Abstract
Hepatic cystogenesis in polycystic liver disease (PLD) is associated with abnormalities in multiple cellular processes, including elevated cAMP and overexpression of histone deacetylase 6 (HDAC6). Disease progression in polycystic kidney (PCK) rats (an animal model of PLD) is attenuated by inhibition of either cAMP production or HDAC6. Therefore, we hypothesized that concurrent targeting of HDAC6 and cAMP would synergistically reduce cyst growth. Changes in hepatorenal cystogenesis were examined in PCK rats treated with a pan-HDAC inhibitor, panobinostat; three specific HDAC6 inhibitors, ACY-1215, ACY-738, and ACY-241; and a combination of ACY-1215 and the somatostatin receptor analogue, pasireotide. We also assessed effects of ACY-1215 and pasireotide alone and in combination on cell proliferation, cAMP production, and expression of acetylated α-tubulin in vitro in cultured cholangiocytes and the length of primary cilia and the frequency of ciliated cholangiocytes in vivo in PCK rats. Panobinostat and all three HDAC6 inhibitors decreased hepatorenal cystogenesis in PCK rats. ACY-1215 was more effective than other HDAC inhibitors and was chosen for combinational treatment. ACY-1215 + pasireotide combination synergistically reduced cyst growth and increased length of primary cilia in PCK rats. In cultured cystic cholangiocytes, ACY-1215 + pasireotide combination concurrently decreased cell proliferation and inhibited cAMP levels. These data suggest that the combination of drugs that inhibit HDAC6 and cAMP may be an effective therapy for PLD.
Polycystic liver disease (PLD) is a genetic cholangiopathy characterized by bile duct dilatation and formation of cholangiocyte-derived hepatic cysts that gradually replace liver tissue. PLD is a liver manifestation of polycystic kidney disease (PKD)—autosomal dominant PKD (ADPKD) and autosomal recessive PKD. ADPKD is associated with mutations in three genes, PKD1, PKD2, and GANAB; mutations in the PKHD1 gene are responsible for cystogenesis in autosomal recessive PKD. PLD also exists alone as autosomal dominant PLD resulting from mutations in multiple genes, PRKCSH, SEC63, LRP5, ALG8, SEC61B, GANAB, and PKHD1.1, 2, 3, 4
Progressive growth of hepatic cysts increases total liver volume by 0.9% to 3.2% and causes serious health problems and decreasing quality of life. Patients may experience constant abdominal pain and distension and may develop multiple complications such as cyst hemorrhage, rupture, or infection.1, 2 The treatment goal in PLD is liver volume reduction to ameliorate symptoms. Interventional therapies include hepatic resection, liver transplantation, cyst aspiration, sclerosis, or fenestration.1, 2, 5 Currently, somatostatin receptor (SSTR) analogues, such as octreotide and lanreotide, are the only available pharmacologic option for PLD patients. However, despite their beneficial effects on hepatic cystogenesis and quality of life, changes in liver volume are modest, and approximately 15% of patients are nonresponders.2, 6, 7, 8, 9 Thus, continued search for new therapies remains of great importance.
Hepatic cystogenesis is associated with multiple abnormal cellular functions (eg, cholangiocyte hyperproliferation, cell cycle disturbances, enhanced fluid secretion) and dysregulated intracellular pathways (eg, cAMP, calcium, mechanistic target of rapamycin, epidermal growth factor receptor, HDAC signaling). Many of these mechanisms have been identified as potential therapeutic targets and tested in preclinical studies.5, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 We have previously suggested and recently demonstrated that combinations of drugs that intervene with several dysregulated pathways or with different components of a single dysregulated pathway might be more effective than single-drug interventions.19, 20 Therefore, here we examined the effects of concurrent targeting of the cAMP signaling pathway by the SSTR agonist, pasireotide, and the HDAC6 pathway by its most potent inhibitor, ACY-1215, on disease progression in polycystic kidney (PCK) rats, an animal model of PLD. We also explore the consequences of drug treatment on cell proliferation, cAMP levels, the length of primary cilia, and the frequency of cilia occurrences.
We found that i) the pan-HDAC inhibitor, panobinostat, and all three specific HDAC6 inhibitors, ACY-1215, ACY-738, and ACY-241, decreased hepatorenal cystogenesis in PCK rats; ii) ACY-1215 was more effective in attenuation of cyst growth than the other tested HDAC inhibitors; iii) the ACY-1215 + pasireotide combination showed greater efficacy on disease progression in PCK rat than each drug alone; iv) the ACY-1215 + pasireotide combination synergistically increased the length of primary cilia in PCK rats; and v) the ACY-1215 + pasireotide combination inhibited cell proliferation and decreased cAMP levels in cultured cholangiocytes more effectively than a single-drug treatment. These data suggest that the concurrent targeting of the cAMP and HDAC6 pathways in cystic cholangiocytes may be a useful therapeutic approach for PLD.
Materials and Methods
Cell Cultures, Animals, and Reagents
PCK cholangiocytes (derived from PCK rats) and ADPKD cholangiocytes (derived from a patient with ADPKD) were used and maintained as described.17 PCK rats were housed in a 12-hour light-dark facility on a standard diet and water ad libitum. Seventy-five PCK rats (our colony, 4 to 5 weeks old) were randomly assigned to assure equal sex representation in each treatment group. The number of animals used in this study was calculated based on our experience, thorough survey of the literature, and after statistical consultation from the Mayo Clinic Center for Translational Science Activities to achieve the total significance level of 0.05. The precise breakdown of animals for each treatment group is shown in the Treatment Protocol that was approved by the Mayo Institutional Animal Care and Use Committee. Pan-HDAC inhibitor, panobinostat, was provided by Novartis Pharmaceutical Inc. (East Hanover, NJ). All three HDAC6 inhibitors, ACY-1215, ACY-738, and ACY-241, were provided by Acetylon Pharmaceuticals Inc. (Boston, MA). Pasireotide and octreotide were purchased, respectively, from MedChem Express (Monmouth Junction, NJ) and Abcam (Cambridge, MA).
Treatment Protocol
First, PCK rats received daily (before noon) i.p. injections of panobinostat (n = 3 females, n = 3 males), ACY-1215 (n = 6 females, n = 5 males), or ACY-241 (n = 6 females, n = 5 males). Doses of HDAC inhibitors (all, 30 mg/kg body weight) are based on published studies21, 22, 23 and were adjusted to the weight of rats weekly. Untreated PCK rats (n = 4 females, n = 4 males) received equal doses of dimethyl sulfoxide (i.p. injections). Second, PCK rats (n = 3 females, n = 4 males) received ACY-738 in a chow diet; untreated PCK rats (n = 4 females, n = 4 males) were kept on a standard chow diet. Third, PCK rats were equally divided into four groups: i) pasireotide-treated (n = 4 females, n = 4 males), ii) ACY-1215–treated (n = 3 females, n = 3 males), iii) ACY-1215 + pasireotide–treated (n = 3 females, n = 3 males), and iv) untreated (n = 4 females, n = 4 males). Pasireotide (20 μg/kg daily) was dissolved in sterile water and administered by osmotic minipumps (model 2002; Alzet Osmotic Pumps, Cupertino, CA) implanted subcutaneously on the animal's back under anesthesia with 1.5% isoflurane (Baxter, Deerfield, IL) as previously described.17 Pumps were replaced every 2 weeks; at this time pasireotide concentrations were adjusted to the animal's weight. ACY-1215 (30 mg/kg body weight) was dissolved in dimethyl sulfoxide and injected (i.p., before noon); doses of ACY-1215 were adjusted to the rat weight every other week. The untreated group received equal doses of dimethyl sulfoxide (i.p. injections) and had implanted pumps filled with sterile water. After 6 weeks of treatment, rats were anesthetized with pentobarbital (50 mg/kg body weight, i.p.), liver and kidneys were harvested, fixed in 10% formaldehyde, and embedded in paraffin for histologic analysis. Body, liver, and kidney weights were assessed. Blood samples were collected from the portal vein for evaluation of serum biochemistry. Cystic and fibrotic areas of liver and kidneys in untreated and drug-treated PCK rats were analyzed as previously described.17 Two liver lobes and two kidney from each rodent were used for analysis. From our previous data demonstrating that the percentage of liver and kidney parenchyma occupied by hepatic and renal cysts in PCK rats do not differ between sexes, measurements of cystic or fibrotic areas in male and female rodents were combined for statistical analysis.16, 17, 24 The primary objective of the study was to assess the changes in hepatic and renal cystic and fibrotic areas, and the secondary objectives were to assess the changes in body, liver, and kidney weights and in serum biochemistries. Experimental outcomes were analyzed in a blinded fashion (M.L.P., T.V.M., and A.I.M.).
Cell Proliferation
As previously described, PCK and ADPKD cholangiocytes (2500 cells/well) were plated for 24 hours before assay.17 Rates of cell proliferation were determined by using CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI). Cystic cholangiocytes were treated with ACY-1215 (10 μmol/L) alone, pasireotide (20 μmol/L) alone, octreotide (20 μmol/L) alone, ACY-1215 + pasireotide combination, and ACY-1215 + octreotide combination for an additional 24 hours. Changes in cell proliferation after treatment were expressed as percentage of change compared with untreated cholangiocytes in which cell proliferation was considered to be equal to 100%.
Immunofluorescence Confocal Microscopy
Livers of untreated and drug-treated PCK rats were first incubated with primary antibodies to acetylated α-tubulin (Sigma-Aldrich, St. Louis, MO; to assess length of primary cilia and frequency of ciliated cholangiocytes), proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology, Dallas, TX; to assess cholangiocyte proliferation), and cytokeratin-19 (Abcam; for cholangiocyte visualization), then with respective secondary fluorescent antibodies (Life Technologies, Eugene, OR). Nuclei were stained with DAPI (Invitrogen, Carlsbad, CA). Images were analyzed by ZeissLSM-510 microscope (Carl Zeiss, Thornwood, NY), and the number of PCNA-positive nuclei was counted per microscopic field.
cAMP
cAMP was measured in PCK and ADPKD cholangiocytes by using cAMP-Screen System (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Cholangiocytes (10,000/well) were incubated with 10 μmol/L ACY-1215, 20 μmol/L pasireotide, or both drugs in combination for 15 to 30 minutes.
Western Blot Analysis
Proteins were separated by 4% to 15% SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and incubated first with primary anti-acetylated α-tubulin and anti–β-actin antibodies (both Sigma-Aldrich) and then with the corresponding secondary horseradish peroxidase-conjugated antibodies (Cell Signaling Technology, Danvers, MA). Bands were visualized with ECL Plus Western Blot Detection kit (BD Biosciences, San Jose, CA). Integrated density of protein bands was assessed by ImageJ software version 1.5.1 (NIH, Bethesda, MD), and data are presented as arbitrary units compared with β-actin.
