In multiple sclerosis, grey matter pathology occurs mostly next to or near the outer surface of the brain. Using quantitative MRI, Liu et al. reveal that white matter abnormalities are also greatest near the surface of the brain, suggesting common elements in the genesis of grey and white matter pathology.
Keywords: multiple sclerosis, magnetization transfer ratio, normal-appearing white matter
In multiple sclerosis, grey matter pathology occurs mostly next to or near the outer surface of the brain. Using quantitative MRI, Liu et al. reveal that white matter abnormalities are also greatest near the surface of the brain, suggesting common elements in the genesis of grey and white matter pathology.
Abstract
In multiple sclerosis, there is increasing evidence that demyelination, and neuronal damage occurs preferentially in cortical grey matter next to the outer surface of the brain. It has been suggested that this may be due to the effects of pathology outside the brain parenchyma, in particular meningeal inflammation or through cerebrospinal fluid mediated factors. White matter lesions are often located adjacent to the ventricles of the brain, suggesting the possibility of a similar outside-in pathogenesis, but an investigation of the relationship of periventricular normal-appearing white matter abnormalities with distance from the ventricles has not previously been undertaken. The present study investigates this relationship in vivo using quantitative magnetic resonance imaging and compares the abnormalities between secondary progressive and relapsing remitting multiple sclerosis. Forty-three patients with relapsing remitting and 28 with secondary progressive multiple sclerosis, and 38 healthy control subjects were included in this study. T1-weighted volumetric, magnetization transfer and proton density/T2-weighted scans were acquired for all subjects. From the magnetization transfer data, magnetization transfer ratio maps were prepared. White matter tissue masks were derived from SPM8 segmentations of the T1-weighted images. Normal-appearing white matter masks were generated by subtracting white matter lesions identified on the proton density/T2 scan, and a two-voxel perilesional ring, from the SPM8 derived white matter masks. White matter was divided in concentric bands, each ∼1-mm thick, radiating from the ventricles toward the cortex. The first periventricular band was excluded from analysis to mitigate partial volume effects, and normal-appearing white matter and lesion magnetization transfer ratio values were then computed for the 10 bands nearest to the ventricles. Compared with controls, magnetization transfer ratio in the normal-appearing white matter bands was significantly lower in patients with multiple sclerosis. In controls, magnetization transfer ratio was highest in the band adjacent to the ventricles and declined with increasing distance from the ventricles. In the multiple sclerosis groups, relative to controls, reductions in magnetization transfer ratio were greater in the secondary progressive multiple sclerosis compared with relapsing remitting multiple sclerosis group, and these reductions were greatest next to the ventricles and became smaller with distance from them. White matter lesion magnetization transfer ratio reductions were also more apparent adjacent to the ventricle and decreased with distance from the ventricles in both the relapsing remitting and secondary progressive multiple sclerosis groups. These findings suggest that in people with multiple sclerosis, and more so in secondary progressive than relapsing remitting multiple sclerosis, tissue structural abnormalities in normal-appearing white matter and white matter lesions are greatest near the ventricles. This would be consistent with a cerebrospinal fluid or ependymal mediated pathogenesis.
Introduction
The pathogenesis of progressive multiple sclerosis remains unknown, but recent studies have raised the possibility of factors external to the brain parenchyma playing a significant role. In people with secondary progressive multiple sclerosis (SPMS), grey matter lesions may be as or more extensive than white matter lesions, (Bø et al., 2003; Lassmann, 2014), and grey matter lesions are much more abundant in the outer (subpial) cortical layers when compared with the cortex next to white matter (Bø et al., 2003). Similarly, neuronal loss in multiple sclerosis may be substantial in the cortex (Wegner et al., 2006), and is also greatest in the outer (subpial) compared with inner cortical layers (Magliozzi et al., 2010). This gradient in cortical grey matter lesions and neuronal pathology has been linked with meningeal inflammation, in particular the presence of B cell follicle-like aggregates in SPMS (Magliozzi et al., 2007, 2010), and it has been suggested that meningeal inflammation or CSF-mediated factors may play a key pathogenic role in cortical pathology
White matter lesions occur more frequently around the ventricle than elsewhere, and a clear explanation for this distribution has not been found (Narayanan et al., 1997). They seem to form around veins, and in the case of periventricular lesions around subependymal veins (Adams et al., 1987), but the mechanism for this has not been determined. Perivascular (venous) inflammatory infiltrates are seen in multiple sclerosis lesions (Adams, 1975), but it is not known if they are necessary for lesion genesis or part of a more chronic process. Tissue hypoxia has been suggested as having a pathogenic role in lesion formation (Lassmann, 2003), and for haemodynamic reasons periventricular white matter may be more likely than other parts of the brain to become hypoxic, explaining the predilection for lesion formation in this region (Beggs, 2013). Patchy ‘granular ependymitis’ has also been observed in a single study by Adams et al. (1987), although only co-localized with ∼1 in 10 periventricular lesions.
