Abstract
Background:
High hepatitis C cure rates have been observed in registration trials with second-generation direct-acting antivirals. Real-world data also indicate high sustained viral response (SVR) rates. Our objective was to determine real-world SVR rates for patients infected with hepatitis C virus (HCV) who were treated with second-generation direct-acting antivirals in the first 18 months of their availability in Canada.
Methods:
Four centres in Calgary contributed their treatment data for a diverse patient population including those who had or had not undergone liver transplantation, those coinfected with HIV and vulnerable populations. We included all patients documented to have started hepatitis C treatment with direct-acting antivirals between October 2014 and April 2016, with follow-up through October 2016. We used multivariate analysis to determine independent predictors of treatment failure.
Results:
Outcome data were available for 351 patients, of whom 326 (92.9%) achieved an SVR (193/206 [93.7%], 57/59 [96.6%] and 44/51 [86.3%] for genotypes 1a, 1b and 3, respectively, p = 0.2). Independent predictors of not achieving SVR were older age (adjusted odds ratio [OR] 0.95 [95% confidence interval (CI) 0.90-1.00]), male sex (adjusted OR 0.30 [95% CI 0.10-0.89]) and, in patients with genotype 1a infection, history of hepatocellular carcinoma (adjusted OR 0.13 [95% CI 0.03-0.53]). In the entire cohort, the presence of cirrhosis, genotype and hepatocellular carcinoma were not associated with a lower SVR rate. There were no differences in SVR rate according to treatment centre, HIV coinfection or liver transplantation. Among patients with genotype 3 infection, a significantly lower SVR rate was observed for those treated outside of standard of care than for those treated within standard of care (33.3% v. 89.6%, p = 0.04). De novo hepatocellular carcinoma developed in 12 patients (3.4%) despite successful direct-acting antiviral therapy.
Interpretation:
We report high SVR rates in a real-world diverse cohort of HCV-infected patients treated with second-generation direct-acting antivirals. The results highlight the importance of conducting real-world analyses to elucidate clinical factors associated with poorer outcomes that may not be identified in registration trials.
Unprecedented cure rates for people chronically infected with hepatitis C virus (HCV) have been achieved with interferon-free regimens. Results of phase III clinical trials of sofosbuvir-ledipasvir and of ombitasvir-paritaprevir-ritonavir and dasabuvir, with or without ribavirin, showed sustained viral response (SVR) rates of 94%-99%1-3 and 97%-99.5%,4 respectively. With other hepatitis C treatments, real-world experiences5 have not shown SVR rates equal to those achieved in clinical trials, including studies of simeprevir-sofosbuvir (81%-87%,6 79%-88%7 and 95%8) and of interferon regimens containing telaprevir or boceprevir.9 In addition, higher complication rates have been observed in real-world studies.5,10 Furthermore, results of direct-acting antiviral trials may have limited generalizability, since most people coinfected with HIV/HCV were excluded.9
In Canada, sofosbuvir-ledipasvir has been available since October 2014, and ombitasvir-paritaprevir-ritonavir and dasabuvir, since 2015. Both regimens were included on the Alberta drug benefits list (i.e., public reimbursement) in 2015. These HCV treatments were available earlier in most western countries, with some real-world experiences reported.11,12 Canadian data on real-world treatment are lacking. Our objective was to determine SVR rates with second-generation direct-acting antivirals in the first 18 months of their availability in Canada. A secondary objective was to assess predictors of SVR.