Statistical Analysis
Data are expressed as means ± SD. Statistical analyses were conducted by one-way analysis of variance for multiple comparisons. The cutoff value for significance was set at P < 0.05.
Results
Pan-HDAC Inhibitor, Panobinostat, Decreases Hepatorenal Cystogenesis in PCK Rats
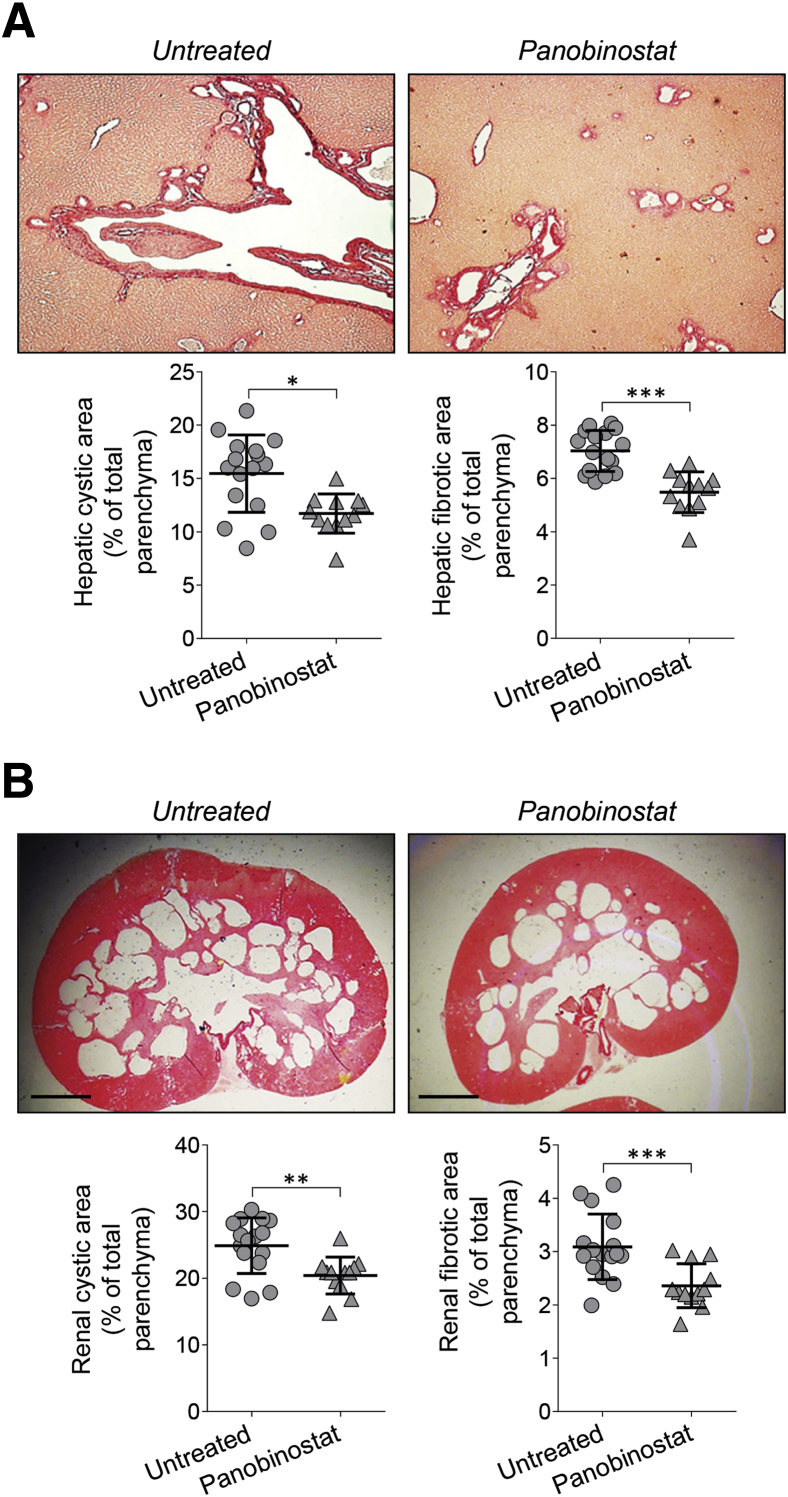
First, the effects of pan-HDAC inhibitor, panobinostat, were assessed on hepatorenal cystogenesis in PCK rats. The treatment protocol is described in Supplemental Figure S1. Panobinostat was well tolerated, without toxicity or death. In response to treatment, hepatic and renal cystogenesis was attenuated as evidenced by decreased hepatic cystic areas (by approximately 24%), hepatic fibrotic areas (by approximately 22%), renal cystic areas (by approximately 18%), and renal fibrotic areas (by approximately 23%) (Figure 1, Table 1). Panobinostat also reduced liver and kidney weights by approximately 10% (Table 1).
Figure 1.
Pan–histone deacetylase (HDAC) inhibitor, panobinostat, decreased hepatic and renal cystogenesis in polycystic kidney (PCK) rats. Drug-treated rats received daily injection of panobinostat (30 mg/kg), and the untreated group were injected with equal doses of dimethyl sulfoxide for 6 weeks. A and B: Representative images of Picrosirius Red–stained liver (A) and kidney (B) sections of untreated and panobinostat-treated PCK rats. Scatter plots depict cystic and fibrotic areas of individual liver lobes and kidneys analyzed. Data are expressed as means ± SD. n = 3 female and 3 male drug-treated rats; n = 4 female and 4 male untreated rats. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars = 250 μm (B). Original magnification, ×4 (A).
Table 1.
Gross Anatomy and Cystic and Fibrotic Areas in Polycystic Kidney Rats Treated with Panobinostat
| Variables | Untreated | Panobinostat |
|---|---|---|
| Body weight, g | ||
| Male | 311.5 ± 13.6 | 299.6 ± 11.8 |
| Female | 211.5 ± 10.2 | 213.1 ± 10.7 |
| Liver weight, g | ||
| Male | 18.9 ± 0.7 | 16.1 ± 1.5∗ |
| Female | 14.0 ± 0.9 | 12.8 ± 1.3∗ |
| Liver/body weight, % | ||
| Male | 6.1 ± 0.8 | 5.5 ± 0.7∗ |
| Female | 6.2 ± 0.7 | 5.8 ± 0.5∗ |
| Kidney weight, g | ||
| Male | 5.9 ± 0.5 | 5.3 ± 0.4∗ |
| Female | 4.2 ± 0.7 | 3.6 ± 0.3∗ |
| Kidney/body weight, % | ||
| Male | 1.9 ± 0.3 | 1.7 ± 0.2∗ |
| Female | 1.9 ± 0.1 | 1.77 ± 0.2∗ |
| Hepatic cystic area, % of total parenchyma | 15.5 ± 3.6 | 11.7 ± 2.8∗∗ |
| Hepatic fibrotic area, % of total parenchyma | 7.0 ± 0.9 | 5.5 ± 0.8∗∗∗ |
| Renal cystic area, % of total parenchyma | 24.9 ± 4.2 | 20.4 ± 2.8∗∗ |
| Renal fibrotic area, % of total parenchyma | 3.1 ± 0.6 | 2.4 ± 0.4∗∗∗ |
Data are expressed as means ± SD.
∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 untreated versus panobinostat.
