., 2002).
All comparisons were adjusted first for the total dose of l-DOPA and then for age, gender, weight, daily dose of l-DOPA and disease duration. Theses prespecified adjustments were made using non-parametric analysis of covariance (Bergersen et al., 2011). In view of our study's exploratory nature and small sample size, we did not adjust for multiple comparisons. Statistical testing was done at two-tailed α-level of 0.05. Data were analysed with SAS version 9.3 (SAS Institute).
Results
Characteristics of the study population
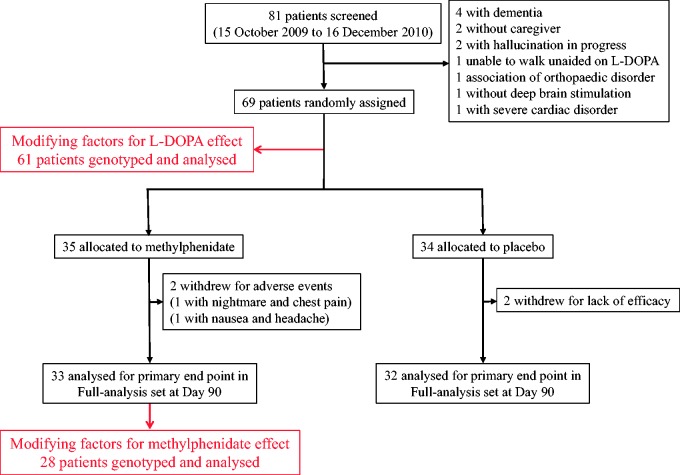
Between 15 October 2009 and 16 December 2011, a total of 81 patients with Parkinson’s disease were screened for inclusion. Of these, 69 patients with severe gait disorders and freezing of gait (despite optimized, stable l-DOPA and subthalamic nucleus stimulation parameters) were prospectively enrolled. Sixty-one of the included patients were genotyped (Fig. 1). The methylphenidate and placebo groups were well balanced in terms of baseline characteristics (Table 1). On the basis of patient and caregiver interviews and fortnightly pill counts, treatment compliance was >90% for all patients, with the exception of three individuals in the placebo group and two in the methylphenidate group with values between 90% and 70%. The mean l-DOPA equivalent daily dose was 710 ± 90.8 mg/day in the methylphenidate group and 720 ± 100 mg/day in the placebo group. The data obtained from the case report form did not differ significantly from those in the video assessment: a difference of ± 2 steps was only recorded for four patients with a high number of steps (two patients from each group).
Figure 1.
Flowchart.
Table 1.
Baseline characteristics of the subjects in each group
|
l-DOPA effect (n = 61) |
Methylphenidate effect (n = 33) |
|||
|---|---|---|---|---|
| rs38367905/5 or 5/6 | rs38367906/6 | rs38367905/5 or 5/6 | rs38367906/6 | |
| (rs283631709/9 or 9/10) | (rs2836317010/10) | (rs283631709/9 or 9/10) | (rs2836317010/10) | |
| Number of patients | 28 | 33 | 19 | 14 |
| Age, years | 63 (58–67) | 62 (59–66) | 62 (57–68) | 60 (58–64) |
| Gender ratio (F/M) | 0.75 | 0.65 | 0.65 | 0.6 |
| Body weight, kg | 79 (64–87] | 80 (73–87) | 78 (64–87) | 80 (73–92) |
| Disease duration, years | 16 (14–18] | 17 (14–22) | 16 (14–18) | 17 (12–24) |
| Subthalamic nucleus stimulation duration, years | 6 (3–8] | 6 (3–8) | 6 (3–8) | 5 (3–9) |
| Mattis DRS (dementia < 130) | 137 (133–140) | 136 (132–140) | 137 (133–140) | 137 (134–140) |
| l-DOPA dose for acute tests | 250 (200–300) | 250 (200–300) | 250 (200–300) | 250 (200–300) |
| l-DOPA daily dose | 750 (462–962) | 700 (450–825) | 700 (500–1100) | 500 (462–950) |
| l-DOPA equivalent daily dose | 875 (537–1062) | 900 (700–1000) | 700 (600–1200) | 850 (800–1200) |
| Use of dopaminergic agonists, n (%) | 16 (57) | 23 (69) | 8 (47) | 9 (81) |
| Use of entacapone, n (%) | 8 (28) | 9 (27) | 6 (35) | 3 (27) |
| Use of amantadine, n (%) | 9 (32) | 8 (24) | 6 (35) | 3 (27) |
| Mean TEED | 102 (70–155) | 101 (72–150) | 102 (72–155) | 100 (71–148) |
Baseline characteristics as taken into account when analysing the association between the SLC6A3 genotype on one hand and the acute l-DOPA response and the chronic methylphenidate treatment response on the other. There were no significant differences between groups with the SLC6A3 rs3836790 and SLC6A3 rs28363170 genotypes. The effect of l-DOPA was studied on the entire genotyped population prior to randomization (n = 61). The effect of methylphenidate was only analysed in the treated group (n = 33). The median (first quartile–third quartile) values and the number (n) and percentage (%) of patients are reported. F = female; M = male; DRS = Dementia Rating Scale. The mean total electrical energy delivered (TEED) for the bilateral subthalamic nucleus stimulation was calculated as TEED = (V2 × frequency × pulse width) / 2 (Koss et al., 2004). There was no significant intergroup difference in total electrical energy delivered. l-DOPA doses are expressed in milligrams.