Methods
Setting and design
In this retrospective cohort study, we extracted and analyzed data from a common database for all HCV-infected patients in our city. Specifically, we included all patients documented to have started hepatitis C treatment between October 2014 to April 2016, with follow-up through October 2016. Patients were excluded if they did not start hepatitis C treatment. Patients were seen at 4 treatment centres in Calgary serving a catchment area of about 2 million people. The diverse cohort of patients was seen at university hepatology clinics at 2 sites (Calgary Liver Unit, Foothills Hospital and South Health Campus), a community clinic treating vulnerable populations (Calgary Urban Project Society) and a community clinic for HIV-coinfected patients (Southern Alberta Clinic). The Calgary Liver Unit sites have an academic profile of research, education and clinical practice served by 7 hepatologists as well as nurses and nurse practitioners. The Calgary Urban Project Society is an inner-city multidisciplinary clinic serving marginalized patients with an infectious diseases specialist and nurses. The hepatologist and infectious diseases specialists at the Southern Alberta Clinic provide care to all HIV-infected patients in southern Alberta, with pharmacy and nursing support. All physicians in this study were local HCV experts, authorized to prescribe direct-acting antivirals under the Alberta Blue Cross insurance plan.
Data sources
Standardized data capture and extraction was used at all sites. A manual chart review was required to extract transient elastography values. Data from the Calgary Liver Unit sites were cross-referenced to electronic medical records. Data from the Southern Alberta Clinic were cross-referenced to pharmacy records (J.K.), and data from the Calgary Urban Project Society were cross-referenced to electronic/paper records (G.M.).
Most outcomes were binary based on the raw data. These did not require dispute resolution. The only outcome that required some discussion was adherence to treatment guidelines, some of which changed minimally during the period of observation. Disagreements were resolved through discussion between 2 authors (A.I.A. and C.S.C.) and review of HCV literature until consensus was reached. Both A.I.A. and C.S.C. have valid International Conference for Harmonization Good Clinical Practice certificates. A.I.A. is a practising hepatologist with more than 12 years' experience in clinical trials, viral hepatitis and liver transplantation. C.S.C. has extensive experience in conducting clinical and translational research on viral hepatitis and is involved in nationwide cohort/epidemiological studies on HCV and hepatitis B virus (HBV).
We have previously published studies using the Calgary Liver Unit viral hepatitis database13,14 including the Southern Alberta Clinic database for HIV-infected patients coinfected with HCV or HBV.15,16 The former database is used by viral hepatitis nurses for patients receiving antiviral therapy and is regularly checked for completeness and accuracy. All data sources are valid and complete. Quality-control measures have been applied to these databases. The completeness of the data was greater than 90% for all variables.
Study variables
We obtained patients' demographic characteristics including age and sex. Clinical and laboratory data included previous HCV treatment (yes/no); liver stiffness measurement by transient elastography at baseline and follow-up; change in liver stiffness measurement between baseline and follow-up; baseline HCV RNA level; previous history of hepatocellular carcinoma (yes/no); hepatocellular carcinoma development after starting treatment (yes/no); coinfection with HBV (yes/no); coinfection with HIV (yes/no), HCV genotype; baseline glomerular filtration rate; having stage 3 or higher chronic kidney disease (yes/no) and cirrhosis at baseline (FibroScan [Echosens] value ≥ 12.5 kPa and/or biopsy showing Metavir F4 fibrosis [cirrhosis]) (yes/no); histologic type (Metavir stage F1-F4); model for end-stage liver disease (MELD) score at baseline; MELD with sodium score at baseline; treatment according to standard of care (yes/no); and treating centre.
Two authors (A.I.A. and C.S.C.) reviewed all data to determine whether the patients had been treated within labelling recommended by Health Canada. Canadian product monographs published during the study period and recommendations based on registration trials for direct-acting antivirals were considered to be standard of care17,18 (Appendix 1, available at www.cmajopen.ca/content/6/1/E12/suppl/DC1).
Outcomes
The primary outcome of interest was undetectable virus 12 weeks after the end of therapy (SVR). We also assessed other short-term outcomes, including change in liver stiffness measurement and development of hepatocellular carcinoma.