Selective HDAC6 Inhibitors, ACY-1215, ACY-241, and ACY-738, Decrease Hepatorenal Cystogenesis in PCK Rats
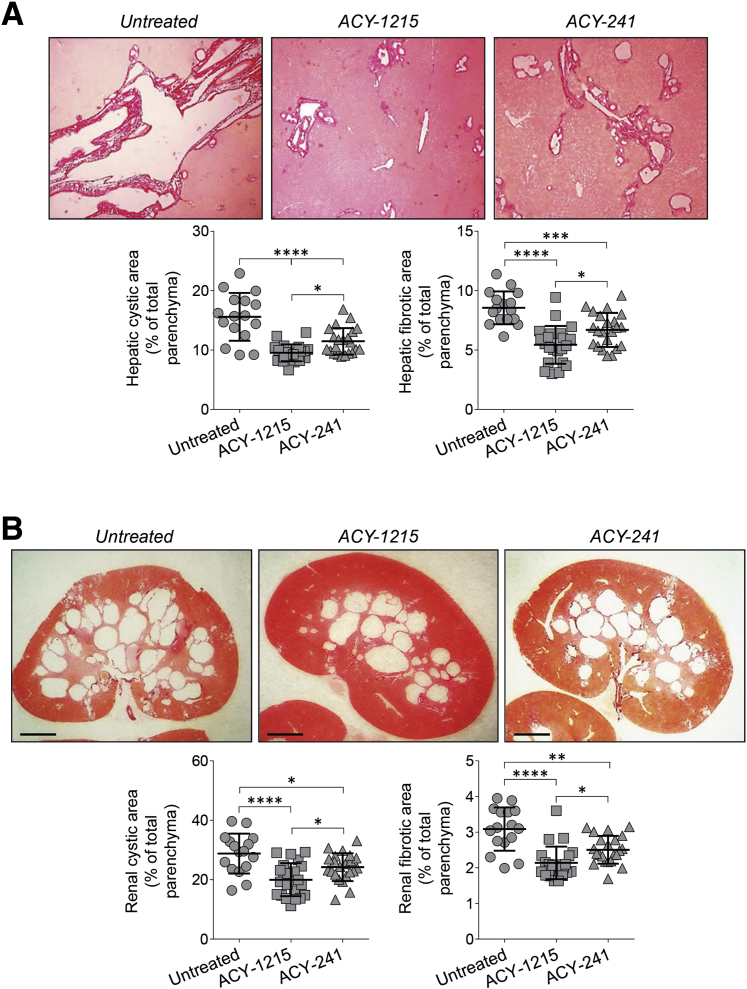
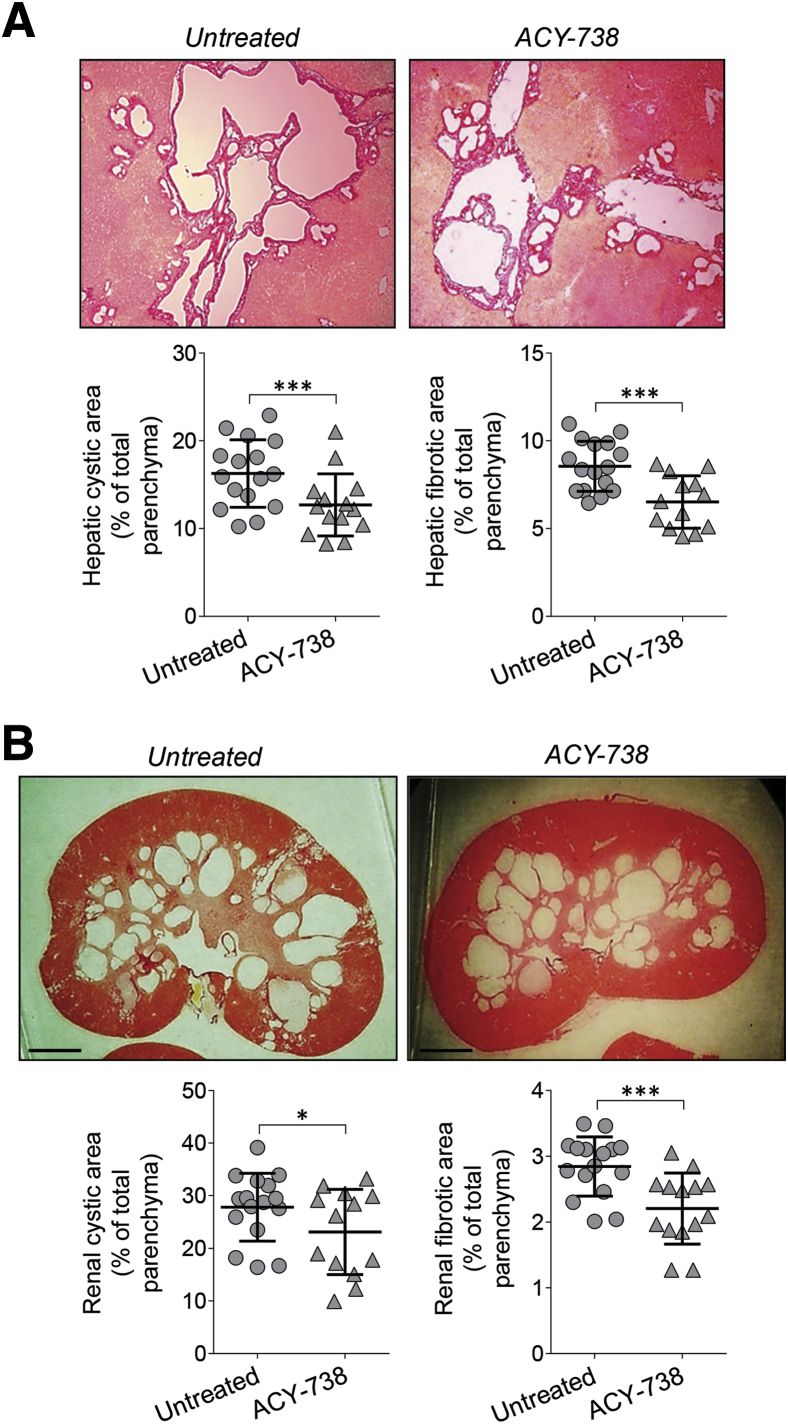
Next, the effects of the three HDAC6-specific inhibitors (ie, ACY-1215, ACY-241, and ACY-738) were examined on hepatorenal cystogenesis in PCK rats. The treatment protocol is described in Supplemental Figure S1. All HDAC6 inhibitors were well tolerated. ACY-1215 and ACY-241 (delivered by i.p. injections) decreased, respectively, hepatic cystic areas by 36% and 27%, hepatic fibrotic areas by 35% and 24%, renal cystic areas by 28% and 16%, and renal fibrotic areas by 30% and 22% (Figure 2, Table 2). Liver and kidney weights in response to treatment were decreased, respectively, by approximately 7% to 8% and 10% to 12% (Table 2). Orally administered ACY-738 decreased hepatic cystic areas by 22%, hepatic fibrotic areas by 24%, renal cystic areas by 18%, and renal fibrotic areas by 24% but had no effects on liver and kidney weights (Figure 3, Table 3).
Figure 2.
Selective histone deacetylase 6 (HDAC6) inhibitors, ACY-1215 and ACY-241, decrease hepatic and renal cystogenesis in polycystic kidney (PCK) rats. ACY-1215–treated rats and ACY-241–treated rats received daily injection of drugs (both, 30 mg/kg), and the control group was injected with equal doses of dimethyl sulfoxide for 6 weeks. A and B: Representative images of Picrosirius Red–stained liver (A) and kidney (B) sections of untreated rats and rodents treated with ACY-1215 and ACY-241. Scatter plots depict cystic and fibrotic areas of individual liver lobes and kidneys analyzed. Data are expressed as means ± SD. n = 6 female and 5 male ACY-1215–treated rats; n = 6 female and 5 male ACY-241–treated rats; n = 4 female and 4 male rats in the control group; n = 2 liver lobes from each rat; n = 2 kidneys from each rat. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bars = 250 μm (B). Original magnification, ×4 (A).
Table 2.
Gross Anatomy and Cystic and Fibrotic Areas in Polycystic Kidney Rats Treated with ACY-1215 and ACY-241
| Variables | Untreated | ACY-1215 | ACY-241 |
|---|---|---|---|
| Body weight, g | |||
| Male | 471.6 ± 52.6 | 395.8 ± 62.9 | 409.2 ± 40.4 |
| Female | 274.0 ± 12.4 | 272.0 ± 11.6 | 278.7 ± 10.6 |
| Liver weight, g | |||
| Male | 22.4 ± 4.2 | 19.6 ± 1.9 | 20.6 ± 3.4 |
| Female | 14.3 ± 0.9 | 13.9 ± 1.3 | 14.0 ± 0.9 |
| Liver/body weight, % | |||
| Male | 4.9 ± 0.2 | 4.6 ± 0.2∗∗ | 4.6 ± 0.2∗ |
| Female | 5.3 ± 0.3 | 4.9 ± 0.3∗ | 4.9 ± 0.2∗ |
| Kidney weight, g | |||
| Male | 6.3 ± 1.4 | 4.9 ± 0.5 | 5.1 ± 0.7 |
| Female | 4.2 ± 0.6 | 3.9 ± 0.9 | 3.6 ± 0.6 |
| Kidney/body weight, % | |||
| Male | 1.7 ± 0.3 | 1.5 ± 0.2∗ | 1.6 ± 0.2∗ |
| Female | 1.4 ± 0.2 | 1.3 ± 0.1∗ | 1.3 ± 0.1∗ |
| Hepatic cystic area, % of total parenchyma | 15.6 ± 4.0 | 10.0 ± 2.0∗∗∗∗ | 11.1 ± 2.5∗∗∗ |
| Hepatic fibrotic area, % of total parenchyma | 8.6 ± 1.4 | 5.5 ± 1.7∗∗∗∗ | 6.5 ± 1.3∗∗∗∗ |
| Renal cystic area, % of total parenchyma | 28.4 ± 7.2 | 20.5 ± 6.0∗∗∗ | 23.7 ± 4.8∗∗ |
| Renal fibrotic area, % of total parenchyma | 3.2 ± 0.4 | 2.2 ± 0.5∗∗∗ | 2.5 ± 0.4∗∗∗ |
Data are expressed as means ± SD.
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 untreated versus ACY-1215 or ACY-241.
Figure 3.
Selective histone deacetylase 6 (HDAC6) inhibitor, ACY-738, decreases hepatic and renal cystogenesis in PCK rats. ACY-738 was given to polycystic kidney (PCK) rats in a chow diet, and the control group received standard chow for 6 weeks. A and B: Representative images of Picrosirius Red–stained liver (A) and kidney (B) sections of untreated rats and rodents treated with ACY-738. Scatter plots depict cystic and fibrotic areas of individual liver lobes and kidneys analyzed. Data are expressed as means ± SD. n = 3 female and 4 male ACY-738–treated PCK rats; n = 4 female and 4 male rats in the control group. ∗P < 0 0.05, ∗∗∗P < 0.001. Scale bars = 250 μm (B). Original magnification, ×4 (A).
Table 3.
Gross Anatomy and Cystic and Fibrotic Areas in Polycystic Kidney Rats Treated with ACY-738
| Variables | Untreated | ACY-738 |
|---|---|---|
| Body weight, g | ||
| Male | 448.0 ± 24.3 | 394.8 ± 15.7 |
| Female | 272.3 ± 5.3 | 234.3 ± 19.6 |
| Liver weight, g | ||
| Male | 20.7 ± 2.9 | 16.5 ± 1.9 |
| Female | 13.9 ± 0.7 | 10.5 ± 1.7 |
| Liver/body weight, % | ||
| Male | 4.6 ± 0.6 | 4.5 ± 0.2 |
| Female | 5.10 ± 0.4 | 4.9 ± 0.5 |
| Kidney weight, g | ||
| Male | 4.7 ± 1.7 | 3.8 ± 0.2 |
| Female | 3.2 ± 0.5 | 2.5 ± 0.3 |
| Kidney/body weight, % | ||
| Male | 1.0 ± 0.2 | 1.0 ± 0.1 |
| Female | 1.1 ± 0.2 | 1.1 ± 0.1 |
| Hepatic cystic area, % of total parenchyma | 16.3 ± 3.8 | 12.7 ± 3.5∗∗∗ |
| Hepatic fibrotic area, % of total parenchyma | 8.6 ± 1.4 | 6.51 ± 1.5∗∗∗ |
| Renal cystic area, % of total parenchyma | 26.9 ± 6.4 | 22.9 ± 7.9∗ |
| Renal fibrotic area, % of total parenchyma | 2.9 ± 0.5 | 2.2 ± 0.5∗∗∗ |
Data are expressed as means ± SD.
∗P < 0.05, ∗∗∗P < 0.001 untreated versus ACY-738.