In light of findings in cortical grey matter, which suggest a possible outside-in pathogenesis, it would be of interest to know if the characteristics of normal-appearing white matter (NAWM) were also dependent on their location relative to the ventricular CSF interface. However, there is a paucity of histopathological or MRI studies that have looked for such an association in periventricular NAWM. One histopathological study looked at white matter lesion features dependent on their distance from the ventricles, specifically looking at glial progenitor cell density, and found that it was highest in the lesions in the subventricular (subependymal) zone (Nait-Ousmesmar et al. 2007). One MRI study looked at white matter lesion features dependent on their distance from the ventricles, which found that lesions are more likely to be hypointense on T-weighted images the closer they are to the ventricles (Papadopoulou et al., 2014).
Magnetization transfer ratio (MTR) is a MRI technique that has proven sensitive to multiple sclerosis pathology in white matter lesions and NAWM (Horsfield, 2005). In combined MRI and histopathological studies, reductions in MTR have been found to correlate with both demyelination and axonal loss, and as such it is sensitive to two major elements of white matter pathology in multiple sclerosis (Schmierer et al., 2004). With recent technical advances, it is now possible to obtain MTR maps at 1-mm isotropic resolutions, and this has allowed us to subdivide the cortex into inner and outer bands. We found a preferential multiple sclerosis disease effect on the outer (subpial) relative to the inner cortex in people with SPMS (Samson et al., 2014).
In this study, using the same high resolution MTR method, we aimed to investigate two questions: (i) in people with relapsing-remitting multiple sclerosis (RRMS) and SPMS, is there a relationship between NAWM and white matter lesion MTR, and distance from the ventricles? And (ii) are periventricular NAWM and lesion MTR abnormalities more prominent in SPMS compared with RRMS?
Materials and methods
Subjects
Participants were between 18 and 65 years old. Those with multiple sclerosis had to have a diagnosis of clinically definitive multiple sclerosis according to McDonald criteria (Polman et al., 2005), and multiple sclerosis subtypes were classified using the Lublin-Reingold (Lublin et al., 1996) criteria. The control group had to have no known neurological disease. All participants provided written informed consent. This study was approved by our local institutional ethics committee.
We included 71 people with multiple sclerosis: 43 with RRMS and 28 with SPMS. The mean ± standard deviation (SD) ages were 42.5 ± 10.0 and 53.0 ± 8.1, respectively, and disease duration from first symptom onset was 11.7 ± 8.1and 21.9 ± 10.4. Forty-eight (68%) were female. The median Expanded Disability Status Scale (Kurtzke, 1983) was 5.5 (range 1–8.5). Thirty-five (49%) of the multiple sclerosis group were receiving a disease-modifying treatment at time of the study. Thirty-eight people served as controls, mean (SD) age 39.1 ± 11.9, 21 (55%) females.
MRI
Using a 3 T Philips Achieva system (Philips Healthcare) with a 32-channel head coil and multi-transmit technology, the following sequences were acquired: 3D sagittal T1-weighted fast field echo (FFE) scan: 1 × 1 × 1 mm3, inversion time = 824 ms, repetition time = 6.9 ms, echo time = 3.1 ms; dual-echo proton density/T2-weighted axial-oblique scans aligned with the anterior to posterior commissure line (1 × 1 × 3 mm3, repetition time = 3500 ms, echo time = 19/85 ms); and high resolution magnetization transfer imaging using a 3D slab-selective FFE sequence with two echoes (1 × 1 × 1 mm3, repetition time = 6.4 ms, echo time = 2.7/4.3 ms, alpha = 9° with and without sinc Gaussian-shaped magnetization transfer pulses of nominal alpha = 360°, offset frequency 1 kHz, duration 16 ms. A turbo field echo (TFE) readout was used, with an echo train length of four, TFE shot interval 32.5 ms, giving a total time between successive magnetization transfer pulses of 50 ms, and scan time ∼25 min). The two echoes were averaged (thereby increasing the signal-to-noise ratio) for both the magnetization transfer on and -off data used to calculate the MTR.
Image analysis
Segmentation of T1-weighted 3D images
The T1-weighted scans were segmented using Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK; www.fil.ion.ucl.ac.uk/; using the new segment pipeline) after white matter lesion filling (Chard et al. 2010), and the resultant grey matter, white matter and CSF probability maps were then binarized. To minimize the potential for partial volume effects between tissues, the probability of each voxel being of a given tissue type had to be ≥90% (Samson et al., 2014). In addition, brain parenchymal fraction (grey matter and white matter tissue volume divided by grey matter, white matter plus CSF volume) was measured using the same SPM8 tissue segmentations, for use as a covariate in the statistical models.