Statistical analysis
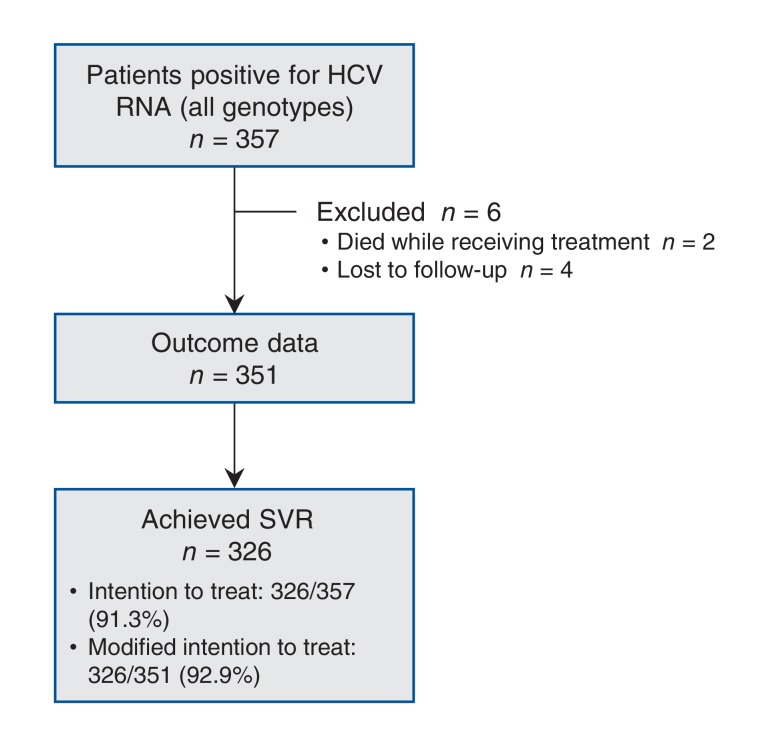
The primary intention-to-treat outcome was SVR. The modified intention-to-treat outcome excluded the very small number of deaths during the treatment period (n = 2) and patients lost to follow up (n = 4) (Figure 1). We did not do a sample size calculation because our intention was to capture all those known to have been treated for hepatitis C within the defined time period (this was a sample of convenience). A post hoc power calculation was done. Specifically, the cohort with genotype 1 infection was compared to a standard reported SVR rate of 95%1,3 (87% power to detect a 5% difference in SVR rate). We determined HCV RNA viral load by means of the RealTime polymerase chain reaction assay (lower limit of detection 12 IU/mL) (Abbott Laboratories).
Figure 1.
Flow diagram showing patients with data available for analysis. Note: HCV = hepatitis C virus, SVR = sustained viral response.
We performed bivariate analyses using the Fisher exact test for categorical data and the Wilcoxon rank sum test for continuous data. Categorical data were expressed as percentages, and continuous data were expressed as medians with interquartile ranges. We created a logistic regression model to evaluate variables that predicted SVR (age, sex, previous treatment, genotype, baseline transient elastography measurement, baseline HCV RNA level, MELD score, MELD with sodium score, presence of cirrhosis, previous hepatocellular carcinoma and treatment per guidelines). Sensitivity analyses were done to assess the regression model among different genotypes. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are reported. We performed all statistical analyses using Stata release 14 (StataCorp). An a priori significance level of 0.05 was used.
Ethics Approval
Ethics approval was obtained through the University of Calgary Conjoint Ethics Review Board and Alberta Health Services Institutional Review Board.
Results
In total, 357 HCV-infected patients were enrolled. Outcome data were available for 351 patients (98.3%) during the first 18 months of direct-acting antiviral availability in Canada (Figure 1). Demographic, clinical and laboratory characteristics of the cohort are presented in Table 1. Of the 6 patients for whom SVR information was not available, 2 died while receiving treatment, and 4 were lost to follow-up; 3 of the latter group had undetectable HCV RNA at the end of treatment.