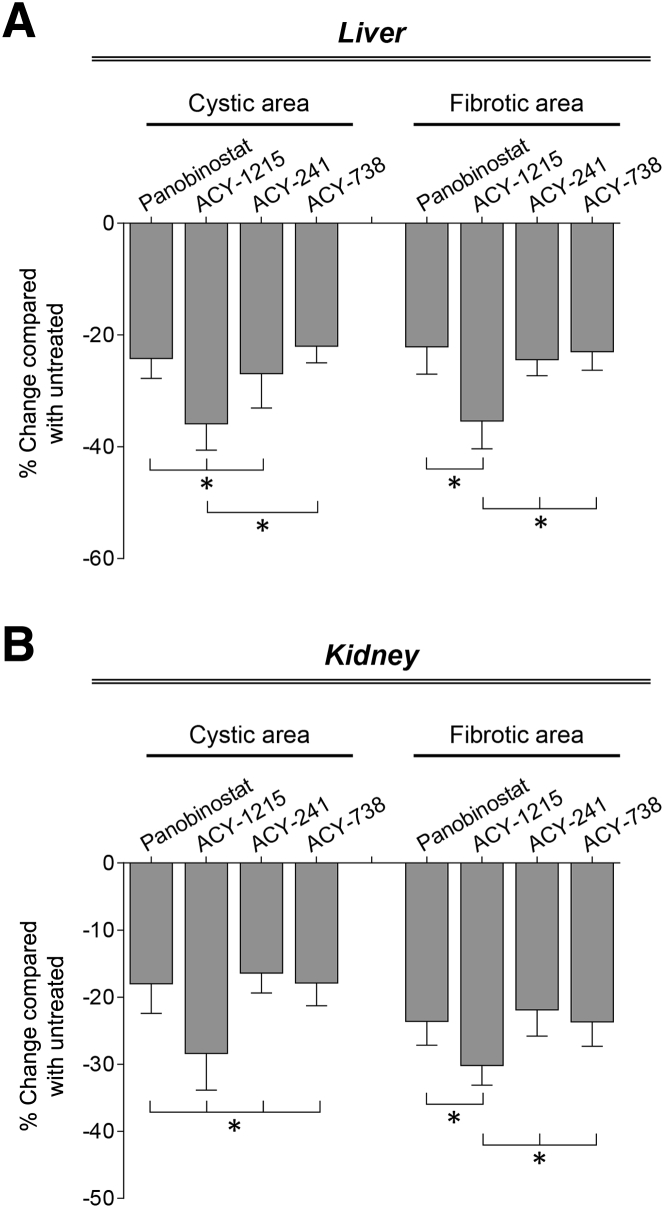
HDAC6 Inhibitor, ACY-1215, Suppress Hepatorenal Cystogenesis More Effectively Than Panobinostat, ACY-241, and ACY-738
By comparing the efficacy of panobinostat and all three HDAC6 inhibitors on PLD progression, it was observed that the selective HDAC6 inhibitor, ACY-1215, suppressed hepatorenal cystogenesis to a significantly greater extent than the other HDAC inhibitors (Figure 4). Thus, ACY-1215 was selected to test its effects in combination with a synthetic agonist of SSTRs, pasireotide, on disease progression in PCK rats.
Figure 4.
Selective histone deacetylase 6 (HDAC6) inhibitor, ACY-1215, shows enhanced efficacy in suppressing cyst growth in polycystic kidney (PCK) rats. A and B: ACY-1215 decreases hepatic (A) and renal (B) cystic and fibrotic areas to a higher extent than panobinostat, ACY-241 and ACY-748. Data are expressed as means ± SD. ∗P < 0.05.
Combination of ACY-1215 and Pasireotide Synergistically Decrease Hepatorenal Cystogenesis in PCK Rats
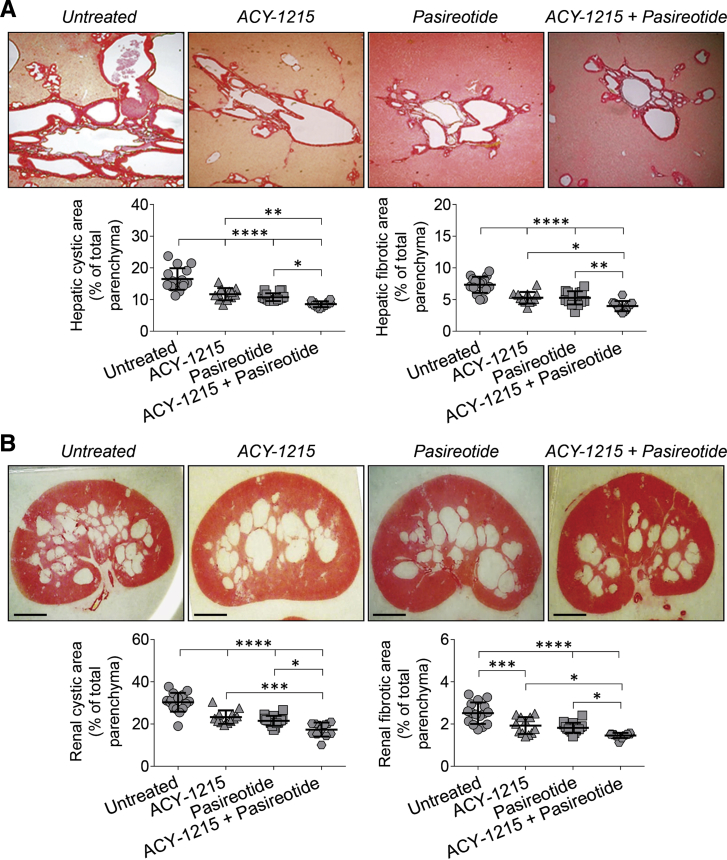
The treatment protocol for the ACY-1215 + pasireotide combination is depicted in Supplemental Figure S2. It was found that the ACY-1215 + pasireotide combination was more effective in reducing hepatic and renal cystogenesis in PCK rats than each drug alone as evident by decreased hepatic cystic areas by 33%, 37%, and 45%; hepatic fibrotic areas by 33%, 32%, and 44%; renal cystic areas by 26%, 32%, and 40%; and renal fibrotic areas by 27%, 33%, and 41% (Figure 5, Table 4). Liver weights were decreased by 26% on treatment with the ACY-1215 + pasireotide combination and by 9% and 17% in response to ACY-1215 and pasireotide, respectively (Table 4). In addition, a reduced kidney weight (by 30%) was observed in PCK rats treated with the ACY-1215 + pasireotide combination compared with the ACY-1215 (14% reduction) and pasireotide (18% reduction) groups (Table 4). Serum biochemistries remained unchanged or diminished slightly in all treatment groups (Table 5).
Figure 5.
Combination of ACY-1215 and pasireotide displays enhanced efficacy on hepatorenal cystogenesis in polycystic kidney (PCK) rats. A and B: Representative images of Picrosirius Red–stained liver (A) and kidney (B) sections of untreated, ACY-1215–treated, pasireotide-treated, and ACY-1215 + pasireotide–treated PCK rats. Scatter plots depict cystic and fibrotic areas of individual liver lobes and kidneys analyzed. ACY-1215 alone and pasireotide alone reduce hepatic and renal cystic and fibrotic areas compared with untreated PCK rats. Concurrent treatment with the ACY-1215 + pasireotide combination has stronger suppressive effects on hepatorenal cystogenesis than the untreated group and each drug alone. Data are expressed as means ± SD. n = 4 female and 4 male untreated PCK rats; n = 3 female and 3 male ACY-1215–treated PCK rats; n = 4 female and 4 male pasireotide-treated PCK rats; n = 3 female and 3 male ACY-1215 + pasireotide–treated PCK rats. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bars = 250 μm (B). Original magnifications, ×4 (A).
Table 4.
Gross Anatomy and Cystic and Fibrotic Areas in Polycystic Kidney Rats Treated with ACY-125 and Pasireotide Alone or in Combination
| Variables | Untreated | ACY-1215 | Pasireotide | ACY-1215 + Pasireotide |
|---|---|---|---|---|
| Body weight, g | ||||
| Male | 446.6 ± 38.3 | 416.3 ± 15.1 | 413.7 ± 10.7 | 418.1 ± 38.7 |
| Female | 286.7 ± 23.5 | 281.1 ± 14.9 | 282.5 ± 8.7 | 282.5 ± 14.6 |
| Liver weight, g | ||||
| Male | 26.1 ± 2.1 | 21.3 ± 1.4∗ | 19.3 ± 1.5∗ | 17.7 ± 1.2∗∗ |
| Female | 15.5 ± 1.3 | 14.5 ± 0.9∗ | 13.0 ± 0.5∗∗ | 11.7 ± 0.6∗∗∗ |
| Liver/body weight, % | ||||
| Male | 6.1 ± 0.5 | 5.1 ± 0.2∗ | 5.0 ± 0.2∗ | 4.3 ± 0.2∗∗ |
| Female | 5.6 ± 0.2 | 3.5 ± 0.3∗ | 3.4 ± 0.2∗∗ | 2.9 ± 0.2∗∗∗ |
| Kidney weight, g | ||||
| Male | 7.7 ± 0.3 | 6.7 ± 0.3∗∗ | 6.5 ± 0.2∗ | 6.0 ± 0.2∗ |
| Female | 4.0 ± 0.2 | 3.5 ± 0.2∗ | 3.4 ± 0.2∗ | 2.9 ± 0.1∗∗ |
| Kidney/body weight, % | ||||
| Male | 1.7 ± 0.1 | 1.6 ± 0.1∗ | 1.6 ± 0.1∗∗ | 1.5 ± 0.1∗∗ |
| Female | 1.4 ± 0.1 | 1.3 ± 0.1∗ | 1.2 ± 0.1∗∗ | 1.0 ± 0.1∗∗∗ |
| Hepatic cystic area, % of total parenchyma | 16.7 ± 3.4 | 11.2 ± 2.7∗∗ | 10.5 ± 1.9∗∗∗ | 9.2 ± 1.3∗∗∗ |
| Hepatic fibrotic area, % of total parenchyma | 7.6 ± 1.3 | 5.1 ± 1.0∗∗∗∗ | 5.2 ± 1.0∗∗∗ | 4.2 ± 0.9∗∗∗∗ |
| Renal cystic area, % of total parenchyma | 31.3 ± 4.5 | 23.3 ± 3.2∗∗∗∗ | 21.2 ± 2.9∗∗∗∗ | 18.8 ± 2.8∗∗∗∗ |
| Renal fibrotic area, % of total parenchyma | 2.5 ± 0.5 | 1.8 ± 0.4∗∗∗∗ | 1.7 ± 0.3∗∗∗∗ | 1.5 ± 0.2∗∗∗∗ |
Data are expressed as means ± SD.
∗P < 0.05, ∗∗P < 0.01,∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 untreated versus with ACY-1215 alone, pasireotide alone, or ACY-1215 + pasireotide combination.
Table 5.
