Abstract
A “concept” refers to what exists in the mind as a representation (as in something comprehended) or as a formulation (as in a plan). It is generally understood as “any idea of what a thing ought to be” (Merriam-Webster). From that premise, an “idea” cannot be compartmentalized or rigidly defined as exclusively belonging to any single individual or school of thought. The utility of a concept is inherently linked to its adaptability to the needs and conditions of the time. I state this upfront because over the past several decades, the concept of the epileptogenic zone (EZ) has become so crucial to the foundation of major schools of surgical epilepsy that discussions and opinions on the topic have essentially sought to legitimize one view while criticizing the other. This review is not a referendum on any specific definition of the EZ but rather a chronological analysis of the historical evolution of this concept and the invasive EEG tools used to study it. The goal is to highlight common ground necessary to tackle the ever-present challenge of defining the ideal resection for a patient with drug-resistant focal epilepsy.
The Epileptogenic Lesion: 1950s – Electrocorticography (ECoG)
Theoretical Definition
As early as 1956, Wilder and Penfield appreciated that epilepsy localization extends beyond the presumably culprit brain structural pathology. They defined epileptogenic lesion as “the foreign tissue lesion itself [and] the structurally and functionally disturbed but still viable surrounding gray matter” (1).
Surgical Definition
The classical Montreal Neurological Institute approach to localizing this epileptogenic lesion was to perform large craniotomies, exposing extensive areas of the cortex. This allowed: 1) the intraoperative identification of visually abnormal convolutions, 2) detailed recording of electrocorticography (ECoG) over large portions of the convexity, 3) electrical cortical stimulation to localize functionally eloquent cortex and its boundaries, and 4) an attempt at reproducing the habitual seizures, particularly the aura. In an era where neuroimaging was restricted to pneumo-encephalography and anesthesia allowed only brief periods of intraoperative ECoG, the prevailing surgical definition of the epileptogenic lesion was derived from a combination of the anatomical and electrical information obtained during surgery: the visible lesion and interictal ECoG spikes.
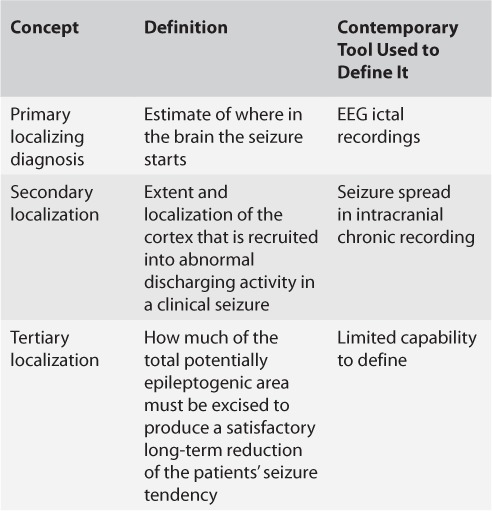
Jasper et al. (2) published evidence supporting this approach in a landmark paper detailing the strong correlation between complete removal of ECoG spikes and favorable seizure outcomes: Thirteen of 19 patients with “clean removal of all epileptogenic tissue as judged by pre- and postexcision corticogram” became completely seizure-free after surgery while there was “questionable or no definite improvement” for 10 of 13 with “partial removal of epileptogenic area and persistence of important epileptiform abnormality in corticogram with postoperative EEG the same or worse than in preoperative studies” (2). Rasmussen later suggested that only acute ECoG “red spikes” seen in abnormal cortical regions surrounding a structural lesion need to be surgically pursued, while “green spikes” seen at variable distances from the lesional border are not as relevant for surgical outcomes (3). Simplistically thinking, all spikes are not created equal, and the spike zone was larger than the epileptogenic lesion and could not be used in isolation to define an epileptogenic focus (Figure 1). Eventually, Rasmussen (3) expanded this idea by proposing “localization concepts” (Table 1). These represented an early perceptive appreciation of the fact that resecting areas of ictal onset on invasive EEG (the primary localizing diagnosis) is not always sufficient to cure epilepsy, a feat that would also require understanding cortical recruitment during seizures (secondary localization) and the extent of resection necessary to produce a favorable seizure outcome (tertiary localization). Rasmussen was very hopeful in 1983 that “some of the increasingly sophisticated EEG techniques now coming into more widespread use may soon yield important progress in the quantification and accuracy of both these latter two localizational aspects of the epileptic spectrum” (3).