Table 1: Demographic, clinical and laboratory characteristics of patients infected with hepatitis C virus according to SVR status.
| Characteristic | No. (%) of patients* | p value | |
|---|---|---|---|
| Achieved SVR n = 326 |
Did not achieve SVR n = 25 |
||
| Age at treatment, median (IQR), yr | 57 (50-61) | 60 (56-64) | 0.02 |
| Sex | |||
| Male (n = 216) | 195 (90.3) | 21 (9.7) | 0.02 |
| Female (n = 135) | 131 (97.0) | 4 (3.0) | |
| Had previous treatment (n = 99) | 91 (91.9) | 8 (8.1) | 0.6 |
| Genotype (n = 350)† | |||
| 1a (n = 206) | 193 (93.7) | 13 (6.3) | 0.5 |
| 1b (n = 59) | 57 (96.6) | 2 (3.4) | 0.3 |
| 2 (n = 25) | 22 (88.0) | 3 (12.0) | 0.4 |
| 3 (n = 51) | 44 (86.3) | 7 (13.7) | 0.1 |
| 4 and 6 (n = 9) | 9 (100.0) | 0 (0.0) | 1.0 |
| FibroScan measurement at baseline, median (IQR), kPa | 10.2 (6.7-20.4) | 12.5 (7.6-26.3) | 0.1 |
| HCV RNA level at baseline, median (IQR), IU/mL | 701 684 (158 376-1 677 684) | 500 000 (94 082-1 500 000) | 0.5 |
| Chronic kidney disease at baseline (n = 16) | 16 (100.0) | 0 (0.0) | 0.6 |
| Estimated glomerular filtration rate at baseline, median (IQR), mL/min | 94 (81-101) | 96 (83-99) | 0.6 |
| Coinfection with HIV (n = 12) | 12 (100.0) | 0 (0.0) | 1.0 |
| Coinfection with HBV (n = 1) | 1 (100.0) | 0 (0.0) | 1.0 |
| Cirrhosis at baseline (n = 350) | |||
| Yes (n = 149) | 133 (89.3) | 16 (10.7) | 0.03 |
| No (n = 201) | 192 (95.5) | 9 (4.5) | |
| Previous history of hepatocellular carcinoma (n = 20) | 16 (80.0) | 4 (20.0) | 0.04 |
| Liver transplantation (n = 9) | 9 (100.0) | 0 (0.0) | 1.0 |
| Site | |||
| 1 (n = 11) | 11 (100.0) | 0 (0.0) | |
| 2 (n = 40) | 37 (92.5) | 3 (7.5) | 0.7 |
| 3 (n = 110) | 100 (90.9) | 10 (9.1) | |
| 4 (n = 190) | 178 (93.7) | 12 (6.3) | |
| Died during follow-up (n = 6) | 3 (50.0) | 3 (50.0) | < 0.01 |
| Development of hepatocellular carcinoma during follow-up (n = 12) | 11 (91.7) | 1 (8.3) | 0.6 |
| MELD score, median (IQR) | 6 (6-6) | 6 (6-6) | 1.0 |
| MELD with sodium score, median (IQR) | 7 (5-8) | 6 (5-8) | 0.9 |
Note: HBV = hepatitis B virus, HCV = hepatitis C virus, IQR = interquartile range, MELD = model for end-stage liver disease, SVR = sustained viral response.
*Except where noted otherwise.
†One patient was treated without an identified genotype and achieved an SVR.
Across all genotypes, by intention-to-treat, 326/357 (91.3%) achieved an SVR, and by modified intention-to-treat, 326/351 (92.9%) achieved an SVR. Patients who achieved an SVR were likely to be younger (age 57 yr v. 60 yr, p = 0.02), female (SVR 97.0% v. 90.3% for males, p = 0.02), noncirrhotic (SVR 95.5% v. 89.3% for those with cirrhosis, p = 0.03) and have no history of hepatocellular carcinoma at diagnosis (93.7% v. 80% for those with a history of hepatocellular carcinoma at diagnosis, p = 0.04) (Table 1).
Of the 351 patients, 6 (4 males [66.7%], median age 60 yr) died after treatment. The SVR rate was significantly lower in this group (3/6 [50.0%] than in those who lived (323/345 [93.6%]) (p < 0.01). There was no impact of baseline MELD score, history of hepatocellular carcinoma or liver transplantation on patient survival. De novo hepatocellular carcinoma developed in 12 patients (3.4%), 11 of whom had achieved an SVR.