Serum Biochemistry in Polycystic Kidney Rats Treated with ACY-1215, Pasireotide, and ACY-1215 + Pasireotide Combination
| Variable | Untreated | ACY-1215 | Pasireotide | ACY-1215 + PAS |
|---|---|---|---|---|
| γ-GT, U/L | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| ALT (GPT), U/L | 60.50 ± 7.22 | 54.25 ± 4.79 | 75.80 ± 20.56 | 47.20 ± 5.89∗ |
| ALP, U/L | 447.52 ± 87.16 | 509.52 ± 148.20 | 451.60 ± 95.69 | 361.75 ± 44.85 |
| Total bilirubin, mg/dL | 0.35 ± 0.06 | 0.35 ± 0.06 | 0.32 ± 0.04 | 0.30 ± 0.00 |
| Albumin, g/dL | 3.98 ± 0.59 | 4.15 ± 0.34 | 4.38 ± 0.41 | 3.60 ± 0.70 |
| Urea nitrogen, mg/dL | 15.75 ± 2.06 | 14.50 ± 1.00 | 14.00 ± 1.22 | 14.61 ± 3.29 |
| Cholesterol, mg/dL | 174.75 ± 18.92 | 161.25 ± 14.31 | 163.60 ± 25.26 | 145.00 ± 19.96 |
Data are expressed as means ± SD.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; GPT, alanine aminotransferase; GT, glutamyl transferase.
P < 0.05 untreated versus ACY-1215 + pasireotide combination.
Combination of ACY-1215 with Pasireotide Synergistically Decreases Cholangiocyte Proliferation in Vitro and in Vivo
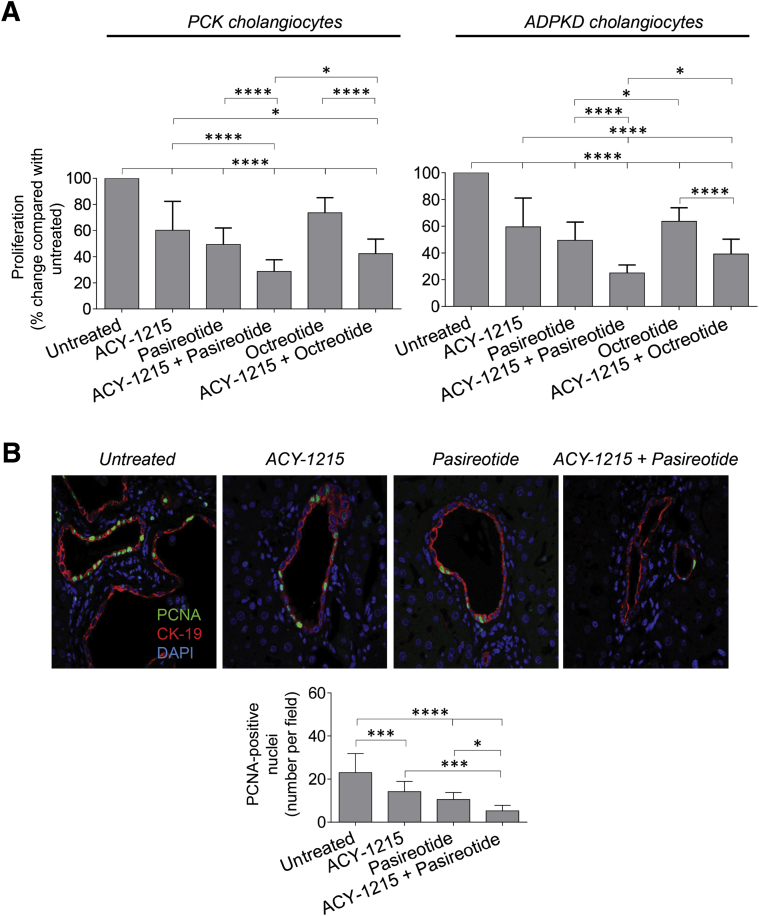
In untreated rat and human cystic cholangiocytes the rate of cell proliferation was considered to be 100%. Relative to untreated cells, proliferation of PCK and ADPKD cholangiocytes was decreased, respectively, to 60.3% ± 22.1% and 59.5% ± 21.6% after ACY-1215 treatment and to 49.4% ± 12.6% and 49.5% ± 13.5% in response to pasireotide application (Figure 6A). When cystic cholangiocytes were exposed to the ACY-1215 + pasireotide combination, the rate of proliferation was reduced to 28.7% ± 9.1% in PCK cholangiocytes and to 25.1% ± 5.9% in ADPKD cholangiocytes (Figure 6A). Another somatostatin analogue, octreotide, also decreased proliferation of PCK cholangiocytes (to 73.6% ± 11.6%) and ADPKD cholangiocytes (to 63.7% ± 10.1%) but not as effectively as pasireotide (Figure 6A). Moreover, the magnitude of decreased cell proliferation was higher when cystic cholangiocytes were treated simultaneously with ACY-1215 and pasireotide (ie, 28.7% ± 9.1% in PCK cholangiocytes and 25.1% ± 5.9% in ADPKD cholangiocytes) than the ACY-1215 + octreotide combination (ie, 42.3% ± 11.2% in PCK cholangiocytes and 39.11% ± 11.8% in ADPKD cholangiocytes) (Figure 6A).
Figure 6.
Combination of ACY-1215 and pasireotide synergistically decreases proliferation of cystic cholangiocytes in vitro and in vivo. A: Proliferation of cultured rat and human cholangiocytes assessed by CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) absorbance decreases in response to 10 μmol/L ACY-1215, 20 μmol/L pasireotide, 20 μmol/L octreotide, and combinations of ACY-1215 + pasireotide and ACY-1215 + octreotide. Compared with a single-drug application, synergistic treatment has stronger inhibitory effects on cholangiocyte growth. Pasireotide alone and in combination with ACY-1215 reduces cholangiocyte growth more effectively than octreotide alone and the ACY-1215 + octreotide combination, respectively. B: Representative images of liver sections stained with antibodies to proliferating cell nuclear antigen (PCNA; green) and cytokeratin (CK)-19 (red), and quantitative analysis show a greater reduction in the number of PCNA-positive nuclei after the ACY-1215 + pasireotide combination than after each drug alone. Nuclei are stained in blue with DAPI. Data are expressed as means ± SD. n = 24 for each data set (A); n = 12 microscopic fields for each group (B). ∗P < 0.05, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Original magnification, ×63. ADPKD, autosomal dominant polycystic kidney disease; PCK, polycystic kidney.
Consistent with these in vitro observations, proliferation of cystic cholangiocytes in PCK rats was decreased on drug treatment. Observed were 23.1 ± 8.8 PCNA-positive cholangiocyte nuclei in untreated PCK rats (Figure 6B). On treatment with ACY-1215 alone, pasireotide alone, and the ACY-1215+ pasireotide combination, the number of PCNA-positive cholangiocyte nuclei was reduced to 14.2 ± 4.8 (1.6-fold decrease), 10.5 ± 3.3 (1.9-fold decrease), and 5.3 ± 2.5 (approximately 3.5-fold decrease), respectively (Figure 6B).
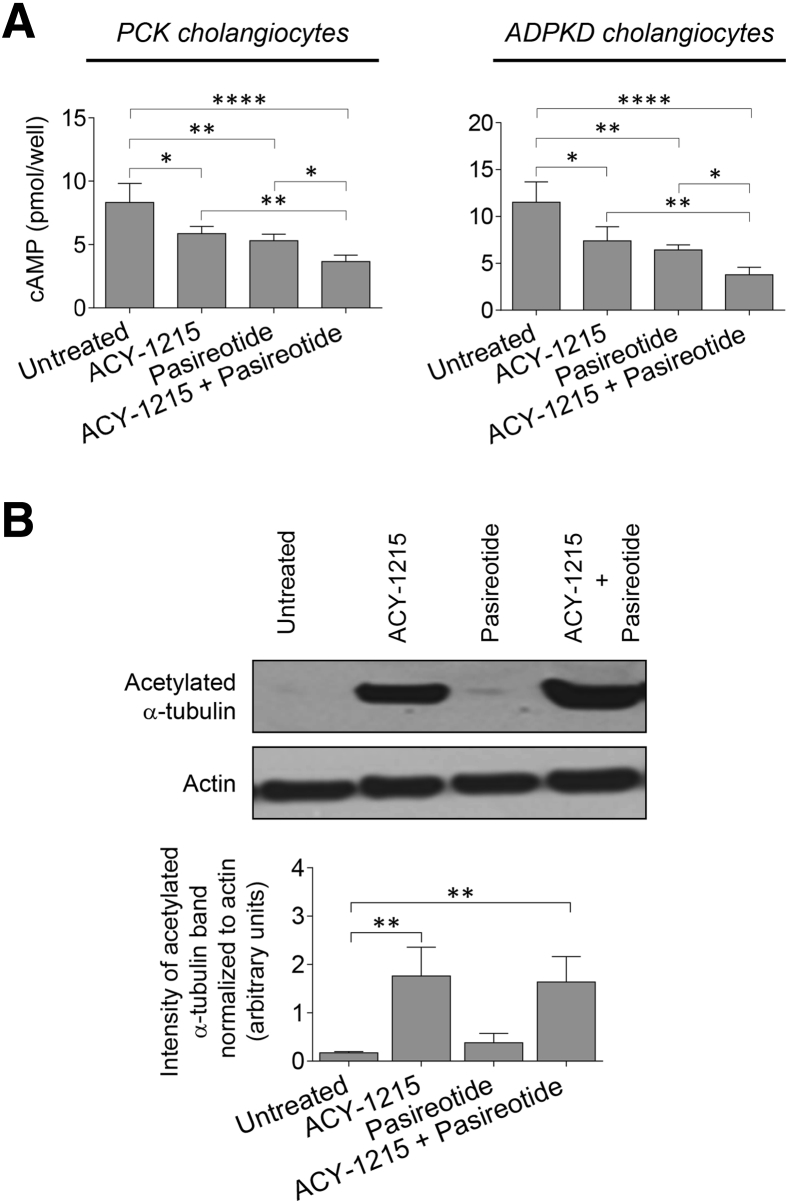
Combination of ACY-1215 and Pasireotide Synergistically Reduces Cholangiocyte cAMP Levels
Levels of cAMP were inhibited in PCK cholangiocytes on treatment with ACY-1215, pasireotide, and the ACY-1215 + pasireotide combination by 29% (5.86 ± 0.57 pmol), 36% (5.30 ± 0.52 pmol), and 56% (3.66 ± 0.51 pmol), respectively, compared with untreated cells (8.32 ± 1.51 pmol) (Figure 7A). Human ADPKD cholangiocytes responded similarly to drug treatment as evident by 35% (7.4 ± 1.51 pmol), 44% (6.42 ± 0.55 pmol), and 67% (3.78 ± 0.81 pmol) decrease in cAMP levels after exposure to ACY-1215, pasireotide, and the ACY-1215 + pasireotide combination, respectively, compared with untreated cells (11.52 ± 2.16 pmol) (Figure 7A).