Extraction of normal-appearing white matter
White matter lesions were identified and contoured on proton density/T2 scans using JIM (Version 6.0, Xinapse Systems). For each participant the white matter lesion masks from the proton density/T2 images were then co-registered to the corresponding T1-weighted 3D scan. To achieve this, a pseudo-T1 image was generated by subtracting the proton density from the T2-weighted image (Hickman et al., 2002), so providing an image with a similar contrast to the T1-weighted 3D scan. Using NiftyReg (Ourselin et al., 2001; Modat et al., 2010), a linear transformation was then computed to co-register the pseudo-T1 image to the T1-weighted 3D image, and then the same transformation was applied to the proton density/T2 lesion mask. A previous study has shown that MTR is significantly more abnormal in the white matter 1–2 mm adjacent to the edge of a multiple sclerosis lesion, as seen on a proton density/T2-weighted scan, when compared with white matter more distant from the lesion margin (Vrenken et al., 2006). To prevent this from affecting NAWM MTR measurements in this study, a perilesional tissue mask was prepared by dilating the proton density/T2 lesion mask 2 mm in each direction. Binarized white matter lesions and perilesional tissue masks were then subtracted from each subject’s binarized white matter mask to create a NAWM mask. As further precaution against lesion contamination of NAWM, the same analysis was rerun using a three voxels dilation of white matter lesion masks. The results of this analysis are reported in the Supplementary material.
Co-registration of magnetization transfer data to T1-weighted images
Magnetizationon (MTon) and magnetizationoff (MToff) images for each subject were affine registered to T1-weighted volume image using NiftyReg, and MTR maps calculated [MTR = (MToff − MTon) / MToff], measured in percentage units (pu).
Segmentation of white matter into concentric periventricular one-voxel thick bands
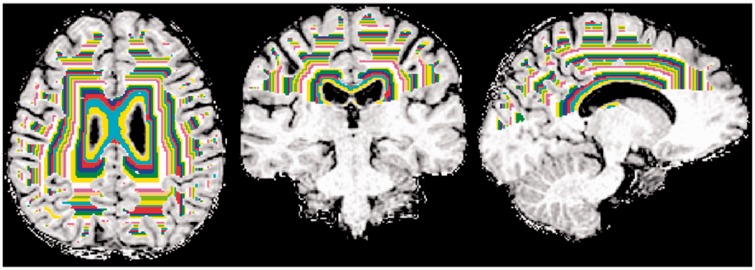
For each subject NAWM was segmented into concentric one-voxel thick bands starting at the ventricular margin and extending in to the brain parenchyma (Fig. 1). To prevent NAWM bands arising simultaneously from the body and temporal horns of the lateral ventricles from clashing, only white matter and ventricular CSF superior to the insula were included in the analysis (Fig. 1).
Figure 1.
NAWM above the superior limit of the insula was segmented into concentric periventricular one-voxel thick bands.
For each subject, a mask of the body of the lateral ventricles was first created by linearly co-registering their thresholded CSF map with the lateral ventricle mask included in the Wake Forest University School of Medicine PickAtlas toolbox (Maldjian et al., 2003). This mask was reviewed and if required manually edited (by Z.L.) to ensure a robust match with lateral ventricle anatomy in each subject. The CSF mask was then repeatedly dilated by one voxel (1 × 1 × 1 mm3) using DilM (part of the FSL software package (http://www.fmrib.ox.ac.uk/fsl/) through NAWM to generate concentric one-voxel thick NAWM bands. In each subject, sequential bands were extracted until the volume of any given band fell below 0.5 ml. This yielded a series of NAWM bands extending from the ventricles through to the cortex. As an extra precaution against periventricular CSF-white matter partial volume effects, data from the first band was not included in subsequent analyses. As the aim of this study was to look for periventricular multiple sclerosis disease effects, MTR values were extracted for the first 10 NAWM bands (i.e. covering ∼1 cm of distance between the ventricles and cortex).
White matter lesion measures
To calculate white matter lesion volumes and MTR values dependent on their distance from the ventricles, a similar procedure to that used to obtain NAWM data was used. Ventricular CSF was sequentially dilated by 1 voxel (as above) through the white matter lesion mask, so generating a set of concentric white matter lesion masks. Mean and SD white matter lesion MTR and volumes were then calculated.
Statistical analyses
Demographic data and MTR values are presented as mean ± SD. Expanded Disability Status Scale values are presented as median (range). Statistical analyses were computed using SPSS (version 22, IBM). In each subject, MTR values within each NAWM band were averaged. The average MTR in each NAWM band was then separately compared between groups using general linear models, including age and gender as potentially confounding factors. Rather than run different models for each possible pairwise combination of groups, all three study groups (healthy controls, RRMS and SPMS) were included in a single model for each NAWM band, so allowing group differences to be tested simultaneously. As a further precaution against CSF partial volume effects, and to allow for potential differences in the relative alignment of NAWM bands due to disease associated atrophy, both the total number of NAWM bands extracted and brain parenchymal fraction were included as additional covariates. Gradients in NAWM MTR were calculated between pairs of bands and divided by the number of intervals spanned (e.g. the MTR gradient between bands 1 and 5 was calculated by subtracting the mean MTR value in band 5 from that in band 1, and dividing by 4). P ≤ 0.05 was considered to be the threshold for statistical significance.