Hepatitis C virus genotype was known for 350 patients, of whom 206 (58.8%) had genotype 1a disease. There was no significant difference in SVR rate between genotypes (Table 1) (p = 0.2). The baseline transient elastography value was similar among patients who achieved an SVR and those who did not (median 10.2 kPa v. 12.5 kPa, p = 0.1). SVR rates were not influenced by baseline renal function, previous treatment, baseline HCV RNA level, MELD score, MELD with sodium score, liver transplantation or HIV coinfection (Table 1). The location of the 4 treating sites (i.e., university v. community) did not affect SVR rates (p = 0.7).
Most patients (338 [96.3%]) were treated with a sofosbuvir-containing regimen. Twenty-four patients (6.8%) received a direct-acting antiviral with pegylated interferon.
Among patients with genotype 1a infection, SVR rates among those who received sofosbuvir-ledipasvir (93.0%) or sofosbuvir-ledipasvir and ribavirin (92.3%) were comparable to rates among those treated with simeprevir-sofosbuvir (100.0%), pegylated interferon and a direct-acting antiviral (92.3%), or ombitasvir-paritaprevir-ritonavir and dasabuvir with or without ribavirin (100.0%) (p = 0.8) (Table 2). Similarly, among patients with genotype 1b disease, the SVR rate for those treated with sofosbuvir-ledipasvir (95.8%) was comparable to the rate for patients treated with all other regimens (100.0%) (p = 1.0).
Table 2: Sustained viral response status by genotype and treatment regimen.
| Regimen | No. (%) of patients | p value | |
|---|---|---|---|
| Achieved SVR n = 326 |
Did not achieve SVR n = 25 |
||
| Genotype 1a (n = 206) | 193 (93.7) | 13 (6.3) | 0.5 |
| Sofosbuvir-ledipasvir (n = 158) | 147 (93.0) | 11 (7.0) | |
| Sofosbuvir-ledipasvir + ribavirin (n = 13) | 12 (92.3) | 1 (7.7) | |
| Simeprevir-sofosbuvir (n = 17) | 17 (100.0) | 0 (0.0) | 0.8 |
| Pegylated interferon + direct-acting antiviral (n = 13) | 12 (92.3) | 1 (7.7) | |
| Ombitasvir-paritaprevir-ritonavir and dasabuvir ± ribavirin (n = 5) | 5 (100.0) | 0 (0.0) | |
| Genotype 1b (n = 59) | 57 (96.6) | 2 (3.4) | 0.2 |
| Sofosbuvir-ledipasvir (n = 48) | 46 (95.8) | 2 (4.2) | |
| Sofosbuvir-ledipasvir + ribavirin (n = 1) | 1 (100.0) | 0 (0.0) | |
| Simeprevir-sofosbuvir (n = 2) | 2 (100.0) | 0 (0.0) | 1.0 |
| Pegylated interferon + direct-acting antiviral (n = 3) | 3 (100.0) | 0 (0.0) | |
| Ombitasvir-paritaprevir-ritonavir and dasabuvir ± ribavirin (n = 5) | 5 (100.0) | 0 (0.0) | |
| Genotype 2 (n = 25) | 22 (88.0) | 3 (12.0) | 0.4 |
| Sofosbuvir + ribavirin (n = 23) | 20 (87.0) | 3 (13.0) | |
| Pegylated interferon + direct-acting antiviral (n = 2) | 2 (100.0) | 0 (0.0) | |
| Genotype 3 (n = 51) | 44 (86.3) | 7 (13.7) | 0.1 |
| Sofosbuvir-ledipasvir + ribavirin (n = 1) | 1 (100.0) | 0 | |
| Sofosbuvir + ribavirin (n = 45) | 38 (84.4) | 7 (15.6) | |
| Pegylated interferon + direct-acting antiviral (n = 5) | 5 (100.0) | 0 (0.0) | |
| Other (n = 9) | 9 (100.0) | 0 (0.0) | 1.0 |
| Sofosbuvir-ledipasvir (n = 4) | 4 (100.0) | 0 (0.0) | |
| Sofosbuvir-ledipasvir + ribavirin (n = 1) | 1 (100.0) | 0 (0.0) | |
| Sofosbuvir + ribavirin (n = 2) | 2 (100.0) | 0 (0.0) | 1.0 |
| Pegylated interferon + direct-acting antiviral (n = 1) | 1 (100.0) | 0 (0.0) | |
| Ombitasvir-paritaprevir-ritonavir and dasabuvir ± ribavirin (n = 1) | 1 (100.0) | 0 (0.0) | |
Note: SVR = sustained viral response.