FIGURE 1.
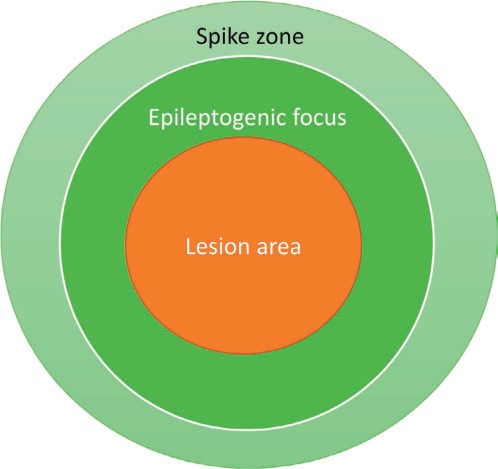
Herbert Jasper's view of the lesion, spike zone, and epileptogenic focus.
TABLE 1.
Rasmussen's Localization Concepts
The Epileptogenic Zone: 1960s – Stereo Electroencephalography (SEEG)
Theoretical Definition
In 1965, Talairach and Bancaud highlighted the inadequacy of the epileptogenic lesion definition given “a certain number of unsatisfactory surgical results” and then introduced the term “epileptogenic zone” (EZ) to reflect “the site of the beginning of the epileptic seizures and of their primary organization” (4).
Surgical Definition
Talairach and Bancaud (4) believed that since epilepsy surgery aimed to cure seizures rather than spikes or lesions, it was critical to understand how ictal patterns led to clinical symptoms (anatomo-electro-clinical correlation). They developed the technique of targeted depth electrode implantations through stereo electroencephalography (SEEG), allowing the three-dimensional exploration of the brain's regions capable of generating a patient's seizures, thereby best understanding how seizures start and spread: the spatiotemporal organization of the epileptic discharge within the brain. In the 1960s, this EZ definition could only be done using intra-operative interical and ictal recordings. Starting in the 1990s, chronic extra-operative recordings became routine with the introduction of chronic video-EEG. Current advances, such as magnetic resonance arteriography and robotic guided surgery, have simplified accurate depth electrode implantation (5). Indeed, multiple factors have influenced the technical aspects of SEEG since its inception, but the electrophysiological principles remain focused on achieving a successful anatomo-electro-clinical correlation that can subsequently delineate a resective surgical strategy. Table 2 highlights a methodical and time-consuming process that Bancaud and Talairach spelled out in 1991 to ultimately ensure that the sequence and constellation of clinical events are explained by the onset and spread of the EEG seizure (6, 7).
TABLE 2.
Principles Necessary to Interpret the Anatomo-Electro-Clinical Correlations in Temporal Lobe Epilepsy

In simplistic terms, the goals of the SEEG evaluation are to rule in a primary localization hypothesis (prove that a network of interest is indeed responsible for the patient's seizures), rule out alternative hypotheses (prove that other networks in our “differential diagnosis” for localization do not provide a better explanation for our EEG findings and semiology), define an ideal resection margin (how much tissue ideally needs to be removed for seizure freedom), define a safe resection margin (how much of the ideal defined resection can be done safely) and, lastly, consider how a lesion—when present—fits with the localization findings and the surgical plan. It is not surprising that the “lesion comes last” in a puristic SEEG evaluation: When the technology was first introduced, all patients were practically nonlesional given the limitations of neuroimaging at the time and the lack of direct cortical visualization in a process relying on depth implantation through burr holes. This emphasis on the anatomo-electro-clinical correlation advanced the understanding of how ictal patterns explain seizure semiology. It also solidified the unique value of electrophysiological data in defining an EZ concept that expands beyond the region of seizure onset to cortical regions necessary for its primary organization. Yet, the process of defining primary organization is challenging to characterize in reproducible terms, and the translation of this theoretical knowledge into a pragmatic surgical plan requires significant expertise in the analysis and interpretation of SEEG findings. Other challenges include difficulty in mapping language cortex (particularly receptive language cortex) and mapping the extent of epileptogenicity to delineate margins of a resection in a predominantly neocortical epilepsy.
The Epileptogenic Zone: 1990s–early 2000s – Subdural Electrodes (SDE)
Theoretical Definition
In 1993, Luders et al. defined the EZ as “the area of cortex that is necessary and sufficient for initiating seizures and whose removal (or disconnection) is necessary for complete abolition of seizures” (8).