Results
Participant demographics, clinical characteristics, and MTR values are given in Table 1. Figure 2 shows the mean (standard error) NAWM MTR in each group. Figure 3A shows the corresponding white matter lesion MTR values and Fig. 3B the white matter lesion volumes.
Table 1.
Participant characteristics
| Healthy controls | RRMS | SPMS | |
|---|---|---|---|
| n | 38 | 43 | 28 |
| Mean age, years ± SD (range) | 39.1 ± 11.9 (23–63) | 42.5 ± 10.0 (25–64) | 53.0 ± 8.1 (36–65) |
| Gender male/female | 17/21 | 14/29 | 9/19 |
| Median EDSS (range) | NA | 2 (1.0-7.0) | 6.5 (4.0–8.5) |
| Disease duration, years ± SD | NA | 11.7 ± 8.1 | 21.9 ± 10.4 |
| Mean MTR in whole NAWM ± SD | 39.7 ± 0.8 | 38.9 ± 1.5 | 38.4 ± 1.9 |
| Mean number of bands extracted ± SD | 44.0 ± 3.2 | 42.7 ± 3.2 | 41.1 ± 3.8 |
| Mean brain parenchymal fraction ± SD | 0.82 ± 0.02 | 0.80 ± 0.02 | 0.80 ± 0.03 |
EDSS = Expanded Disability Status Scale; NA = not available.
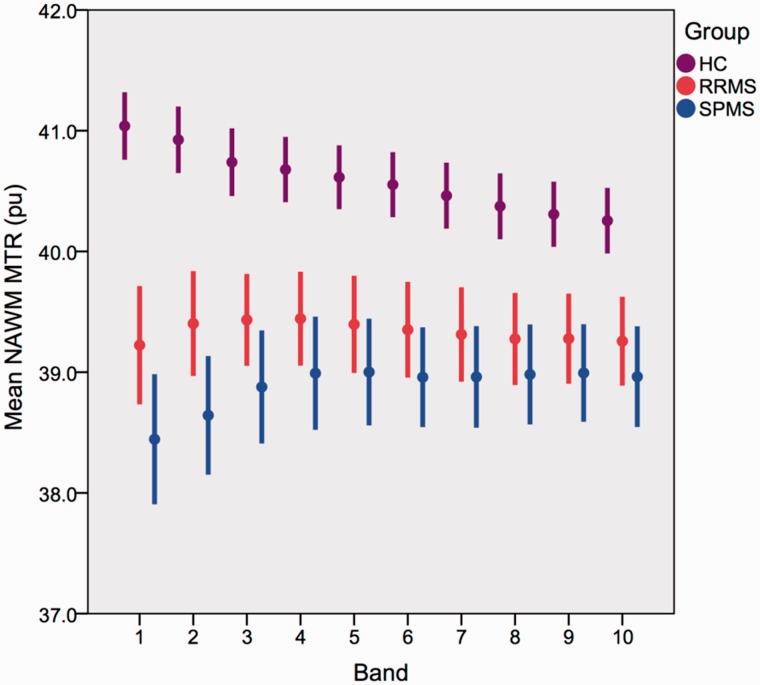
Figure 2.
NAWM band MTR in each subgroup. Mean ± 2 × standard error. MTR is given in per cent units (pu).
