Abstract
Background
Racial disparities in cancer outcomes have been described. It is still unclear if patients of Hispanic ethnicity have better or worse survival outcomes. In this study we evaluated whether Hispanic participants in SWOG clinical trials had different survival outcomes compared to non-Hispanics.
Methods
Adult patients registered in SWOG phase II/III clinical trials between 1986 and 2012 were analyzed. Studies of similar histology and stage were combined. Within each analysis, Kaplan-Meier survival curves were generated to examine differences in outcome by ethnicity. Multivariate Cox regression was used to estimate the association of ethnicity and survival outcomes, controlling for major disease-specific prognostic factors and demographic variables plus area-level income and education to account for socioeconomic status.
Results
29,338 patients registered to 38 trials were included; 5% of them were Hispanic. Hispanics were more likely to be younger and from lower income and education areas (all p<0.05). No differences in survival were observed across tumor types except in the advanced-stage prostate cancer group, where we observed an association between Hispanic ethnicity and worse overall survival (HR=1.40, p=0.006), progression-free survival (HR=1.36, p=0.007), and cancer specific survival (HR=1.42, p=0.013). After adjusting for multiple comparisons, no differences in outcomes were seen.
Conclusions
Hispanic patients participating in SWOG trials receiving uniform treatment and follow-up had similar survival outcomes compared to non-Hispanic patients, with the single exception of the advanced stage prostate cancer group.
Impact
Our study shows that Hispanic patients receiving uniform treatment and follow-up have similar outcomes compared to non-Hispanics.
Keywords: Race, Ethnicity, Hispanics, Outcome, Disparities
BACKGROUND
Over the last few decades a sustained decline in cancer mortality has been evident1. Declines in cancer death rates have been noted for men and women across all ethnic and racial groups. However, the mortality decline has been consistently greater among whites compared to African Americans (AA) or Hispanics1 suggesting that therapeutic advances are not benefiting all patients equally2, 3. While differences in tumor biology are likely contributing, differences found within tumor subsets suggest that socio-demographic factors like access to quality care or timely treatment could play a role.
Hispanics represent the largest ethnic minority group in the United States, accounting for 17.4% of the total population4. Projections indicate that the size of this population will double by 20505, suggesting the importance of evaluating outcomes for this ethnic group. Hispanics have different behavioral and environmental exposures and a high prevalence of important cancer risk factors like obesity and diabetes. Among Hispanics, cancer is the leading cause of death6, and Hispanics are more likely to be diagnosed with advanced stages compared with their non-Hispanic counterparts. Recent reports suggest that the cancer death rates among Hispanics are 30% lower compared with non-Hispanic whites, however these observed discrepancies may be exaggerated due to incomplete death ascertainment and challenges associated with follow-up6. Comprehensive analyses using prospective data across a broad range of cancers comparing outcomes among Hispanics and non-Hispanics have not been conducted.
In this study, we evaluated outcomes of cancer patients treated in clinical trials according to Hispanic ethnicity. Studying clinical trials participants allows for the analysis of a homogenous patient population with known baseline prognostic factors, controls for treatment, and follows patients similarly at regular intervals with reliable assessment of outcomes. To the best of our knowledge this is the first study evaluating outcomes according to Hispanic ethnicity in a large cohort of clinical trial participants controlling for demographic and clinically-relevant variables.
METHODS
The data included in this analysis originate from SWOG, an NCI-sponsored clinical trials consortium. Beginning in the late 1980s, SWOG routinely collected self-reported data on ethnicity (Hispanic vs. non-Hispanic). We systematically examined the SWOG trials from 1986–2012. We considered only published phase III or large phase II trials enrolling adult patients with available ethnicity data.
Given that the Hispanic population in the U.S. has a younger age distribution, and cancer occurs primarily in older individuals, the proportion of patients with Hispanic ethnicity in trials is often small. To increase power, we combined trials according to disease site, histology and stage. The primary outcome was overall survival (OS), measured as days from registration to last contact (censored) or death by any cause. We also examined progression-free survival (PFS) and cancer specific survival (CSS) as secondary outcomes. PFS was defined as days from registration to last contact (censored), death by any cause, or evidence of relapse or progressive disease. CSS was measured as days from registration to cancer specific death, death by another cause (censored), or last contact (censored). Approximately 29% of patient deaths were accompanied by detailed cause of death information. For the remaining 71%, any death preceded by documented relapse or progression was considered a cancer specific death. All analyses were limited to the first five years after registration in order to focus on cancer-related and treatment-related survival. Patients with last contact date or death date greater than five years post-registration were censored at five years.