For patients with genotype 3 infection treated with sofosbuvir-ribavirin, the SVR rate of 84.4% was similar to that with other regimens (p = 0.6) (Table 2), but the small number of patients treated with other regimens limited meaningful comparisons. Nonetheless, in this group, most of whom were treated with sofosbuvir-ribavirin, 28/51 (54.9%) had cirrhosis, compared to 121/299 (40.5%) for all other genotypes (p = 0.07). Despite the overrepresentation of patients with cirrhosis in the genotype 3 group, genotype 3 by itself did not influence the SVR rate (Table 2).
Most patients were treated within established treatment guidelines (Appendix 1). For most patients with a genotype other than genotype 3, treatment within or outside of treatment guidelines did not affect SVR (Appendix 1). However, for the small number of patients with genotype 3 disease who were treated with sofosbuvir-ribavirin outside of standard of care (n = 3), a significantly lower SVR rate was noted compared to those treated within standard of care (33.3% v. 89.6%) (p = 0.04).
We calculated the change in transient elastography value between baseline and follow-up for 183 patients (data not shown). The reduction in transient elastography value was similar for those who achieved an SVR (n = 167) and those who did not (n = 16) (-2.8 kPa v. -2.7 kPa) (p = 0.3). However, among patients with genotype 1a infection, the reduction in transient elastography value was significantly greater among those who achieved an SVR than among those who did not (-2.9 kPa v. -0.3 kPa) (p = 0.02).
Among the overall cohort of 357 patients, the end-of-treatment polymerase chain reaction assay for HCV RNA gave a positive result with the viral load at or below the level of quantification in 49 patients (13.7%), most of whom (40 [81.6%]) achieved an SVR. We did not observe any cases of HBV reactivation.19
Significant predictors of not achieving an SVR identified by univariate analysis were older age, male sex, the presence of baseline cirrhosis and previous history of hepatocellular carcinoma. However, in multivariate modelling, only older age (adjusted OR 0.95 [95% CI 0.90-1.00]) and male sex (adjusted OR 0.30 [95% CI 0.10-0.89]) were associated with not achieving an SVR (Table 3). In a sensitivity analysis limiting the cohort to patients with genotype 1a infection, only male sex (adjusted OR 0.12 [95% CI 0.02-0.98]) and previous history of hepatocellular carcinoma (adjusted OR 0.13 [95% CI 0.03-0.53]) were associated with not achieving an SVR (data not shown). Similar results were observed in the modified intention-to-treat subgroup.
Table 3: Predictors of achieving an SVR among all patients.
| Variable | Univariate analysis OR (95% CI) |
Multivariate analysis adjusted OR (95% CI) |
|---|---|---|
| Age | 0.95 (0.90-0.99) | 0.95 (0.90-1.00) |
| Male sex | 0.28 (0.10-0.84) | 0.30 (0.10-0.89) |
| Previous treatment | 0.82 (0.34-1.97) | - |
| Genotype 1a | 1.34 (0.59-3.03) | - |
| FibroScan measurement at baseline (per kPa) | 0.99 (0.97-1.02) | - |
| Viremia (per 1000 IU) | 1.00 (0.99-1.02) | - |
| Cirrhosis at baseline | 0.39 (0.17-0.91) | 0.56 (0.23-1.36) |
| Treated according to guidelines | 1.09 (0.36-3.32) | - |
| Previous history of hepatocellular carcinoma | 0.27 (0.08-0.88) | 0.37 (0.11-1.28) |
| MELD score at baseline | 1.76 (0.35-8.97) | - |
| MELD with sodium score at baseline | 1.01 (0.78-1.31) | - |
Note: CI = confidence interval, MELD = model for end-stage liver disease, OR = odds ratio, SVR = sustained viral response.