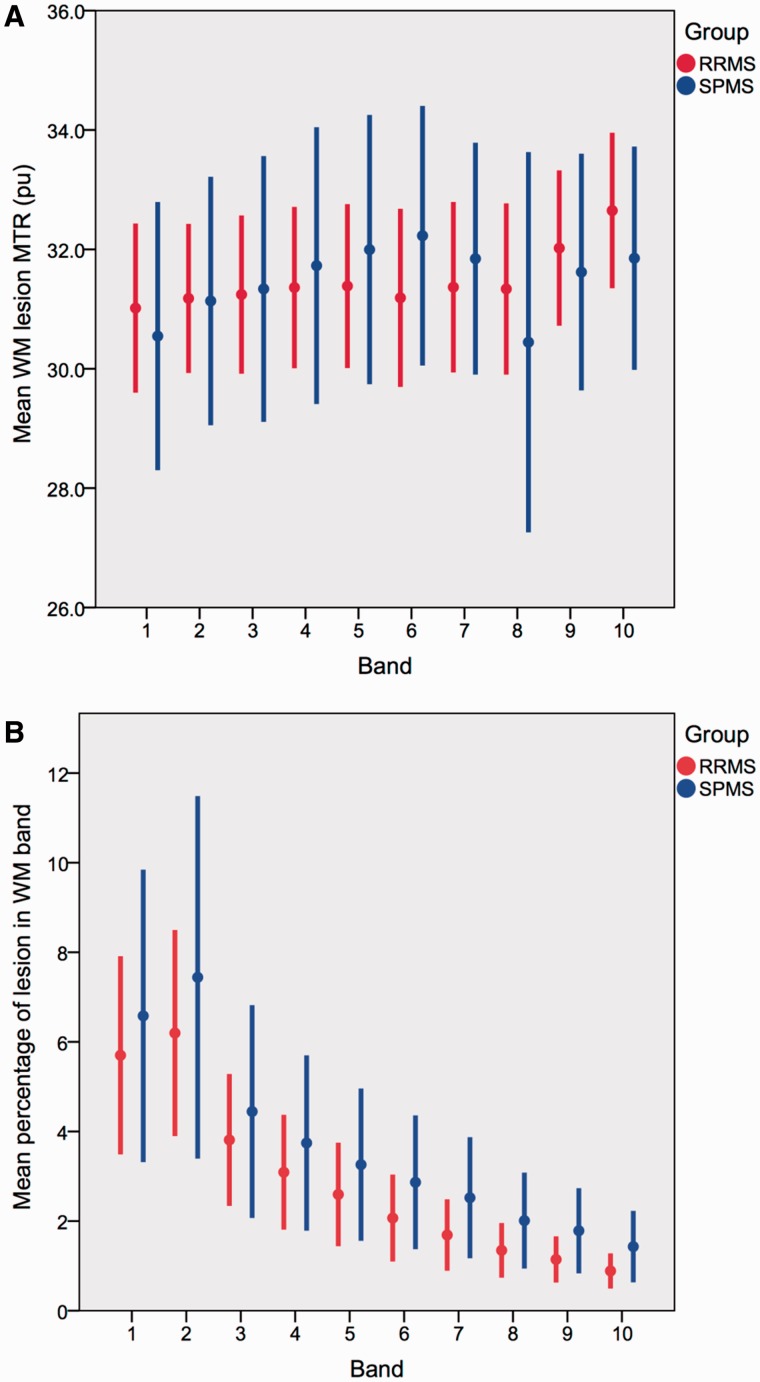
Figure 3.
White matter lesion MTR and volumes. (A) White matter lesion MTR for each band in the RRMS and SPMS groups. (B) White matter lesion volume for each band in the RRMS and SPMS groups. Data are mean ± 2 × standard error.
From Fig. 2 it can be seen that in healthy controls, the NAWM MTR was highest in the immediate periventricular NAWM band, and then declined with distance from the ventricles. In people with multiple sclerosis, NAWM MTR was lower than that in controls (Table 2). The multiple sclerosis-associated reduction in MTR was greatest in the NAWM adjacent to the ventricles, and noticeably declined with increasing distance from the ventricles until band 5. Whereas in controls MTR was higher in band 1 than band 5, in multiple sclerosis MTR was higher in band 5 than band 1. We therefore tested between group differences in NAWM MTR in bands 1, 5 and (for reference) 10, and MTR gradients between bands 1 and 5, and 5 and 10.
Table 2.
Mean MTR values in NAWM bands 1, 5 and 10 from lateral ventricle
| Band | Type | Mean (SE) MTR (pu) | Differences |
|---|---|---|---|
| 1 | Healthy controls | 41.0 (0.14) | HC versus RRMS: P < 0.001 HC versus SPMS: P < 0.001 RRMS versus SPMS: P = 0.019 |
| All multiple sclerosis | 38.9 (0.19) | ||
| RRMS | 39.2 (0.24) | ||
| SPMS | 38.4 (0.27) | ||
| 5 | Healthy controls | 40.6 (0.13) | HC versus RRMS: P < 0.001 HC versus SPMS: P < 0.001 RRMS versus SPMS: P = 0.152 |
| All multiple sclerosis | 39.2 (0.15) | ||
| RRMS | 39.4 (0.20) | ||
| SPMS | 39.0 (0.22) | ||
| 10 | Healthy controls | 40.3 (0.14) | HC versus RRMS: P < 0.001 HC versus SPMS: P < 0.001 RRMS versus SPMS: P = 0.258 |
| All multiple sclerosis | 39.1 (0.14) | ||
| RRMS | 39.3 (0.18) | ||
| SPMS | 39.0 (0.21) |
HC = healthy controls; pu = per cent units.