The distribution of the genetic variants complied with Hardy-Weinberg equilibrium (P > 0.05) (Supplementary Tables 1–6). The allele frequencies for SLC6A3 polymorphisms did not differ when comparing our Parkinson’s disease population with 100 control subjects (data not shown). The linkage disequilibrium between SLC6A3 rs3836790 and rs28363170 was D' = 0.89 (0.73–0.97) (r2 = 0.62), and the linkage disequilibrium between DDC rs921451 and rs3837091 was D' = 0.939 (0.76–0.99) (r2 = 0.58). We did not observe any influence of the SLC6A3 genotypes or other genotypes on (i) the effectiveness of subthalamic nucleus stimulation efficacy; (ii) the levodopa dose reduction during stimulation (analysed 1 year after electrode implantation); or (iii) the stimulation parameters after 1 year and at the time of the study (data not shown).
Association between genotypes and the motor response to l-DOPA
In a co-dominant model, genotypes at SLC6A3 rs3836790 (the VNTR in intron 8) and rs28363170 (the VNTR in the 3'UTR) were associated with the response to l-DOPA (Table 2). There were two major and three minor haplotypes of rs3836790 and rs28363170. Analysis of the haplotype structures showed that rs3836790 allele 6 and rs28363170 allele 10 were on the same haplotype, as were rs3836790 allele 5 and rs28363170 allele 9, giving two major haplotypes: 6/10 and 5/9 (Supplementary Table 5). Haplotype trend regression analyses revealed that rs3836790 and rs28363170 haplotypes were associated with the response to l-DOPA (P = 0.0003) and that the association was based on the two major haplotypes. A comparison with allelic associations showed that haplotypes were not more informative than the data on rs3836790 alone. Thus, only rs3836790 was further investigated. Regarding the relationship between rs3836790 and the response to l-DOPA, a Tukey-Kramer test revealed a significant difference between 6/6 and 5/6 genotypes (P < 0.0001), but not between the 5/5 genotype and the other genotypes. In view of the mean response to l-DOPA (a score of 14.0) for the three patients with the 5/5 genotype relative to the mean responses for the 5/6 and 6/6 genotypes (12.2 and 17.2, respectively), we chose to pool 5/5 and 5/6 patients and compare them with 6/6 patients. Using this model for a recessive allele 6, a significant association (P < 0.0001) with the response to l-DOPA was detected when comparing 6/6 genotypes (mean ± SD: 17.2 ± SD) with 5/5 and 5/6 genotypes (mean ± SD: 12.3 ± 2.5).
Table 2.
Effect of genotypes on the UPDRS motor score improvement following administration of l-DOPA
|
Genotypes at SLC6A3 | |||||
|---|---|---|---|---|---|
| rs3836790 (the VNTR in intron 8) | 5/5 (n = 3) | 5/6 (n = 25) | 6/6 (n = 33) | - | P-value |
| Response to l-DOPA | 14.0 ± 1.0 | 12.2 ± 2.6 | 17.2 ± 4.0 | <0.0001 | |
| rs28363170 (VNTR in 3'UTR) | 10/10 (n = 32) | 9/10 (n = 21) | 5/9 (n = 1) | 9/9 (n = 5) | |
| Response to l-DOPA | 16.8 ± 3.9 | 12.4 ± 3.4 | 14 | 13.8 ± 3.5 | 0.0003 |
Mean (SD) difference in the motor UPDRS score when comparing OFF and ON conditions.
In a multivariate analysis, this association was still statistically significant when adjusted for age, gender, weight, disease duration and l-DOPA equivalent daily dose (P = 0.0004).
In co-dominant models, there were no significant associations between the response to l-DOPA on one hand and the DDC rs921451, rs3837091, MAOB rs1799836, and COMT rs4680 variants on the other (with P-values ranging from 0.402 to 0.748) (Supplementary Table 6).
Associations between genotypes and improvement of gait by l-DOPA
In a co-dominant models, the genotypes at SLC6A3 rs3836790 were significantly associated with the number of freezing of gait episodes (P = 0.0132) but not with the number of steps (P = 0.062) and the completion time (P = 0.0551) (Table 3). In view of the low number of patients (n = 3) with the 5/5 genotype, associations between the number of freezing of gait episodes, the number of steps, the completion time and genotypes were analysed in a recessive model (i.e. 6/6 versus 5/5 + 5/6 genotypes). The analysis revealed that the SLC6A3 rs3836790 genotype was significantly associated with the number of freezing of gait episodes, the number of steps and the completion time (with P-values of 0.004, 0.02 and 0.016, respectively).