Interpretation
In this multicentre retrospective Canadian cohort study, we observed high SVR rates in a real-world, diverse cohort of HCV-infected patients treated with second-generation direct-acting antivirals. All 11 patients treated with ombitasvir-paritaprevir-ritonavir and dasabuvir with or without ribavirin achieved an SVR. There was a high proportion of patients with cirrhosis (42.4%), likely stemming from the "warehousing" of patients that occurred before October 2014. A relatively small number of patients (n = 24) were treated with pegylated interferon, ribavirin and a second-generation direct-acting antiviral; a higher than expected SVR rate, 95.8%, was seen in this group. We observed no differences in SVR rate between the 4 treatment sites, including the single site that treated patients coinfected with HIV. All liver transplant recipients achieved an SVR. Treatment outside of established standard of care did not affect SVR in most patients; however, the SVR rate was significantly lower among a small number of patients with genotype 3 disease treated outside of standard of care compared to those treated within standard of care. Consistent with other observations,20 de novo hepatocellular carcinoma developed in 12 patients (3.4%) up to 6 months after treatment. This highlights the need for screening for hepatocellular carcinoma after hepatitis C treatment, especially among patients with cirrhosis. Most of the 49 patients with a positive result on the end-of-treatment HCV RNA polymerase chain reaction assay ultimately achieved SVR, perhaps reflecting a more sensitive HCV RNA assay compared to previous eras of hepatitis C treatment. Independent predictors of not achieving an SVR were male sex, older age and, in patients with genotype 1a disease, history of hepatocellular carcinoma.
Our patient population was similar to other reported real-world cohorts in age, previous hepatitis C treatment and presence of cirrhosis.11,12 Our high SVR rates are also comparable to other registration trial and real-world data for patients treated with sofosbuvir-ledipasvir with or without ribavirin (ION-1 study 98%-99%,1 ION-2 study 94%-99%,2 ION-3 study 93%-95%,3 Trio Health study 94%,11 HCV-TARGET study 96%-97%12), ombitasvir-paritaprevir-ritonavir and dasabuvir (SAPPHIRE-I study 95%-98%,21 TURQUOISE-II study 92%-96%22) simeprevir-sofosbuvir (COSMOS study 95%7) and pegylated interferon, ribavirin and direct-acting antiviral (NEUTRINO study 90%23). Disparate findings from registration trial data that we identified in this real-world cohort included pretreatment patient characteristics predictive of treatment failure. Our findings add to a growing body of real-world literature that has identified other risk factors predictive of treatment failure (i.e., concomitant treatment with proton pump inhibitors).11
Limitations
Although our study was powered to detect SVR variability in patients with genotype 1 infection, one should be cautious in interpreting results for other genotypes owing to the small sample. Furthermore, for some treatment regimens (ombitasvir-paritaprevir-ritonavir and dasabuvir, simeprevir-sofosbuvir, and pegylated interferon, ribavirin and direct-acting antiviral) there were relatively few patients, which limits the generalizability of those observations. Our follow-up period of 6-18 months was relatively short. Future studies assessing long-term outcomes are ongoing. A total of 31.8% of patients with genotype 1 infection who could have been treated for 8 weeks with sofosbuvir-ledipasvir received 12 weeks of treatment. Results from the ION-3 trial2 indicate that there would be no expected decrement in SVR for selected patients, and this is an area for improvement and potential substantial cost savings.