Statistical Analysis
Kaplan-Meier survival curves were generated for each analysis, separately for Hispanic and non-Hispanic patients. Multivariate Cox regression was used to estimate the effect of Hispanic ethnicity on OS, PFS and CSS while controlling for disease-specific prognostic factors and socio-demographic variables. Each analysis accounted for age, race, and sex. To account for differences in socioeconomic status (SES) and educational level, SES was estimated using patient zip code. Zip code was linked to median household income and percent of residents who completed high school via 2000 U.S. census data. If the median household income in a patient’s zip code was higher than the overall 2000 U.S. median household income, the patient was designated as high income. A similar method used percent of residents who completed high school in a given zip code to define the area as having high or low education level. In addition, each analysis accounted for important disease-specific clinical adjustment variables. Only clinical adjustment variables with a known effect on survival that were measured in all studies within an analysis were included. Supplementary Table-1 provides a complete list of the variables included in the multivariable model for each tumor subtype category. Each analysis was stratified by study. Results are expressed as Hazard Ratios (HR) and 95% Confidence Intervals (CI). Follow-up time was truncated at 5 years to emphasize survival related to cancer diagnosis and treatment. In addition to disease-specific analyses, we also conducted an omnibus test of the global trend across the different analyses, based on a one-sample t-test of the analysis-specific (signed) chi-square statistics representing the test of the statistical significance of the association of ethnicity and outcome in the multivariable regression models.
Since males represented a very small fraction of the breast cancer patients, they were removed from the breast cancer analyses. Among patients with Non-Hodgkin Lymphoma (NHL) the International Prognostic Index (IPI) was included as a covariate; since the calculation of this index considers age, NHL analyses did not include age. Finally, because there were relatively few patients in the advanced breast cancer group who were ER and PR-negative, only patients who were ER and/or PR-positive were included. We conducted multiple sensitivity analyses: 1) we compared survival outcomes between Hispanics and non-Hispanic whites (rather than all non-Hispanics); 2) we excluded income and education level; 3) we limited follow-up to the first 10 years after randomization (rather than the first five years only); 4) we examined alternative approaches to identifying cancer-specific deaths; and 5) we examined the potential for different trends in the association between ethnicity and outcomes between patients enrolled prior to vs. after the year 2000. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), all tests were two-sided and used an exploratory significance level of α=0.05. Observed p-values were also compared to a multiple comparisons Bonferroni-adjusted p-value level. Assuming independent testing for a given endpoint (e.g. overall survival), this level was calculated at p=.0036 (p=.05 divided by 14 analyses per endpoint type). Power calculations encompassing the range of sample sizes used in this analysis are shown in the appendix (Supplementary Table-2).
RESULTS
In total, we obtained patient data from 38 unique trials totaling 37,548 enrollments. 8,210 patients were missing data on Hispanic ethnicity, zip code, or one of the important clinical adjustment variables, leaving 29,338 patients available for inclusion in 14 separate analyses (Table-1).
Table-1.