Table 3.
Associations between the SLC6A3 rs3836790 genotype (Int8 VNTR) and changes in the severity of gait disorders during l-DOPA treatment
|
SLC6A3 |
||||
|---|---|---|---|---|
| Parameter | Condition | 5/5 or 5/6 (n = 28) | 6/6 (n = 33) | P*,† |
| Number of steps in the SWS | ||||
| OFF | 33 (28–53) | 34 (27–40) | ||
| ON | 34 (25–42) | 25 (20–28) | ||
| Difference (%) | 9.2 (–4.6–29.7) | 27.6 (17.2–34.3) | 0.001/<0.001 | |
| SWS completion time, s | ||||
| OFF | 23 (19–58) | 25 (18–34) | ||
| ON | 19 (15–33) | 15 (11–19) | ||
| Difference (%) | 10.2 (4.8–32.4) | 42.1 (18.9–56.6) | 0.002/0.001 | |
| Number of freezing of gait episodes during the freezing of gait trajectory | ||||
| OFF | 7 (5–12) | 6 (4–10) | ||
| ON | 5 (2–10) | 2 (1–5) | ||
| Difference (%) | 11.8 (0.0–57.3) | 50.0 (25.0–83.3) | 0.005/0.005 | |
Data are reported as the median (interquartile range) values in the OFF and ON conditions and the difference between the two. *In a Wilcoxon test; †In a non-parametric covariance analysis adjusted for the dose of l-DOPA. SWS = Stand-Walk-Sit Test.
A multivariate analysis adjusted for the dose of l-DOPA gave similar significant results for the number of steps (P = 0.0009), the completion time (P = 0.001) and the number of freezing of gait episodes (P = 0.005). A multivariate analysis adjusted for age, gender, weight, disease duration and l-DOPA equivalent daily dose (or l-DOPA daily dose) revealed significant associations for the number of steps (P = 0.0005) the completion time (P = 0.001) and the number of freezing of gait episodes (P = 0.004).
In co-dominant models, there were no significant associations between the number of steps, the completion time, and the number of freezing of gait episodes on one hand and the DDC rs921451, rs3837091, MAOB rs1799836, and COMT rs4680 variants on the other (with P-values ranging from 0.165 to 0.976).
Association between the SLC6A3 rs3836790 genotype with motor and gait improvements after methylphenidate treatment
A non-parametric covariance analysis adjusted for the baseline value revealed a significant association between the SLC6A3 rs3836790 genotype and an improvement in motor symptoms and gait in the ON l-DOPA condition after methylphenidate treatment (Table 4). No significant associations between SLC6A3 rs3836790 genotype and efficacy criteria in the OFF l-DOPA condition were detected, except for the Stand-Walk-Sit Test completion time. In a multivariate analysis adjusted for the dose of l-DOPA, the SLC6A3 rs3836790 genotype was strongly correlated with the motor UPDRS score ON l-DOPA (P = 0.002) the number of steps ON l-DOPA (P = 0.0003), the completion time OFF l-DOPA (P = 0.027), the completion time ON l-DOPA (P = 0.0009) and the number of freezing of gait episodes ON l-DOPA (P = 0.017).
Table 4.