Figure 7.
Combination of ACY-1215 and pasireotide synergistically decreases cAMP levels in cystic cholangiocytes and ACY-1215 increases acetylation of α-tubulin. A: Levels of cAMP are inhibited in response to 10 μmol/L ACY-1215, 20 μmol/L pasireotide, or the ACY-1215 + pasireotide combination. Drug combination decreases cAMP levels more effectively than a single-drug treatment. B: Representative Western blot analysis and quantitation of band density show an increased expression of acetylated α-tubulin in polycystic kidney (PCK) cholangiocytes on ACY-1215 and ACY-1215 + pasireotide treatment. Data are expressed as means ± SD. n = 4 for each data set (A); n = 3 for each experimental condition (B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001. ADPKD, autosomal dominant polycystic kidney disease.
ACY-1215 Increases Tubulin Acetylation in Cystic Cholangiocytes
The effects of HDAC6 inhibitor, ACY-1215, were examined on the expression of one of the main down-stream targets of HDAC6, acetylated α-tubulin. An increase level of acetylated α-tubulin was observed in cultured PCK cholangiocytes on ACY-1215 but not pasireotide treatment (Figure 7B).
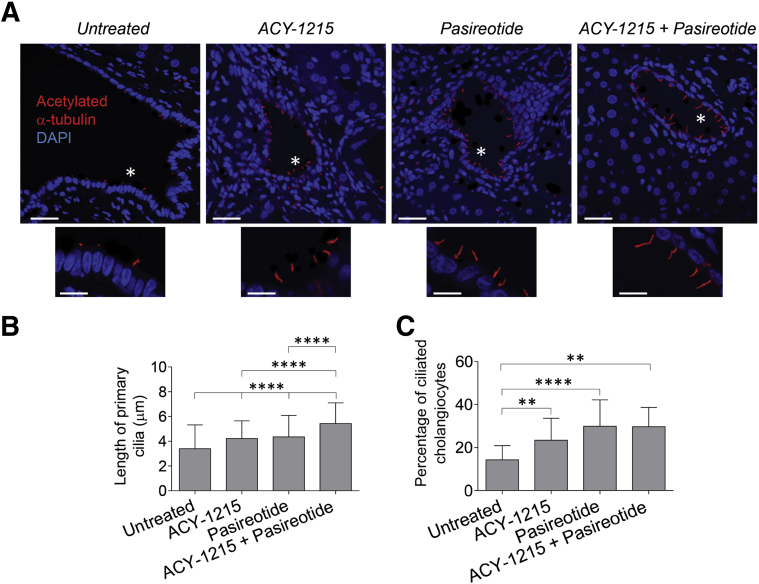
HDAC6 and cAMP Targeting Increases the Length of Primary Cilia and Frequency of Ciliated Cholangiocytes in the PCK Rat
Primary cilia in cholangiocytes of untreated PCK rats were approximately 3.4 ± 1.9 μm in length. ACY-1215, pasireotide, and the ACY-1215 + pasireotide combination increased the length of cilia, respectively, to 4.2 ± 1.4 μm, 4.5 ± 1.7 μm, and 5.4 ± 1.7 μm (Figure 8, A and B). The percentage of ciliated cholangiocytes was also increased on treatment with ACY-1215 (23.4% ± 10.2%), pasireotide (29.9% ± 12.3%), and the ACY-1215 + pasireotide combination (29.7% ± 9.1%) compared with cholangiocytes of untreated PCK rats (14.3% ± 6.5%) (Figure 8, A and C).
Figure 8.
Combination of ACY-1215 and pasireotide increases ciliary length and frequency of their occurrences in cholangiocytes of polycystic kidney (PCK) rats. A: Representative images of liver sections from untreated and drug-treated PCK rats are stained with antibody to ciliary marker, acetylated α-tubulin (red). Nuclei are stained in blue with DAPI. Area depicted by asterisk is shown with a higher power underneath. B and C: The length of primary cilia (B) and the percentage of ciliated cholangiocytes (C) increase in response to treatment with ACY-1215, pasireotide, and drug combination. Randomly selected microscopic fields from each group of untreated and drug-treated PCK rats were analyzed. Data are expressed as means ± SD. n = 5 microscopic fields. ∗∗P < 0.01, ∗∗∗∗P < 0.0001. Scale bars = 10 μm (A, main images and insets).
Discussion
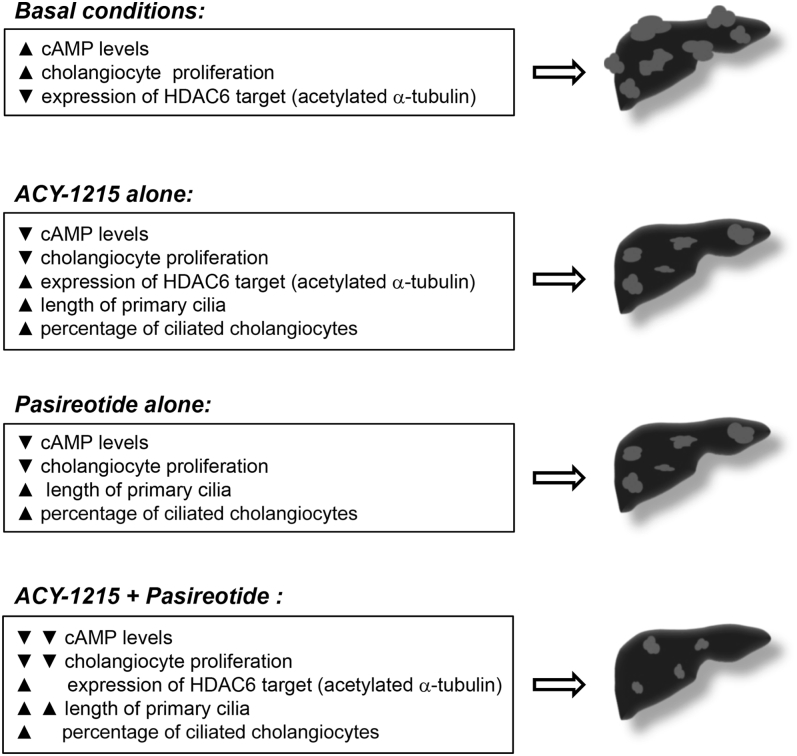
The key findings reported here are i) HDAC inhibitors decrease hepatic and renal cystogenesis in an animal model of PLD, the PCK rat; ii) the selective HDAC6 inhibitor, ACY-1215, is more effective in suppression of cyst growth in PCK rats than other HDAC inhibitors; iii) the combination of ACY-1215 with the agonist of SSTR, pasireotide, reduced hepatorenal cystogenesis in PCK rats and increased the length of primary cilia to a greater extent than either drug alone; and iv) a combination of ACY-1215 and pasireotide inhibited cholangiocyte proliferation and cAMP production (ie, two main mechanisms that promote hepatic cystogenesis) in vitro more significantly than each drug alone (Figure 9). These data suggest that the concurrent targeting of the cAMP and HDAC6 pathways in cystic cholangiocytes may be a useful therapeutic approach for PLD.
Figure 9.
Combination of ACY-1215 and pasireotide synergistically decreases hepatic cystogenesis by inhibiting cholangiocyte proliferation and cAMP levels. Under basal conditions, cystic cholangiocytes are characterized by elevated cAMP, decreased expression of acetylated α-tubulin, and cell hyperproliferation, which lead to hepatic cystogenesis. ACY-1215 alone decreases cAMP levels, inhibits cholangiocyte proliferation, increases expression of acetylated α-tubulin, and increases ciliary length and frequency of their occurrences, subsequently decreasing hepatic cystogenesis. Reduced cAMP levels, decreased cholangiocyte proliferation, increased length of primary cilia, and percentage of ciliated cholangiocytes and attenuated hepatic cystogenesis are observed in response to pasireotide treatment. ACY-1215 + pasireotide combination had additive effects on cAMP levels, cholangiocyte proliferation, and length of primary cilia and, subsequently, on hepatic cystogenesis compared with each drug alone. HDAC6, histone deacetylase 6.
We and others have reported that hepatic cystogenesis is linked to disturbances in multiple cellular functions.25, 26, 27, 28, 29 These aberrant processes represent potential therapeutic targets in PLD, and some of them have been evaluated in preclinical trials.5, 16, 17, 18, 19, 20, 24 We have also previously suggested and recently demonstrated that simultaneous targeting of different abnormal pathways in cystic cholangiocytes or affecting several components of a single dysregulated pathway might be more beneficial for disease treatment than targeting a single pathway.19, 20 Here, we tested this hypothesis by concurrently inhibiting HDAC6 and cAMP in cystic cholangiocytes.
Various HDAC inhibitors have been developed over the years.30, 31, 32 Pan-HDAC inhibitors are known to have a broad spectrum of action simultaneously affecting multiple HDACs.33 One of the pan-HDAC inhibitors, trichostatin, has been previously tested in an animal model of ADPKD and showed an inhibitory effect on renal cyst growth.34 Here, we extended these data demonstrating that another potent pan-HDAC inhibitor, panobinostat,33, 35 reduced both hepatic and renal cystogenesis in PCK rats.
In addition to pan-HDAC inhibitors, drugs that target isoform-specific HDACs, including HDAC6, have been developed.36 We have reported that HDAC6 is overexpressed in rodents and humans with PLD and that specific HDAC6 inhibitors, tubastatin-A and ACY-1215, decreased growth of hepatic cysts in PCK rats.15 Given that multiple HDAC6 inhibitors exist, the role of several such drugs was assessed in PLD treatment. Each of the three tested HDAC6 inhibitors, ACY-1215, ACY-738, and ACY-241, effectively suppressed hepatorenal cystogenesis in PCK rats. The effects of panobinostat, ACY-738, and ACY-241 were comparable, whereas ACY-1215 decreased hepatic and renal cyst growth to a significantly greater degree.