Compared with controls MTR was significantly lower in bands 1, 5 and 10 in overall multiple sclerosis groups, as well as in RRMS and SPMS subgroups (Table 2). In band 1 MTR was significantly lower in the SPMS compared with RRMS group (Table 2). Age effects were not significant. Gender had a significant effect in band 5 and 10; however, this did not materially alter the observed inter-group differences and so unadjusted results are presented (Table 2). Covarying for brain parenchymal fraction or for the number of bands extracted did not materially alter the results, except for the comparison of MTR in band 1 between RRMS and SPMS groups, which reduced the statistical significance of differences between them to a borderline level (Supplementary Table 1). Compared with controls, MTR gradients between bands 1 and 5 differed significantly with both the multiple sclerosis subgroups or overall multiple sclerosis groups (Table 3). Between bands 5 and 10, changes in MTR differed significantly between the control and the multiple sclerosis groups (Table 3). Comparing the multiple sclerosis subgroups, NAWM MTR gradients between bands 1 and 5 were significantly greater in the SPMS compared with RRMS group. Age and gender were not significant in these models, and covarying for the total number of bands or brain parenchymal fraction did not materially affect them (Supplementary Table 2), and so unadjusted results are presented.
Table 3.
MTR gradients between NAWM bands 1, 5 and 10
| Bands | Type | Mean (SE) MTR change (pu, per band interval) | Differences |
|---|---|---|---|
| 1 to 5 | HC | −0.11 (0.01) | HC versus RRMS: P < 0.001 |
| All multiple sclerosis | 0.08 (0.03) | HC versus SPMS: P < 0.001 | |
| RRMS | 0.04 (0.03) | RRMS versus SPMS: P = 0.027 | |
| SPMS | 0.14 (0.04) | HC versus RRMS: P = 0.007 | |
| 5 to 10 | HC | −0.07 (0.01) | HC versus SPMS: P = 0.001 |
| All multiple sclerosis | −0.02 (0.01) | RRMS versus SPMS: P = 0.253 | |
| RRMS | −0.03 (0.01) | ||
| SPMS | −0.01 (0.02) |
HC = health controls; pu = per cent units.
Lesion MTR and volume by distance from the ventricles is shown in Fig. 3A and B. It can be seen that with distance from the ventricles lesion volume decreases, and from bands 1 to 5 lesion MTR increases. Beyond band 5, lesions volumes are very small, and lesion MTR measures becomes more variable, but as with NAWM, they appear to plateau and then decline, so mirroring (at a lower level) the MTR gradient between bands seen in control white matter. Dilating the white matter lesion masks by three voxels did not materially alter the results (Supplementary Tables 3 and 4 and Supplementary Fig. 1).
Discussion
In the multiple sclerosis groups MTR was reduced throughout the NAWM; however, the distribution of MTR values did not simply mirror, at a lower level, those in healthy controls. Instead, there was a clearly greater multiple sclerosis disease effect close to the ventricles. Similar findings were noted in white matter lesions, with the magnitude of MTR reductions within lesion decreasing with distance from the ventricles. These findings strongly indicate that not only are lesions more likely to occur around the ventricles (Narayanan et al., 1997) (Fig. 3B), but pathological changes within NAWM and white matter lesions are also greater around the ventricles (Figs 2 and 3A). This suggests that pathology in white matter occurs preferentially at or near the inner surface of the brain.
Considering technical factors that may have influenced these results, inclusion of ventricular CSF in the NAWM and white matter lesions masks could spuriously reduce periventricular MTR. However, we think this is highly unlikely for four reasons. First, we used a stringent threshold (90%) to binarize the SPM8 tissue segmentations. Second, we discarded MTR data in the first NAWM and white matter lesion bands in each subject. Third, covarying for the total number of NAWM bands or brain parenchymal fraction (a measure of brain atrophy) in the statistical analyses did not materially alter the results. Fourth, there was no periventicular reduction in MTR seen in controls, and we would expect to see at least a hint of this if partial volume with CSF was significantly affecting periventricular NAWM MTR measures.
It is also highly unlikely that there was any lesion contamination of NAWM (that would reduce NAWM MTR). First, based on work by Vrenken et al. (2006), who demonstrated that perilesional reductions in MTR were detectable for 1 to 2 mm, we dilated our lesion masks by two voxels i.e. the linear equivalent of at least 2 mm, making it very unlikely that any residual lesions or perilesional tissues could have contributed significantly to NAWM measures. We also re-ran the analysis dilating lesions by three voxels, and this did not materially alter the results (Supplementary material). Secondly, the pattern of changes between bands in NAWM MTR (Fig. 2) and white matter lesion MTR (Fig. 3A) are similar, and this would be difficult to account for through consistent partial volume between them. Further investigation of whether or not periventricular NAWM MTR abnormalities occur independently of lesions could be undertaken by studying subjects who do not have periventricular lesions. We could not investigate this in our cohort as almost all had periventricular lesions, which is usually the case in established multiple sclerosis. A future study of people with clinically isolated syndromes or clinically very early multiple sclerosis—more of whom may not have periventricular lesions—would be of interest.