Surgical Definition
The EZ concept of Luder and colleagues becomes meaningful only when viewed in the context of a five cortical zone definition proposed for presurgical evaluation (Table 3). As one visualizes the overlap between these zones (Figure 2) and the tests used to define them, a few implications can be considered.
TABLE 3.
Five Cortical Zones Defined in the Presurgical Evaluation
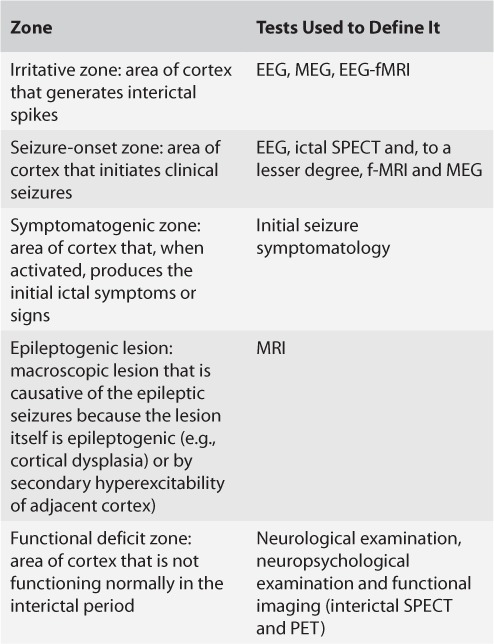
FIGURE 2.
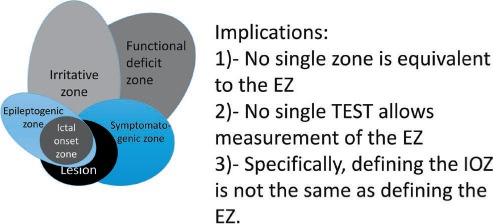
The variable overlap of the epileptogenic zone with the major cortical areas.
First, there is no direct preoperative measurement of the EZ: Its delineation is a purely conceptual exercise incorporating data derived from multiple tests and various components of a presurgical evaluation. This requires a clear understanding of the role played by each zone in defining the epilepsy and elevates the definition of a surgical plan to an endeavor that extends beyond delineating the lesion.
Second, removing the cortex causing the seizures (ictal onset zone [IOZ]) is typically necessary but not sufficient to achieve lasting seizure freedom: The EZ extends beyond the IOZ, aligning with the idea of a potentially epileptic cortex beyond the current zone and representing a more advanced version of the localization concepts introduced by Rasmussen.
Third, by definition, one can be certain that the EZ was correctly identified only after the surgery renders the patient seizure free, which complicates the pragmatic usefulness of this definition as a surgical plan is being developed.
Fourth, the concept of the EZ-visualized disorder with distinct dysfunctional zones around an epileptic pathology—the epileptogenic lesion—inherently limits the grasp of epilepsy in the absence of a lesion.
SDE represents the ideal evaluation tool for such an EZ definition: extensive cortical coverage allowed meticulous cortical distribution of spikes (irritative zone), ictal patterns (ictal onset zone) with their contiguous cortical spread, and functional mapping of eloquent cortex—ultimately facilitating the delineation of the five cortical zones. This EZ definition recognizes that a potential seizure onset zone capable of generating seizures in the future exists beyond the actual seizure onset zone that is triggering the patient's current seizures, and that both need to be resected to achieve seizure freedom (9). Long-term surgical outcome studies have documented reasonable success rates, with 66% of patients seizure free 1 year after surgery, 50% at 3 years, and 33% at 10 years (10). The main limitations of SDE (highlighted by outcomes at study centers) are an inability to capture epileptic activity in the deep regions of the brain or to evaluate the EZ as the product of distant, yet connected, brain regions. Predictors of seizure freedom include a single IOZ (as opposed to multiple ictal onsets, suggesting either multifocal epilepsy or variable spread patterns from an unexplored “deep” IOZ; 10), ictal onset in the center of the SDE suggesting definite localization of the IOZ (as opposed to seizures seemingly starting from the edge of a grid, which may represent spread from an unexplored cortex; 11) and complete resection of the IOZ (12). The addition of some free-hand depth electrode recording to SDE grew in the mid-2000s as an attempt to remedy some of these limitations and to sample from deep brain regions, such as the hippocampus, the depths of a well-visualized malformation of cortical development, and rarely from the insula.