We and others have experimentally shown the importance of elevated intracellular cholangiocyte cAMP in hepatic cyst growth.2, 5, 16, 17, 24 To date, the lowering of cAMP in cystic cholangiocytes by agonists of somatostatin receptors is the only available pharmacologic treatment for patients with symptomatic liver disease.2, 5, 7, 8 The efficacy of two synthetic somatostatin analogues, octreotide and pasireotide, has been previously evaluated by us in preclinical trials, and our data demonstrate that pasireotide is more effective than octreotide in suppressing hepatorenal cystogenesis.16, 17 Furthermore, we recently examined the synergistic effects of pasireotide and hydroxychloroquine on disease progression in PCK rats.19 Hydroxychloroquine is an inhibitor of autophagy, an intracellular degradative pathway dysregulated in PLD. The combination of pasireotide with hydroxychloroquine attenuated hepatic cystogenesis in PCK rats more effectively than a single-drug therapy.19
Thus, on the basis of our present and previous observations,15, 17, 19 ACY-1215 and pasireotide were chosen for combinational studies. As expected, concurrent administration of these drugs decreased hepatic and renal cystic and fibrotic areas to a higher degree than each drug alone. Furthermore, the ACY-1215 + pasireotide combination resulted in greater suppression of cholangiocyte proliferation than a single drug. Consistent with these in vitro data, ACY-1215 and pasireotide concurrently decreased the number of proliferating cholangiocytes in vivo in PCK rats more competently than each drug alone.
The rate of cholangiocyte proliferation was also examined in vitro in response to another somatostatin analogue, octreotide, alone and in combination with ACY-1215. As previously reported,17 pasireotide inhibited cholangiocyte proliferation more effectively than octreotide. Synergistic treatment of PCK and ADPKD cholangiocytes with octreotide and ACY-1215 suppressed cell growth to a lesser extent than the ACY-1215 + pasireotide combination.
We have previously reported and have confirmed here that pasireotide inhibits cAMP production in cystic cholangiocytes, subsequently decreasing growth of hepatic cysts.17 In addition, the HDAC6 inhibitor, ACY-1215, suppressed cAMP levels. Decreased rates of proliferation and cAMP production in response to HDAC6 inhibition has been reported in different cell types.15, 37, 38, 39, 40 Furthermore, the selective HDAC6 inhibitor, tubacin, down-regulated cAMP production in renal epithelial cells and reduced their growth.40 Of importance, we demonstrated that in cultured rat and human cystic cholangiocytes, inhibition of cAMP in response to the ACY-1215 + pasireotide combination was greater than each drug alone. Although the mechanism by which ACY-1215 affects cAMP levels in cystic cholangiocytes remains to be clarified, inhibition of the components of cAMP machinery (such as adenylyl cyclases) may be considered.
HDAC6 inhibitors are known to modulate the activity of this histone deacetylase as evident by the changes in expression of HDAC6 targets.41 One of the main downstream targets of HDAC6 is acetylated α-tubulin. We previously showed that decreased levels of acetylated α-tubulin in cystic cholangiocytes are associated with HDAC6 overexpression.15 As expected, ACY-1215 increased the acetylation of α-tubulin in cultured cystic cholangiocytes. Consistent with our data, up-regulation of α-tubulin acetylation in response to another HDAC6 inhibitor, tubastatin A, was previously reported.42, 43 Inhibition of HDAC6 by ACY-1215 also increased the length of primary cilia and the percentage of ciliated cholangiocytes in PCK rats in vivo. Likewise, in pasireotide-treated PCK rats, the length of primary cilia and the frequency of ciliated cholangiocytes were increased. Moreover, the ACY-1215 + pasireotide combination had stronger effects on ciliogenesis than each drug alone.
In addition, one of the variables of liver functional test (namely, alanine aminotransferase) was decreased after the ACY-1215 and pasireotide combination. This observation is encouraging, considering our previous published observations demonstrating that because of short (ie, 6 weeks) duration of treatment liver function tests remain unchanged in response to different drugs given alone.16, 17, 24, 27
Recently, the combinational drug approach as a potential therapeutic avenue for the treatment of PLD and PKD has begun to emerge. The somatostatin analogue, octreotide, has been shown to prevent an increase of liver cystic areas in Pkd2cKO mice because of paradoxical activation of Raf/extracellular single-regulated kinase 1/2 in response to the Raf inhibitor, sorafenib.44 However, the mechanistic target of rapamycin inhibitor, everolimus, combined with octreotide did not have an additive effect on liver volume reduction in autosomal dominant PLD patients.45 Targeting of cAMP machinery by tolvaptan, an agonist of the vasopressin receptor II, and pasireotide reduced renal cystogenesis in an animal model of PKD by inhibiting cAMP.46 We recently reported that concurrent interference with cAMP signaling and autophagy pathway attenuates hepatic cytogenesis in an animal model of PLD to a higher extent than single-drug therapy.19 Thus, there is increasing recognition that a combination of drugs is a reasonable approach to treat hepatorenal cystogenesis.
Conclusions
Our data demonstrate that the simultaneous targeting of HDAC6 and cAMP in cystic cholangiocytes suppressed hepatorenal cystogenesis in PCK rats by inhibiting cholangiocyte proliferation and decreasing cAMP levels to a greater extent than either drug alone. These data support the benefit of this combination regimen for patients with PLD.
Acknowledgments
M.L.P., T.V.M., and N.F.L. supervised the project and wrote the manuscript; and M.L.P., T.V.M., S.A.G., A.I.M., J.F.D., and P.-Y.L. performed experiments and contributed to discussion.
Footnotes
Supported by NIH grants DK24031 (N.F.L.) and CA183764 (S.A.G.), the Mayo Clinic, the Clinical Core and Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology grant P30DK084567 (N.F.L.), the Mayo Translational Polycystic Kidney Disease (PKD) Center supported by National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK090728 (N.F.L.), the Mayo Translational PKD Center Pilot and Feasibility award (T.V.M.), and the Eileen Creamer O'Neill award from the PKD Foundation (T.V.M.).
M.L.P. and T.V.M. contributed equally to this work.
Disclosures: S.A.G. and N.F.L. are named as inventors on patents filed by Mayo Clinic claiming methods for using histone deacetylase 6 inhibitors to treat polycystic liver disease. T.V.M. and S.A.G. received partial funding support for this study from Acetylon Pharmaceuticals Inc. The sponsor was not involved in the study design, analysis, or data interpretation. The manuscript was prepared by the authors and reviewed by the sponsor.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2017.12.016.
Supplemental Data
The treatment protocol to study the effects of histone deacetylase (HDAC) inhibitors on hepatorenal cystogenesis in polycystic kidney (PCK) rats. Rodents received daily i.p. injections of panobinostat, ACY-1215, or ACY-241 (all, 30 mg/kg body weight). Untreated PCK rats received equal doses of dimethyl sulfoxide (DMSO). Doses of HDAC inhibitors are based on published studies and were adjusted to the weight of rats weekly. ACY-738 was given in a chow diet; untreated PCK rats were kept on a standard chow diet. Rats were sacrificed after 6 weeks of treatment. The following variables were analyzed: body weights, liver and kidney weights, renal and hepatic cystic areas, and renal and hepatic fibrotic areas. F, female; M, male.
The treatment protocol to study the combinatorial effects of ACY-1215 and pasireotide on hepatorenal cystogenesis in polycystic kidney (PCK) rats. Pasireotide (20 μg/kg daily) was dissolved in sterile water and administered by osmotic minipumps implanted subcutaneously on the animal's back. Pumps were replaced every 2 weeks; at this time pasireotide concentrations were adjusted to the animal's weight. ACY-1215 (30 mg/kg body weight) was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally. Doses of ACY-1215 were adjusted to the animal weight every week. The untreated group received equal doses of DMSO (i.p. injections) and had implanted pumps filled with sterile water. Rats were sacrificed after 6 weeks of treatment. The following variables were analyzed: body weights, liver and kidney weights, renal and hepatic cystic areas, and renal and hepatic fibrotic areas. F, female; M, male.