Brain atrophy could lead to the relative position of NAWM bands differing between people, e.g. band 10 in one person may fall a quarter of the way from the ventricular margin to the cortex, whereas in another person it may be halfway between them. However, misalignment of NAWM bands due to atrophy could not account for the MTR gradients seen (as it will tend to flatten gradients within each group) or differences between groups (as it will increase variability in group-wide MTR measures within each band). However, as a further precaution we also included both the total number of NAWM bands extracted and brain parenchymal fraction as covariates in the statistical models, and this did not materially alter the results.
A gradient in disease-associated MTR reductions was seen in all multiple sclerosis groups, but the periventricular reduction was significantly greater in the SPMS group when compared with RRMS. Several possible mechanisms might contribute to preferential periventricular abnormalities in multiple sclerosis, although with the currently limited information available it is not possible to determine which is the primary one. First, CSF mediated factors may be relevant. This could provide a unifying explanation for the predilection for cortical grey matter pathology to occur next to the outer surface of the brain (Bø et al., 2003; Magliozzi et al., 2007, 2010; Samson et al., 2014) and the periventricular NAWM MTR gradients seen in the present study. CSF from people with multiple sclerosis has been shown to induce both oligodendrocyte (Hughes and Field, 1967) and neuronal damage in cell cultures (Vidaurre et al., 2014). Secondly, inflammatory activity in the ependyma may be a factor. In a single histopathological study, Adams et al. (1987) found the ependyma overlying periventricular white matter to be focally abnormal, and interpreted this as representing ependymitis. Thirdly, it has been suggested that periventricular white matter is more likely than other white matter regions to become hypoxic when oxygen availability is reduced (Beggs, 2013) although hypoxia is also only thought to be a significant factor in the pathogenesis of a minority of white matter lesions (Lucchinetti et al., 2000; Lassmann, 2003).
The differences in the observed MTR gradients between people with multiple sclerosis and healthy controls are very difficult to explain on the basis of technical factors, such as partial volume, alone. However, there are some other limitations to consider. Because of the morphological complexity of dilating the whole ventricular system through white matter, and the potential for bands arising from the body of lateral ventricles overlapping with those from the temporal horns, we restricted our analysis to tissues above the insula. In addition, specific pathological interpretation of white matter MTR is difficult as it is sensitive to both demyelination and axonal loss (Schmierer et al., 2004), demyelination and axonal loss are highly correlated (Schmierer et al., 2004), and so we cannot determine which is mostly responsible for the gradient seen. In a recent spinal cord study in people with multiple sclerosis, a reduction in MTR has also been observed in the outermost cord voxel layer and, in addition to demyelination and axonal loss, the possibility of a direct effect of inflammatory infiltrates was also raised (Kearney et al., 2014). Quantitative histopathological assessment of the ependyma and periventricular white matter is warranted to provide further insight into the cause of the MTR abnormalities observed in this study.
In conclusion, we have found a gradient in MTR abnormalities from periventricular through to deep NAWM in people with multiple sclerosis, with the most marked abnormality seen in the immediate periventricular region and in SPMS. In combination with previous observations about cortical pathology, this suggests that there is a predilection for pathology to occur near the surface of the brain, particularly in SPMS. The present results highlight the need for further work clarifying the potential role of factors such as CSF mediators, ependymal inflammation, and periventricular tissue hypoxia, in the pathogenesis of lesional and non-lesional abnormalities in multiple sclerosis, and of SPMS.
Acknowledgements
We thank all the people who participated in this study and Daniel Altmann for statistical support.
Glossary
Abbreviations
- MTR
magnetization transfer ratio
- NAWM
normal-appearing white matter
- RRMS
relapsing remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
Funding
Z.L. was supported by an ECTRIMS (European Committee for Treatment and Research in Multiple Sclerosis) clinical training fellowship. M.P. thanks the non-profit AKWO association, Lavagna (GE, Italy), for their unrestricted financial support. The NMR Research Unit is supported by the MS Society of Great Britain and Northern Ireland and the National Institute for Health Research University College London Hospitals Biomedical Research Centre. The MS Society of Great Britain and Northern Ireland has provided financial support for staff and research costs directly associated with this study (grant 917-09).
Conflict of interest
Ö.Y. received honoraria for lectures from Teva and Bayer Schering (paid to the University Hospital Basel). V.S. received research support from Biogen Idec and Novartis. NM reports no disclosures. M.P. is supported by the Non-profit Karol Wojtila Association (Lavagna, Italy) and has received research support from Novartis. R.S. reports no disclosures. C.W.K. was on the advisory board for BG12 (Biogen) and is editor of Functional Neurology. D.H.M has received honoraria from Biogen Idec, Novartis, GlaxoSmithKline, and Bayer Schering, and research grant support for undertaking MRI analysis in multiple sclerosis trials sponsored by GlaxoSmithKline, Biogen Idec and Novartis. D.T.C. has received honoraria (paid to UCL) from Bayer, Teva and the Serono Symposia International Foundation for faculty-led education work; Teva for advisory board work; meeting expenses from Teva; and holds stock in GlaxoSmithKline.