Network Epilepsy: 2000s to Present – SEEG/Depth Recordings
Theoretical Definition
Multiple definitions exist as the interest in epilepsy networks grew exponentially over the past few decades (1 PubMed Index publication on “epilepsy networks” in 1975 as opposed to 255 in 2016). The earliest well-formulated definition in North America came from Spencer in 2002: “A network [is] a functionally and anatomically connected, bilaterally represented, set of cortical and subcortical brain structures and regions in which activity in any one part affects activity in all the others” (13).
Surgical Definition
The surgical implications of the Spencer definition, as highlighted in her seminal paper, state that “broadly applied treatment (directed at any region of the network) should theoretically be just as effective as treatments directed at a specific ‘focus’ of seizure activity” (13). Cited examples of this idea include vagus nerve stimulation and thalamic stimulation. This expanded concept of the EZ opened doors for sophisticated neuroimaging and electrophysiological analyses that explore the connections between various nodes of this epileptic network, using tools such as functional MRI and cortico-cortical evoked potentials (14), connectivity analyses (15), graph theory (16), and dynamic computational modeling (17, 18). For example, a recent spatiotemporal model of seizure dynamics distinguishes various components of the network by their ability to sustain autonomous seizure activity, as well as whether or not epileptiform activity builds up preictally, generating a representation of the network that covers large areas of the brain surface (17).
The pragmatic implications of this peaked appreciation of epilepsy as a network remain to be seen. So far, the benefits of modulating individual network regions either electrically (e.g., responsive neurostimulation; 19) or anatomically (e.g., thermal ablation; 20) are limited to palliation when compared to the more definitive results achieved with resective surgery. Similarly, additional work is needed to translate the wealth of these newly developed imaging and EEG tools into clinically applicable testing with added value beyond the current standard of practice.
Future Direction
As discussed so far, an under-recognized common thread through the EZ definitions is the appreciation—albeit to varying degrees—that removing the cortex generating seizures at the time of surgery is not always enough for a definitive cure. The tertiary localization of Rasmussen, the emphasis on the zone of primary organization in SEEG, the recognition of a potential seizure onset zone by Luders—are all glimpses of an understanding of a dynamic EZ, one that evolves over time rather than remains static in a given brain region. Failure to remove the current EZ leads to immediate ongoing postoperative seizures. Failure to remove the potential EZ leads to late seizure recurrence after an initial period of seizure freedom (21). The challenge is in our inability to define the potential EZ. Our localization tools have significantly evolved since Rasmussen's days, as have our tools for invasive EEG: We went from brief intraoperative EcOG when the EZ was a lesion, to subdural recordings when the EZ was more lesion and perilesional cortex, to SDE with depths, and then SEEG when the EZ became a network—yet our surgical outcomes remain the same. A simple visual of the evolving representations of the EZ (Figure 3) clearly shows an unremitting trend towards including more brain in the EZ. The future may be in our recognition that in some patients, the epileptogenic zone is the brain: It is in the genetic, molecular, subcellular structure of the brain predisposing some of our surgical patients to redevelop epilepsy even after we remove their current EZ (22). The future may be in our focusing more on the “epileptogenic” rather than on the “zone” component of the epileptogenic zone.
FIGURE 3.
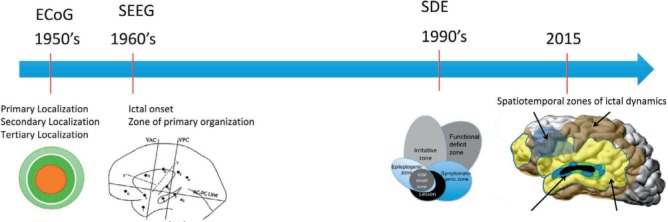
The evolving representations of the epileptogenic zone (EZ).