References
- 1.Cnossen W.R., Drenth J.P. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis. 2014;9:69. doi: 10.1186/1750-1172-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larusso N.F., Masyuk T.V., Hogan M.C. Polycystic liver disease: the benefits of targeting cAMP. Clin Gastroenterol Hepatol. 2016;14:1031–1034. doi: 10.1016/j.cgh.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse W., Dong K., Choi J., Punia S., Fedeles S.V., Choi M., Gallagher A.R., Huang E.B., Gulati A., Knight J., Mane S., Tahvanainen E., Tahvanainen P., Sanna-Cherchi S., Lifton R.P., Watnick T., Pei Y.P., Torres V.E., Somlo S. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest. 2017;127:3558. doi: 10.1172/JCI96729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porath B., Gainullin V.G., Cornec-Le Gall E., Dillinger E.K., Heyer C.M., Hopp K., Edwards M.E., Madsen C.D., Mauritz S.R., Banks C.J., Baheti S., Reddy B., Herrero J.I., Banales J.M., Hogan M.C., Tasic V., Watnick T.J., Chapman A.B., Vigneau C., Lavainne F., Audrezet M.P., Ferec C., Le Meur Y., Torres V.E., Genkyst Study Group HALT Progression of Polycystic Kidney Disease Group. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease Group. Harris P.C. Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet. 2016;98:1193–1207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perugorria M.J., Masyuk T.V., Marin J.J., Marzioni M., Bujanda L., LaRusso N.F., Banales J.M. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nat Rev Gastroenterol Hepatol. 2014;11:750–761. doi: 10.1038/nrgastro.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan M.C., Masyuk T.V., Page L.J., Kubly V.J., Bergstralh E.J., Li X., Kim B., King B.F., Glockner J., Holmes D.R., III, Rossetti S., Harris P.C., LaRusso N.F., Torres V.E. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peces R., Cuesta-Lopez E., Peces C., Perez-Duenas V., Vega-Cabrera C., Selgas R. Octreotide reduces hepatic, renal and breast cystic volume in autosomal-dominant polycystic kidney disease. Int Urol Nephrol. 2011;43:565–569. doi: 10.1007/s11255-010-9748-1. [DOI] [PubMed] [Google Scholar]
- 8.Gevers T.J., Drenth J.P. Somatostatin analogues for treatment of polycystic liver disease. Curr Opin Gastroenterol. 2011;27:294–300. doi: 10.1097/MOG.0b013e328343433f. [DOI] [PubMed] [Google Scholar]
- 9.Gevers T.J., Nevens F., Torres V.E., Hogan M.C., Drenth J.P. Alkaline phosphatase predicts response in polycystic liver disease during somatostatin analogue therapy: a pooled analysis. Liver Int. 2016;36:595–602. doi: 10.1111/liv.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renken C., Fischer D.C., Kundt G., Gretz N., Haffner D. Inhibition of mTOR with sirolimus does not attenuate progression of liver and kidney disease in PCK rats. Nephrol Dial Transplant. 2011;26:92–100. doi: 10.1093/ndt/gfq384. [DOI] [PubMed] [Google Scholar]
- 11.Spirli C., Locatelli L., Fiorotto R., Morell C.M., Fabris L., Pozzan T., Strazzabosco M. Altered store operated calcium entry increases cyclic 3′,5′-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology. 2012;55:856–868. doi: 10.1002/hep.24723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S., Tian X., Somlo S., Strazzabosco M. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology. 2010;51:1778–1788. doi: 10.1002/hep.23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spirli C., Okolicsanyi S., Fiorotto R., Fabris L., Cadamuro M., Lecchi S., Tian X., Somlo S., Strazzabosco M. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystin-2 defective mice. Gastroenterology. 2010;138:360–371.e7. doi: 10.1053/j.gastro.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banales J.M., Masyuk T.V., Gradilone S.A., Masyuk A.I., Medina J.F., LaRusso N.F. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradilone S.A., Habringer S., Masyuk T.V., Howard B.N., Masyuk A.I., Larusso N.F. HDAC6 is overexpressed in cystic cholangiocytes and its inhibition reduces cystogenesis. Am J Pathol. 2014;184:600–608. doi: 10.1016/j.ajpath.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masyuk T.V., Masyuk A.I., Torres V.E., Harris P.C., Larusso N.F. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Masyuk T.V., Radtke B.N., Stroope A.J., Banales J.M., Gradilone S.A., Huang B., Masyuk A.I., Hogan M.C., Torres V.E., Larusso N.F. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58:409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masyuk T.V., Radtke B.N., Stroope A.J., Banales J.M., Masyuk A.I., Gradilone S.A., Gajdos G.B., Chandok N., Bakeberg J.L., Ward C.J., Ritman E.L., Kiyokawa H., LaRusso N.F. Inhibition of Cdc25A suppresses hepato-renal cystogenesis in rodent models of polycystic kidney and liver disease. Gastroenterology. 2012;142:622–633.e4. doi: 10.1053/j.gastro.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masyuk A.I., Masyuk T.V., Lorenzo Pisarello M.J., Ding J.F., Loarca L., Huang B.Q., LaRusso N.F. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology. 2018;67:1088–1108. doi: 10.1002/hep.29577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masyuk T.V., Masyuk A.I., LaRusso N.F. Therapeutic targets in polycystic liver disease. Curr Drug Targets. 2017;18:950–957. doi: 10.2174/1389450116666150427161743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P., Almeciga-Pinto I., Jarpe M., van Duzer J.H., Mazitschek R., Yang M., Jones S.S., Quayle S.N. Selective HDAC inhibition by ACY-241 enhances the activity of paclitaxel in solid tumor models. Oncotarget. 2017;8:2694–2707. doi: 10.18632/oncotarget.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North B.J., Almeciga-Pinto I., Tamang D., Yang M., Jones S.S., Quayle S.N. Enhancement of pomalidomide anti-tumor response with ACY-241, a selective HDAC6 inhibitor. PLoS One. 2017;12:e0173507. doi: 10.1371/journal.pone.0173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benoy V., Vanden Berghe P., Jarpe M., Van Damme P., Robberecht W., Van Den Bosch L. Development of improved HDAC6 inhibitors as pharmacological therapy for axonal Charcot-Marie-Tooth disease. Neurotherapeutics. 2017;14:417–428. doi: 10.1007/s13311-016-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masyuk T.V., Masyuk A.I., Lorenzo Pisarello M., Howard B.N., Huang B.Q., Lee P.Y., Fung X., Sergienko E., Ardecky R.J., Chung T.D., Pinkerton A.B., LaRusso N.F. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Galphas signaling. Hepatology. 2017;66:1197–1218. doi: 10.1002/hep.29284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz-Garrido P., Marin J.J., Perugorria M.J., Urribarri A.D., Erice O., Saez E., Uriz M., Sarvide S., Portu A., Concepcion A.R., Romero M.R., Monte M.J., Santos-Laso A., Hijona E., Jimenez-Aguero R., Marzioni M., Beuers U., Masyuk T.V., LaRusso N.F., Prieto J., Bujanda L., Drenth J.P., Banales J.M. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. J Hepatol. 2015;63:952–961. doi: 10.1016/j.jhep.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masyuk T.V., Lee S.O., Radtke B.N., Stroope A.J., Huang B., Banales J.M., Masyuk A.I., Splinter P.L., Gradilone S.A., Gajdos G.B., LaRusso N.F. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. Am J Pathol. 2014;184:110–121. doi: 10.1016/j.ajpath.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gradilone S.A., Masyuk T.V., Huang B.Q., Banales J.M., Lehmann G.L., Radtke B.N., Stroope A., Masyuk A.I., Splinter P.L., LaRusso N.F. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology. 2010;139:304–314.e2. doi: 10.1053/j.gastro.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banales J.M., Masyuk T.V., Bogert P.S., Huang B.Q., Gradilone S.A., Lee S.O., Stroope A.J., Masyuk A.I., Medina J.F., LaRusso N.F. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. Am J Pathol. 2008;173:1637–1646. doi: 10.2353/ajpath.2008.080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waanders E., Van Krieken J.H., Lameris A.L., Drenth J.P. Disrupted cell adhesion but not proliferation mediates cyst formation in polycystic liver disease. Mod Pathol. 2008;21:1293–1302. doi: 10.1038/modpathol.2008.115. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Li G., Xu W. Histone deacetylase inhibitors: an attractive strategy for cancer therapy. Curr Med Chem. 2013;20:1858–1886. doi: 10.2174/0929867311320140005. [DOI] [PubMed] [Google Scholar]
- 31.Lapinska K., Housman G., Byler S., Heerboth S., Willbanks A., Oza A., Sarkar S. The effects of histone deacetylase inhibitor and calpain inhibitor combination therapies on ovarian cancer cells. Anticancer Res. 2016;36:5731–5742. doi: 10.21873/anticanres.11156. [DOI] [PubMed] [Google Scholar]
- 32.Ceccacci E., Minucci S. Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br J Cancer. 2016;114:605–611. doi: 10.1038/bjc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mottamal M., Zheng S., Huang T.L., Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20:3898–3941. doi: 10.3390/molecules20033898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan L.X., Li X., Magenheimer B., Calvet J.P., Li X. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 2012;81:76–85. doi: 10.1038/ki.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennika T., Hu G., Olaciregui N.G., Barton K.L., Ehteda A., Chitranjan A., Chang C., Gifford A.J., Tsoli M., Ziegler D.S., Carcaboso A.M., Becher O.J. Pre-clinical study of panobinostat in xenograft and genetically engineered murine diffuse intrinsic pontine glioma models. PLoS One. 2017;12:e0169485. doi: 10.1371/journal.pone.0169485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falkenberg K.J., Johnstone R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 37.Rivieccio M.A., Brochier C., Willis D.E., Walker B.A., D'Annibale M.A., McLaughlin K., Siddiq A., Kozikowski A.P., Jaffrey S.R., Twiss J.L., Ratan R.R., Langley B. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gradilone S.A., Radtke B.N., Bogert P.S., Huang B.Q., Gajdos G.B., LaRusso N.F. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riolo M.T., Cooper Z.A., Holloway M.P., Cheng Y., Bianchi C., Yakirevich E., Ma L., Chin Y.E., Altura R.A. Histone deacetylase 6 (HDAC6) deacetylates survivin for its nuclear export in breast cancer. J Biol Chem. 2012;287:10885–10893. doi: 10.1074/jbc.M111.308791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cebotaru L., Liu Q., Yanda M.K., Boinot C., Outeda P., Huso D.L., Watnick T., Guggino W.B., Cebotaru V. Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int. 2016;90:90–99. doi: 10.1016/j.kint.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuji G., Okiyama N., Villarroel V.A., Katz S.I. Histone deacetylase 6 inhibition impairs effector CD8 T-cell functions during skin inflammation. J Allergy Clin Immunol. 2015;135:1228–1239. doi: 10.1016/j.jaci.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Leng Y., Wang J., Liao H.M., Bergman J., Leeds P., Kozikowski A., Chuang D.M. Tubastatin A, an HDAC6 inhibitor, alleviates stroke-induced brain infarction and functional deficits: potential roles of alpha-tubulin acetylation and FGF-21 up-regulation. Sci Rep. 2016;6:19626. doi: 10.1038/srep19626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asthana J., Kapoor S., Mohan R., Panda D. Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells. J Biol Chem. 2013;288:22516–22526. doi: 10.1074/jbc.M113.489328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spirli C., Morell C.M., Locatelli L., Okolicsanyi S., Ferrero C., Kim A.K., Fabris L., Fiorotto R., Strazzabosco M. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56:2363–2374. doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Chrispijn M., Gevers T.J., Hol J.C., Monshouwer R., Dekker H.M., Drenth J.P. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. J Hepatol. 2013;59:153–159. doi: 10.1016/j.jhep.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Hopp K., Hommerding C.J., Wang X., Ye H., Harris P.C., Torres V.E. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol. 2015;26:39–47. doi: 10.1681/ASN.2013121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The treatment protocol to study the effects of histone deacetylase (HDAC) inhibitors on hepatorenal cystogenesis in polycystic kidney (PCK) rats. Rodents received daily i.p. injections of panobinostat, ACY-1215, or ACY-241 (all, 30 mg/kg body weight). Untreated PCK rats received equal doses of dimethyl sulfoxide (DMSO). Doses of HDAC inhibitors are based on published studies and were adjusted to the weight of rats weekly. ACY-738 was given in a chow diet; untreated PCK rats were kept on a standard chow diet. Rats were sacrificed after 6 weeks of treatment. The following variables were analyzed: body weights, liver and kidney weights, renal and hepatic cystic areas, and renal and hepatic fibrotic areas. F, female; M, male.
The treatment protocol to study the combinatorial effects of ACY-1215 and pasireotide on hepatorenal cystogenesis in polycystic kidney (PCK) rats. Pasireotide (20 μg/kg daily) was dissolved in sterile water and administered by osmotic minipumps implanted subcutaneously on the animal's back. Pumps were replaced every 2 weeks; at this time pasireotide concentrations were adjusted to the animal's weight. ACY-1215 (30 mg/kg body weight) was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally. Doses of ACY-1215 were adjusted to the animal weight every week. The untreated group received equal doses of DMSO (i.p. injections) and had implanted pumps filled with sterile water. Rats were sacrificed after 6 weeks of treatment. The following variables were analyzed: body weights, liver and kidney weights, renal and hepatic cystic areas, and renal and hepatic fibrotic areas. F, female; M, male.