Associations between the SLC6A3 rs3836790 genotype (Int8 VNTR) and the changes in the severity of gait and motor symptoms after methylphenidate administration
|
Dopamine transporter |
|||||
|---|---|---|---|---|---|
| Symptoms | Conditions | Visit | 5/5 or 5/6 (n = 17) | 6/6 (n = 11) | P*,† |
| Motor UPDRS score | OFF | Baseline | 29 (24–37) | 27 (24–37) | |
| 3 months | 24 (19–34) | 23 (19–27) | |||
| Difference % | 16.7 (−3.2–21.9) | 15.4 (3.7–41.0) | 0.40/0.42 | ||
| ON | Baseline | 20 (14–28) | 17 (10–24) | ||
| 3 months | 21 (15–29) | 11 (8–20) | |||
| Difference % | 3.8 (−7.7–10.0) | 26.7 (8.3–40.0) | 0.003/0.007 | ||
| Number of steps | OFF | Baseline | 34 (31–55) | 32 (28–38) | |
| 3 months | 33 (26–42) | 30 (27–32) | |||
| Difference % | 3.7 (0.0–26.1) | 10.2 (–1.7–25.0) | 0.94/0.37 | ||
| ON | Baseline | 28 (25–31) | 26 (24–32) | ||
| 3 months | 28 (24–31) | 23 (19–29) | |||
| Difference % | 3.3 (−11.6–10.2) | 16.7 (9.2–28.3) | 0.003/<0.001 | ||
| Completion time, s | OFF | Baseline | 22 (18–25) | 22 (19–30) | |
| 3 months | 19 (17–25) | 17 (14–20) | |||
| Difference % | 6.7 (–2.9–14.7) | 26.7 (9.4–35.9) | 0.01/0.008 | ||
| ON | Baseline | 17 (15–20) | 19 (16–22) | ||
| 3 months | 17 (16–20) | 15 (12–17) | |||
| Difference % | 0.0 (–15.6–10.5) | 18.5 (13.5–25.9) | <0.001/<0.001 | ||
| Number of freezing | OFF | Baseline | 6 (3–7) | 8 (3–14) | |
| 3 months | 4 (1–8) | 4 (0–6) | |||
| Change | 1 (1–4) | 3 (1–7) | 0.58/0.65 | ||
| ON | Baseline | 4 (2–5) | 5 (3–8) | ||
| 3 months | 3 (3–5) | 2 (0–3) | |||
| Difference % | 1 (1–2) | 3 (2–5) | 0.01/0.03 | ||
Data are reported as the median (interquartile range) value and the change from baseline (expressed in %; except for the number of freezing of gait episodes because of a value of zero at baseline). The number of steps and the completion time at the Stand-Walk-Sit Test and the number of freezing of gait (FOG) at the freezing of gait trajectory are displayed. *P-values for intergroup comparisons were calculated using a Wilcoxon test or a non-parametric analysis of covariance adjusted for baseline values (for the number of freezing of gait episodes); †A non-parametric covariance analysis adjusted for the total dose of l-DOPA.
In a multivariate analysis adjusted for age, gender, weight, disease duration and l-DOPA equivalent daily dose (or l-DOPA daily dose), the association was still statistically significant for the motor UPDRS score ON l-DOPA (P = 0.0005) the number of steps ON l-DOPA (P = 0.0005), the completion time OFF l-DOPA (P = 0.030), the completion time ON l-DOPA (P = 0.0001) and the number of freezing of gait episodes ON l-DOPA (P = 0.017). The same effect was observed for the SLC6A3 rs3836790 genotype (data not shown).
Association of SLC6A3 rs3836790 genotype with the striatal SLC6A3 binding
After 3 months of treatment with methylphenidate, striatal SLC6A3 expression was significantly lower (by 35%) in the methylphenidate group than in the placebo group (Table 5). The rs3836790 genotype was significantly associated with lower post-treatment level of SLC6A3 binding in the methylphenidate group but not the baseline (pretreatment) level of DaT binding.
Table 5.
Association between the SLC6A3 binding potential (in SPECT measurements) and SLC6A3 rs3836790 genotypes
|
Overall study population |
Methylphenidate-treated population |
|||||
|---|---|---|---|---|---|---|
| Placebo | Methylphenidate | P-value* | 5/5 or 5/6 | 6/6 | P-value* | |
| n | 14 | 14 | 7 | 7 | ||
| Baseline | 0.35 (0.20–0.47) | 0.34 (0.21–0.38) | 0.32 (0.19–0.40) | 0.34 (0.20–0.38) | ||
| 3 months | 0.35 (0.19–0.48) | 0.22 (0.10–0.25) | 0.25 (0.16–0.32) | 0.20 (0.10–0.22) | ||
| Difference (%) | 6.1 (0.8–10.2) | 31.5 (19.7–51.1) | <0.001 | 21.3 (17.6–21.9) | 50.6 (41.0–51.5) | 0.01 |
Median (interquartile range) of the dopamine transporter density parameter at baseline and 3 months, together with the difference between the two (in %). *Mann-Whitney U-test.
Discussion
This study is the first to show that some genotypes of the SLC6A3 gene (encoding SLC6A3, previously known as DaT1) may be associated with differences in the acute motor response to l-DOPA and the chronic response of gait parameters to methylphenidate in patients with Parkinson’s disease. We observed a significant mean difference of 6 points (i.e. a 17% difference) in the motor UPDRS scores when comparing patients with the genotype associated with a greater effect of l-DOPA after an acute challenge (the SLC6A3 rs3836790 6/6 genotype) and patients with the genotypes associated with a lower effect (the SLC6A3 rs3836790 5/6 or 5/5 genotypes). This association was independent of gender, weight, the challenge dose of l-DOPA and the genotypes of some other genes that may be involved in the metabolism of dopamine and l-DOPA.