Conclusion
We found high SVR rates in HCV-infected patients, even in difficult-to-treat patients and those with complex conditions, in both university and community centres. Our study also highlights risk factors for failure of hepatitis C treatment, identifies areas for optimizing management of the disease (i.e., treatment within standard-of-care guidelines, irrelevance of end-of-treatment viral load testing, ongoing need for hepatocellular carcinoma surveillance following treatment) and illustrates the relevance of obtaining real-world treatment data.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/6/1/E12/suppl/DC1.
Supplementary Material
Acknowledgements
The authors thank the patients, staff and other physicians of the Calgary Liver Unit, Southern Alberta Clinic and Calgary Urban Project Society.
References
- 1.Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 2.Kowdley KV, Gordon SC, Reddy KR, et al. ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 4.Ferenci P, Bernstein D, Lalezari J, et al. PEARL-III StudyPEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 5.Hézode C, Fontaine H, Dorival C, et al. CUPIC Study Group. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132–42.e4. doi: 10.1053/j.gastro.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. HCV-TARGET Study Group. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150:419–29. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–65. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 8.Lawitz E, Matusow G, DeJesus E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: a phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360–9. doi: 10.1002/hep.28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeed S, Strumpf EC, Walmsley SL, et al. How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis. 2016;62:919–26. doi: 10.1093/cid/civ1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SC, Muir AJ, Lim JK, et al. HCV-TARGET study group. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real world experience from HCV-TARGET. J Hepatol. 2015;62:286–93. doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper EB, Bacon BR, Curry MP, et al. Real-world effectiveness for 12 weeks of ledipasvir-sofosbuvir for genotype 1 hepatitis C: the Trio Health study. J Viral Hepat. 2017;24:22–7. doi: 10.1111/jvh.12611. [DOI] [PubMed] [Google Scholar]
- 12.Terrault NA, Zeuzem S, Di Bisceglie AM, et al. HCV-TARGET Study Group. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–40.e5. doi: 10.1053/j.gastro.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaheen AA, AlMattooq M, Yazdanfar S, et al. Tenofovir disoproxil fumarate significantly decreases serum lipoprotein levels compared with entecavir nucleos(t)ide analogue therapy in chronic hepatitis B carriers. Aliment Pharmacol Ther. 2017;46:599–604. doi: 10.1111/apt.14218. [DOI] [PubMed] [Google Scholar]
- 14.Coffin CS, Rezaeeaval M, Pang JX, et al. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment Pharmacol Ther. 2014;40:1262–9. doi: 10.1111/apt.12990. [DOI] [PubMed] [Google Scholar]
- 15.O'Neil CR, Pang JX, Lee SS, et al. Treatment outcomes with telaprevir-based therapy for HIV/hepatitis C coinfected patients are comparable with hepatitis C monoinfected patients. Can J Infect Dis Med Microbiol. 2015;26:293–6. doi: 10.1155/2015/974871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffin CS, Osiowy C, Myers RP, et al. Virology and clinical sequelae of long-term antiviral therapy in a North American cohort of hepatitis B virus (HBV)/human immunodeficiency virus type 1 (HIV-1) co-infected patients. J Clin Virol. 2013;57:103–8. doi: 10.1016/j.jcv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.HARVONI (ledipasvir/sofosbuvir tablets [product monograph]. Foster City (CA): Gilead Sciences Inc.; 2012. [accessed 2017 Dec. 11]. Available www.gilead.ca/application/files/3214/9754/4243/harvoni_pm_english.pdf.
- 18.SOVALDI (sofosbuvir) tablets [product monograph]. Foster City (CA): Gilead Sciences; 2016:1-58. [accessed 2017 Dec. 11]. Available www.gilead.ca/application/files/9714/9704/5557/sovaldi_pm_english.pdf.
- 19.Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus. Ann Intern Med. 2017;167:760. doi: 10.7326/L17-0477. [DOI] [PubMed] [Google Scholar]
- 20.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–33. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 22.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973–82. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 23.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.