Supplementary material
Supplementary material is available at Brain online.
References
- Adams CW. The onset and progression of the lesion in multiple sclerosis. J Neurol Sci. 1975;25:165–82. doi: 10.1016/0022-510x(75)90138-0. [DOI] [PubMed] [Google Scholar]
- Adams CW, Abdulla YH, Torres EM, Poston RN. Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol. 1987;13:141–52. doi: 10.1111/j.1365-2990.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Beggs CB. Venous hemodynamics in neurological disorders: an analytical review with hydrodynamic analysis. BMC Med. 2013;11:142. doi: 10.1186/1741-7015-11-142. doi: 10.1186/1741-7015-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–32. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CAM. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging. 2010;32:223–8. doi: 10.1002/jmri.22214. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Barker GJ, Molyneux PD, Miller DH. Technical note: the comparison of hypointense lesions from ‘pseudo-T1’ and T1-weighted images in secondary progressive multiple sclerosis. Mult Scler. 2002;8:433–435. doi: 10.1191/1352458502ms824xx. [DOI] [PubMed] [Google Scholar]
- Horsfield MA. Magnetization transfer imaging in multiple sclerosis. J Neuroimaging. 2005;15:58S–67S. doi: 10.1177/1051228405282242. [DOI] [PubMed] [Google Scholar]
- Hughes D, Field EJ. Myelotoxicity of serum and spinal fluid in multiple sclerosis: a critical assessment. Clin Exp Immunol. 1967;2:295–309. [PMC free article] [PubMed] [Google Scholar]
- Kearney H, Yiannakas MC, Samson RS, Wheeler-Kingshott CA, Ciccarelli O, Miller DH. Investigation of magnetization transfer ratio-derived pial and subpial abnormalities in the multiple sclerosis spinal cord. Brain. 2014;137:2456–68. doi: 10.1093/brain/awu171. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci. 2003;206:187–91. doi: 10.1016/S0022-510X(02)00421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Multiple sclerosis: lessons from molecular neuropathology. Exp Neurol. 2014;262:2–7. doi: 10.1016/j.expneurol.2013.12.003. http://dx.doi.org/10.1016/j.expneurol.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–93. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Modat M, Ridgway GR, Taylor ZA, Lehmann M, Bames J, Hawkes DJ, et al. Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed. 2010;98:278–84. doi: 10.1016/j.cmpb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Muhlert N, Sethi V, Schneider T, Daga P, Cipolotti L, Haroon HA, et al. Diffusion MRI-based cortical complexity alterations associated with executive function in multiple sclerosis. J Magn Reson Imaging. 2013;38:54–63. doi: 10.1002/jmri.23970. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B, Picard-Riera N, Kerninon C, Decker L, Seilhean D, Hoglinger GU, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci USA. 2007;l04:4694–9. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Fu L, Pioro E, De Stefano N, Collins DL, Francis GS. Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol. 1997;41:385–91. doi: 10.1002/ana.410410314. [DOI] [PubMed] [Google Scholar]
- Ourselin S, Roche A, Subsol G, Pennec X, Ayache N. Reconstructing a 3D structure from serial histological sections. Image Vis Comput. 2001;19:25–31. [Google Scholar]
- Papadopoulou A, Menegola M, Kuhle J, Ramagopalan SV, D’Souza M, Sprenger T, et al. Lesion-to-ventricle distance and other risk factors for the persistence of newly formed black holes in relapsing-remitting multiple sclerosis. Mult Scler. 2014;20:322–30. doi: 10.1177/1352458513495583. [DOI] [PubMed] [Google Scholar]
- Polman CH, Wolinsky JS, Reingold SC. Multiple sclerosis diagnostic criteria: three years later. Mult Scler. 2005;11:5–12. doi: 10.1191/1352458505ms1135oa. [DOI] [PubMed] [Google Scholar]
- Samson RS, Cardoso MJ, Muhlert N, Sethi V, Wheeler-Kingshott CA, Ron M, et al. Investigation of outer cortical magnetisation transfer ratio abnormalities in multiple sclerosis clinical subgroups. Mult Scler. 2014;20:1322–30. doi: 10.1177/1352458514522537. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–15. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- Vidaurre OG, Haines JD, Katz Sand I, Adula KP, Huynh JL, McGraw CA, et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain. 2014;137:2271–86. doi: 10.1093/brain/awu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrenken H, Geurts JJ, Knol DL, Polman CH, Castelijns JA, Pouwels PJ, et al. Normal-appearing white matter changes vary with distance to lesions in multiple sclerosis. AJNR Am J Neuroradiol. 27:2005–11. [PMC free article] [PubMed] [Google Scholar]
- Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology. 2006;67:960–7. doi: 10.1212/01.wnl.0000237551.26858.39. [DOI] [PubMed] [Google Scholar]