A systematic literature search did not detect publications dealing with a possible association between SLC6A3 genotypes on one hand and the acute motor response to l-DOPA and the acute and chronic motor response to methylphenidate on the other. More than 100 studies have assessed possible associations between variants in the SLC6A3 gene and psychiatric disorders (notably attention deficit hyperactivity disorder and substance abuse). To a lesser extent, SLC6A3 gene variants have also been investigated in Parkinson’s disease as modifiers of the disease risk modifiers or the occurrence of dopaminergic-related complications (such as hallucinations, dyskinesia, and depression) (Supplementary Table 1). To the best of our knowledge, only one previous study has assessed (and failed to detect) a putative association between SLC6A3 genotypes and the response pattern to an acute oral l-DOPA challenge (Contin et al., 2004). In contrast to the latter study, the requirement for a stable l-DOPA regimen was an inclusion criterion in our present work. Furthermore, we studied a large population of subthalamic nucleus-stimulated patients, all of whom were at the same advanced stage of Parkinson’s disease; this is likely to have reduced interindividual variability in the motor response and thus strengthens our results.
Dopamine transporters and the l-DOPA response
Our results suggest the presence of a highly significant association between SLC6A3 genotypes and the response to l-DOPA. SLC6A3 is the most powerful determinant of dopamine metabolism in the striatum (Gainetdinov et al., 1998). In studies of conditions other than Parkinson’s disease, it has been suggested that patients with SLC6A3 rs3836790 6/6 or SLC6A3 rs28363170 10/10 genotypes have a higher baseline levels of dopamine transporter expression than patients with other SLC6A3 genotypes (rs3836790 5/5 and 6/6, and rs28363170 9/9 and 9/10) and thus lower synaptic dopamine levels (due to greater dopamine reuptake) (Fuke et al., 2001; VanNess et al., 2005; Dreher et al., 2009; Forbes et al., 2009). In advanced Parkinson’s disease, the situation could be more critical because the presynaptic neurons within the striatum degenerate markedly (as shown by the very low baseline binding in the dopamine transporter imaging analysis). Hence, one can resonantly hypothesize that exogenous l-DOPA administration may be more effective in patients with SLC6A3 rs3836790 6/6 or SLC6A3 rs28363170 10/10 genotypes, as larger numbers of SLC6A3 in the striatum would lower baseline extracellular dopamine levels through greater dopamine reuptake. This might result in greater clinical benefit than in patients with other genotypes and lower numbers of SLC6A3 transporters (i.e. with lower dopamine reuptake and thus higher baseline extracellular dopamine levels in the striatum). However, this hypothesis cannot be proved by a SLC6A3 density analysis, which was only performed here on a subpopulation of 14 patients receiving their usual dopaminergic treatment. In fact, the objective of the SLC6A3 density was to assess the effect of methylphenidate (Table 5).
The SLC6A3 genotype appears to be associated with the DOPA-sensitivity of gait disorders observed in the population of patients with advanced Parkinson’s disease (i.e. hypokinesia and freezing of gait) studied here. Our results suggest that to better relieve gait disorders, it may be worth increasing the dose of l-DOPA in patients harbouring the SLC6A3 rs3836790 6/6 or SLC6A3 rs28363170 10/10 genotypes. Indeed, it seems that higher dopaminergic doses are required to control gait disorders than to control segmental symptoms, as shown by the sole reduction in the frequency of freezing of gait in our l-DOPA condition. This observation also suggests that better knowledge of SLC6A3 genotypes could help the physician to define the l-DOPA treatment strategy in patients with severe gait disorders.
Dopamine transporters and the methylphenidate response
We found that SLC6A3 genotypes were also strongly associated with less severe motor handicap and gait disorders in patients receiving l-DOPA and methylphenidate (relative to placebo). This association was independent of gender, weight, and the challenge dose of l-DOPA. Interestingly, methylphenidate improved symptoms more in the OFF condition than it did in the ON condition, for which the results were more variable (Moreau et al., 2012). The SLC6A3 genotype might explain this variability (at least in part). In the present study, methylphenidate produced an effective pharmacological blockade of dopamine transporters, as reflected by lower striatal radioligand binding relative to the placebo group (a difference of 35%). The greater benefit of the genotypes SLC6A3 rs3836790 6/6 or SLC6A3 rs28363170 10/10 seemed to be related to a higher level of SLC6A3 inhibition (by up to 29 when compared with other genotypes). One can thus hypothesize that methylphenidate inhibits a larger number of SLC6A3 transporters in patients with the SLC6A3 rs3836790 6/6 or rs28363170 10/10 genotypes, which are likely to be associated with a higher striatal extracellular dopamine levels after exogenous l-DOPA administration, greater potentiation of dopamine neurotransmission and thus greater clinical benefit. This observation might be of great value in helping to predict the risk/benefit balance of prescribing methylphenidate for severe gait disorders in advanced Parkinson’s disease. Indeed, long-term methylphenidate use may be risky in elderly patients and those with a cardiac risk. Hence, the benefit of methylphenidate seems to be more pronounced for patients with SLC6A3 rs3836790 6/6 or rs28363170 10/10 genotypes, even though some patients with other genotypes also displayed clinical improvements. The established consequences of the SLC6A3 rs3836790 genotype suggest that the association reported here is based on functional involvement (Guindalini et al., 2006).