Supplementary Material
References
- 1. Penfield W. Epileptogenic lesions. 1956; 56: 75– 88. [PubMed] [Google Scholar]
- 2. Jasper HH, Arfel-Capdeville G, Rasmussen T.. Evaluation of EEG and cortical electrographic studies for prognosis of seizures following surgical excision of epileptogenic lesions. 1961; 2: 130– 137. [PubMed] [Google Scholar]
- 3. Rasmussen T. Characteristics of a pure culture of frontal lobe epilepsy. 1983; 24: 482– 493. [DOI] [PubMed] [Google Scholar]
- 4. Talairach J, Bancaud J.. Lesion, “irritative” zone and epileptogenic focus. 1966; 27: 91– 94. [DOI] [PubMed] [Google Scholar]
- 5. Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P, Gonzalez-Martinez J.. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. 2016; 57: 386– 401. [DOI] [PubMed] [Google Scholar]
- 6. Garcia Sola R, Miravet J.. Surgical treatment for epilepsy. results after a minimum follow-up of five years. 1991; 52: 157– 160. [DOI] [PubMed] [Google Scholar]
- 7. Kahane P, Landre E, Minotti L, Francione S, Ryvlin P.. The Bancaud and Talairach view on the epileptogenic zone: A working hypothesis. 2006; 8: S16– 26. [PubMed] [Google Scholar]
- 8. Luders HO, Engel J, Munari C.. General principles. : ( 2nd ed.). ( Engel JJ, ) New York: Raven Press, 1993: 137– 153. [Google Scholar]
- 9. Luders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W.. The epileptogenic zone: General principles. 2006; 8: S1– 9. [PubMed] [Google Scholar]
- 10. Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, Najm I, Bingaman W.. Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. 2012; 53: 1722– 1730. [DOI] [PubMed] [Google Scholar]
- 11. Widdess-Walsh P, Jeha L, Nair D, Kotagal P, Bingaman W, Najm I.. Subdural electrode analysis in focal cortical dysplasia: Predictors of surgical outcome. 2007; 69: 660– 667. [DOI] [PubMed] [Google Scholar]
- 12. See SJ, Jehi LE, Vadera S, Bulacio J, Najm I, Bingaman W.. Surgical outcomes in patients with extratemporal epilepsy and subtle or normal magnetic resonance imaging findings. 2013; 73: 68– 77. [DOI] [PubMed] [Google Scholar]
- 13. Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. 2002; 43: 219– 227. [DOI] [PubMed] [Google Scholar]
- 14. Jones SE, Zhang M, Avitsian R, Bhattacharyya P, Bulacio J, Cendes F, Enatsu R, Lowe M, Najm I, Nair D, Phillips M, Gonzalez-Martinez J.. Functional magnetic resonance imaging networks induced by intracranial stimulation may help defining the epileptogenic zone. 2014; 4: 286– 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Mierlo P, Carrette E, Hallez H, Raedt R, Meurs A, Vandenberghe S, Van Roost D, Boon P, Staelens S, Vonck K.. Ictal-onset localization through connectivity analysis of intracranial EEG signals in patients with refractory epilepsy. 2013; 54: 1409– 1418. [DOI] [PubMed] [Google Scholar]
- 16. Panzica F, Varotto G, Rotondi F, Spreafico R, Franceschetti S.. Identification of the epileptogenic zone from stereo-EEG signals: A connectivity-graph theory approach. 2013; 4: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinha N, Dauwels J, Kaiser M, Cash SS, Brandon Westover M, Wang Y, Taylor PN.. Predicting neurosurgical outcomes in focal epilepsy patients using computational modelling. 2017; 140 pt 2: 319– 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khambhati AN, Davis KA, Oommen BS, Chen SH, Lucas TH, Litt B, Bassett DS.. Dynamic network drivers of seizure generation, propagation and termination in human neocortical epilepsy. 2015; 11: e1004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, Srinivasan S, Jobst B, Gross RE, Shields DC Barkley G, Salanova V, Olejniczak P, Cole A, Cash SS, Noe K, Wharen R, Worrell G, Murro AM, Edwards J, Duchowny M, Spencer D, Smith M, Geller E, Gwinn R, Skidmore C, Eisenschenk S, Berg M, Heck C, Van Ness P, Fountain N, Rutecki P, Massey A, O'Donovan C, Labar D, Duckrow RB, Hirsch LJ, Courtney T, Sun FT, Seale CG.. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. 2015; 84: 810– 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bourdillon P, Isnard J, Catenoix H, Montavont A, Rheims S, Ryvlin P, Ostrowsky-Coste K, Mauguiere F, Guénot M.. Stereo electroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: Results from a 10-year experience. 2017; 58: 85– 93. [DOI] [PubMed] [Google Scholar]
- 21. Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W.. Temporal patterns and mechanisms of epilepsy surgery failure. 2013; 54: 772– 782. [DOI] [PubMed] [Google Scholar]
- 22. Jehi L. Improving seizure outcomes after epilepsy surgery: Time to break the “find and cut” mold. 2015; 15: 189– 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