Other genotypes
Our analysis of the other main gene variants involved in dopamine neurotransmission failed to show any significant associations. MAOB genotypes have been frequently studied as potential disease modifier of Parkinson’s disease, with conflicting results. Only two retrospective studies have investigated the association between MAOB variants and the effect of chronic l-DOPA therapy. The results of a clinical study of a group of 95 patients with Parkinson’s disease in Poland indicated that l-DOPA therapy may be efficient in individuals carrying the MAOB A allele (Białecka et al., 2004). A recent study of 103 patients did not find any significant differences in genotype distributions when comparing those receiving daily doses of l-DOPA above 500 mg and below 500 mg in the fifth year of treatment (Torkaman-Boutorabi et al., 2012). In accordance with previous studies, neither the DDC nor COMT variants studied here were associated with the peak motor response to l-DOPA (Contin et al., 2005; Corvol et al., 2011; Devos et al., 2014). In fact, DDC genotypes may be associated with the central bioavailability of dopamine (rather than peripheral bioavailability) because of the concomitant use of a DDC inhibitor. This results in a greater area under the curve for the motor response during an acute l-DOPA challenge (Devos et al., 2014).
Limitations
Our study had several limitations. The l-DOPA dose for the acute challenge was 150% of the usual morning l-DOPA equivalent dose used by patients to relieve their symptoms. This dose was chosen with a view to obtaining the best possible motor state on l-DOPA. However, it would also have been interesting to give a fixed dose of l-DOPA to assess the effect of the patient’s genotype. However, this latter paradigm would have prevented us from minimizing sources of bias influencing DOPA-sensitivity (bodyweight, etc.). Lastly, the doses of l-DOPA used in the present study did not differ greatly from one patient to another or from one genotype group to another. In view of the large number of parameters and sources of possible bias, pharmacogenetic studies usually require very large numbers of patients to achieve sufficient statistical power. In an a posterior power calculation, we calculated the smallest significant difference in the change in the motor UPDRS score (expressed as an effect size using the standardized mean difference) between SLC6A3 genotypes (collapsed into two groups) in patients treated with l-DOPA and methylphenidate, respectively. Based on our study sample sizes and the SLC6A3 genotype distribution, we were able to detect an effect size of 0.7 for the l-DOPA group and 1.0 for methylphenidate group, with a power of 80% and an alpha risk of 5%. Both these effect sizes can be considered to be large (Cohen et al., 1992). In view our study's exploratory nature and small sample size, we did not adjust for multiple comparisons. However, our highly standardized conditions of treatment administration and our very homogenous study population enabled us to observe statistically significant results for SLC6A3 genotypes with a smaller number of patients. All of our patients were receiving subthalamic nucleus stimulation at the same, advanced stage of Parkinson’s disease. This population is highly DOPA-sensitive (i.e. with a marked difference between OFF and ON conditions). We excluded patients with Parkinson’s disease with low DOPA sensitivity and the severe cognitive disorders that are frequently observed in older and non-stimulated Parkinson’s disease populations. Moreover, patients with late-stage Parkinson’s disease are mostly dependent on exogenous l-DOPA, which reduces bias related to the influence of endogenous dopamine when seeking to identify pharmacogenetic factors.
Although the dopamine transporter genotypes probably influenced dopamine bioavailability in the nigrostriatal pathway, our present data could not provide insight into the underlying mechanisms. To establish the dopamine transporter polymorphisms' impact on the duration and amplitude of l-DOPA efficacy, we would have had to score the motor part of the UPDRS for each patient every 15 min and calculate the area under the curve.
Conclusions and perspectives
The concept of personalized medical care guided by pharmacogenetic parameters is attractive but complex because of the many associated factors (i.e. the physician’s and the patient’s beliefs and fears). Hence, the goal is to establish whether or not genetic knowledge will enable physicians to adapt the dose of l-DOPA more rapidly and thus reduce long-term, l-DOPA-related complications in Parkinson’s disease. We hypothesize that patients bearing SLC6A3 rs3836790 6/6 or rs28363170 10/10 genotypes would gain greater benefit from l-DOPA treatment and would require slightly lower doses and longer time intervals between doses. This knowledge would also guide the use of methylphenidate in elderly patients with cardiac risks. We also hypothesize that patients with the SLC6A3 rs3836790 6/6 or rs28363170 10/10 genotype would gain greater benefit from long-term treatment with methylphenidate. The decisional value of genotype data should be analysed in term of the risk/benefit balance and the health economic impact (i.e. the relatively low cost of genotyping versus the high cost of severe motor and gait complications). The present work is the first of several studies designed to establish guidelines on dose modulation. Our findings must first be replicated in an independent cohort. A very large, randomized, standardized, prospective study could then provide information on how the daily dose of l-DOPA might be modulated as a function of genotype in a non-stimulated population.
Acknowledgements
The authors wish to thank Valerie Vasseur, Francine Niset, Carine Piatek, Gregory Mercier, Pascal Bechu, Patrick Gele, Bertrand Accard, Laetitia Boidin and the Fédération de la Recherche Clinique du CHU de Lille for collecting the data and Dr David Fraser (Biotech Communication, Damery, France) for helpful comments on the manuscript's English. We thank Novartis Pharma for providing the methylphenidate and placebo for the present investigator-driven study. Novartis Pharma provided the methylphenidate and the placebo but had no involvement in the study design, data collection, data analysis, data interpretation or report writing. Lastly, we thank all the study patients and their physicians for their participation.
Glossary
Abbreviations
- UPDRS
Unified Parkinson’ Disease Rating Scale
- VNTR
variable number tandem repeat
Appendix 1
The Parkgait-II Study Group (alphabetic order):
Bertrand Accard, Amélie Bichon, Séverine Bleuse, Michel Borg, Pierre Burbaud, Maxime Bubrovszky, Walid Chucha, Maria-Clara Cohelo-Braga, Philippe Damier, Bérangère Debilly, Gilles Defer, Marie Delliaux, Dominique Deplanque, Pascal Derkinderen, Sandrine Dupouy, Frédérique Fluchere, Olivier Fleury, Patrick Gelé, Nathalie Girault, David Grabli, Claude Hossein-Foucher, Paul Krack, Alexandre Kreisler, Pierre Krystkowiak, Romain Lefaucheur, Eugénie Lhommée, François Mahieu, Thavarak Ouk, Pierre Pollak, Pierre Renou, Nathalie Rouaix, Emmanuelle Schmitt, Clémence Simonin, Violaine Talmant, Marc Verin, Frédérique Warembourg, Marie-Laure Welter, Tatiana Witjas
Funding
This academic study was funded by a PHRC grant from the French Ministry of Health. The present research has received funding from the French government’s “Investissements d’Avenir” programme (reference: ANR-10-IAIHU-06).
Conflict of interest
Details of any conflict of interest are provided in the Supplementary material available at Brain online.
Supplementary material
Supplementary material is available at Brain online.
References
- Bergersen L, Gauvreau K, Marshall A, Kreutzer J, Beekman R, Hirsch R, et al. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circ Cardiovasc Interv 2011; 4: 188–94. [DOI] [PubMed]
- Berry MD, Juorio AV, Li XM, Boulton AA. Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res. 1996;21:1075–87. doi: 10.1007/BF02532418. [DOI] [PubMed] [Google Scholar]
- Białecka M, Droździk M, Kłodowska-Duda G, Honczarenko K, Gawrońska-Szklarz B, Opala G, et al. The effect of monoamine oxidase B (MAOB) and catechol-O-methyltransferase (COMT) polymorphisms on levodopa therapy in patients with sporadic Parkinson's disease. Acta Neurol Scand. 2004;110:260–6. doi: 10.1111/j.1600-0404.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- Białecka M, Kurzawski M, Klodowska-Duda G, Opala G, Tan EK, Drozdzik M. The association of functional catechol-O-methyltransferase haplotypes with risk of Parkinson's disease, levodopa treatment response, and complications. Pharmacogenet Genomics. 2008;18:815–21. doi: 10.1097/FPC.0b013e328306c2f2. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Contin M, Martinelli P, Mochi M, Albani F, Riva R, Scaglione C, et al. Dopamine transporter gene polymorphism, spect imaging, and levodopa response in patients with Parkinson disease. Clin Neuropharmacol. 2004;27:111–5. doi: 10.1097/00002826-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Contin M, Martinelli P, Mochi M, Riva R, Albani F, Baruzzi A. Genetic polymophism of catechol-O-methyltransferase and Levodopa pharmacodynamic pattern in patients with Parkinson’s disease. Mov Disord. 2005;20:734–9. doi: 10.1002/mds.20410. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Bonnet C, Charbonnier-Beaupel F, Bonnet AM, Fiévet MH, Bellanger A, et al. The COMT Val158Met Polymorphism affects the response to entacapone in Parkinson’s disease: A randomized Crossover Clinical Trial. Ann Neurol. 2011;69:111–8. doi: 10.1002/ana.22155. [DOI] [PubMed] [Google Scholar]
- Costa-Mallen P, Costa LG, Checkoway H. Genotype combinations for monoamine oxidase-B intron 13 polymorphism and dopamine D2 receptor TaqIB polymorphism are associated with ever-smoking status among men. Neurosci Lett. 2005;385:158–62. doi: 10.1016/j.neulet.2005.05.035. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Schulzer M, Mak E, Calne DB, Stoessl AJ. Presynaptic mechanisms of motor fluctuations in Parkinson's disease: a probabilistic model. Brain. 2004;127(Pt 4):888–99. doi: 10.1093/brain/awh102. [DOI] [PubMed] [Google Scholar]
- de Lau L, Breteler M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA. 2009;106:617–22. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Mouradian MM, Juncos JL, Schlegel J, Mohr E, Chase TN. Motor fluctuations in Parkinson's disease: central pathophysiological mechanisms, Part I. Ann Neurol. 1988;24:366–71. doi: 10.1002/ana.410240303. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nat Neurosci. 2009;12:1062–8. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos D, Moreau C, Delval A, Dujardin K, Defebvre L, Bordet R. Methylphenidate: a treatment for Parkinson's disease? CNS Drugs. 2013;27:1–14. doi: 10.1007/s40263-012-0017-y. [DOI] [PubMed] [Google Scholar]
- Devos D, Lejeune S, Cormier-Dequaire F, Tahiri K, Charbonnier-Beaupel F, Rouaix N, et al. Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:170–5. doi: 10.1016/j.parkreldis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–6. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev. 1998;26:148–53. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Gibb WRG, Lees AJ. The prevalence of the lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindalini C, Howard M, Haddley K, Laranjeira R, Collier D, Ammar N, et al. A dopamine transporter gene functional variant associated with cocaine abuse in a Brazilian sample. Proc Natl Acad Sci USA. 2006;103:4552–7. doi: 10.1073/pnas.0504789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BD, Kurth JH, Barnes RI, Bowcock AM, Kurth MC. Linkage analysis of the monoamine A and B genes using newly-defined polymorphisms. Cytogenet Cell Genet. 1993;62:236–7. doi: 10.1159/000133485. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Auinger P, Oakes D Parkinson Study Group. Levodopa response in early Parkinson's disease. Mov Disord. 2009;24:2328–36. doi: 10.1002/mds.22759. [DOI] [PubMed] [Google Scholar]
- Hoogland J, de Bie RM, Williams-Gray CH, Muslimović D, Schmand B, Post B. Catechol-O-methyltransferase val158met and cognitive function in Parkinson's disease. Mov Disord. 2010;25:2550–4. doi: 10.1002/mds.23319. [DOI] [PubMed] [Google Scholar]
- Klebe S, Golmard JL, Nalls MA, Saad M, Singleton AB, Bras JM, et al. French Parkinson's disease genetics study group; international Parkinson's disease genomics consortium (IPDGC). The Val158Met COMT polymorphism is a modifier of the age at onset in Parkinson's disease with a sexual dimorphism. J Neurol Neurosurg Psychiatry. 2013;84:666–73. doi: 10.1136/jnnp-2012-304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss AM, Alterman RL, Tagliati M, Shils JL. Calculating total electrical energy delivered by deep brain stimulation systems. Ann Neurol. 2005;58:168. doi: 10.1002/ana.20525. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Nanko S, Ueki A, Otsuka E, Hattori M, Hoda F, et al. High and low activity alleles of catechol-O-methyltransferase gene: ethnic difference and possible association with Parkinson's disease. Neurosci Lett. 1997;221:202–4. doi: 10.1016/s0304-3940(96)13289-4. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lyoo ChH, Ulmanen I, Syvanen AC, Rinne JO. Genotypes of catechol-O-methyltransferase and response to l-Dopa treatment in patients with Parkinson’s disease. Neurosci Lett. 2001;298:131–4. doi: 10.1016/s0304-3940(00)01749-3. [DOI] [PubMed] [Google Scholar]
- Martínez-Martín P, García Urra D, del Ser Quijano T, Balseiro Gómez J, Gómez Utrero E, Piñeiro R, et al. A new clinical tool for gait evaluation in Parkinson's disease. Clin Neuropharmacol. 1997;20:183–94. doi: 10.1097/00002826-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Moreau C, Delval A, Defebvre L, Dujardin K, Duhamel A, Petyt G, et al. Parkgait-II study group. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson's disease undergoing subthalamic stimulation: a multicentre, parallel, randomized, placebo-controlled trial. Lancet Neurol. 2012;11:589–96. doi: 10.1016/S1474-4422(12)70106-0. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord. 2008;23(Suppl 3):S580–4. doi: 10.1002/mds.22037. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: Clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18:149–54. doi: 10.1016/j.parkreldis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Torkaman-Boutorabi A, Shahidi GA, Choopani S, Rezvani M, Pourkosary K, Golkar M, et al. The catechol-O-methyltransferase and monoamine oxidase B polymorphisms and levodopa therapy in the Iranian patients with sporadic Parkinson's disease. Acta Neurobiol Exp (Wars) 2012;72:272–82. doi: 10.55782/ane-2012-1900. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov Disord. 2003;18(Suppl 7):S71–80. doi: 10.1002/mds.10578. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–6. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Harada S, Nakamura T, Ohkoshi N, Yoshizawa K, Hayashi A, et al. Association between catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson’s disease. Neuropsychobiology. 2003;48:190–3. doi: 10.1159/000074637. [DOI] [PubMed] [Google Scholar]
- Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in samples of unrelated individuals. Hum Hered. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]