Clinical trials included in the analysis classified according to disease site and stage
| Type of cancer | Trials by study number | Enrollment date range | Major eligibility criteria | Important clinical adjustment variables* | Total sample size (No. Hispanic-% Hispanic) |
|---|---|---|---|---|---|
| Adjuvant breast, ER− and PR− | S8897, S9313, S9623, S0012, S0221, S0307 | 1989–2012 | Early stage (I-III), no prior chemotherapy for this breast cancer** | • Number of positive lymph nodes: ≥4 vs. <4 • Tumor size:≤5cm vs. >5cm • Pre-menopausal vs. post-menopausal |
4,387 (270–6%) |
| Adjuvant breast, ER+ and/or PR+ | S8814, S8897, S9313, S9623, S0012, S0221, S0307 | 1989–2012 | Early stage (I-III), no prior chemotherapy for this breast cancer** | • Number of positive lymph nodes: ≥4 vs. <4 • Tumor size: ≤5cm vs. >5cm • Pre-menopausal vs. post-menopausal |
9,991 (482–5%) |
| Advanced breast, ER+ and/or PR+ | S0226, S0500 | 2004–2012 | Stage IV, no prior chemotherapy for metastatic disease | • Her2- vs. Her2+ • Pre-menopausal vs. post-menopausal |
958 (43–4%) |
| Advanced colorectal | S8905, S9420 | 1989–1999 | Metastatic (disseminated), maximum 1 prior adjuvant chemo/immunotherapy | • Performance status: 0–1 vs. 2 | 1,038 (81–8%) |
| Adjuvant gastric | S9008 | 1991–1998 | Stages IB-IV (M0) | • Number of involved lymph nodes: none vs. 1 – 3 vs. ≥4 • T Stage:T1–T2 vs. T3–T4 |
474 (50–11%) |
| Adjuvant colorectal | S9304, S9415 | 1994–2000 | Stage M0, no prior systemic or radiation therapy | • Number of nodes involved: N0 vs. N1 vs. N2–N3 • Performance status: 0–1 vs. 2 • Invasion of perirectal fat or adjacent organs: T1–T2 vs. T3–T4 |
2,521 (87–3%) |
| Advanced prostate | S8894, S9346 | 1989–2008 | D2 disease | • Performance status: <2 vs. ≥2 • Severity of disease: minimal vs. extensive |
2,810 (100–4%) |
| Advanced prostate, hormone refractory | S9916, S0421 | 1999–2010 | Metastatic prostate cancer that is unresponsive or refractory to hormone therapy. Maximum one prior systemic therapy. | • Extraskeletal metastatic disease: yes vs. no • PSA progression only: yes vs. no • Performance status: < 2 vs. ≥ 2 |
1,547 (81–5%) |
| AML | S8600, S9031, S9333, S0106 | 1986–2009 | No previous systemic chemotherapy for acute leukemia | • SWOG performance status: <2 vs. ≥2 | 979 (45–5%) |
| Advanced NSCLC | S9308, S9509, S0003 | 1993–2002 | No previous systemic chemotherapy or biologic therapy for NSCLC | • Weight loss: <5% vs. ≥5% • LDH: Normal (≤ULN) vs. abnormal (>ULN) • Stage of disease: IIIB vs. IV |
1,137 (31–3%) |
| Advanced Aggressive NHL | S8516, S9704, S9349, S9125, S9240 | 1986–2007 | Intermediate or high grade histology, no prior chemotherapy or radiation therapy for lymphoma*** | • IPI risk: Low vs. low-int vs. high-int vs. high | 699 (37–5%) |
| Advanced Indolent NHL | S0016, S8809, S9800, S9911 | 1988–2008 | Stages IIB-IV, no prior chemotherapy or radiation therapy for lymphoma | • IPI risk: Low vs. low-int vs. high-int vs. high | 974 (25–3%) |
| Multiple Myeloma | S9210, S9321, S0232 | 1993–2007 | No prior chemotherapy for this disease | • Stage: I-II vs. III | 1,211 (43–4%) |
| Advanced GIST | S0033 | 2000–2001 | Metastatic or unresectable | • Performance status: 0–2 vs. 3 • Measurable vs. Non-Measurable disease |
612 (28–5%) |
| Total | 38 unique trials | 1986–2012 | 29,338 (1,403–5%) |
Including age (<65 vs. ≥65), race (African American vs. other), and sex (where appropriate).
For S0307, chemotherapy was allowed concurrent with registration.
Study S9704 allows a single course of CHOP/CHOP-R chemotherapy.
Patient Characteristics
Patient characteristics and clinical prognostic factors according to cancer type are shown in Table-2. Five percent of the participants were Hispanic. Patient characteristics and clinical prognostic factors according to Hispanic ethnicity are shown in Table-3. Hispanic patients participating in SWOG clinical trials were younger and less likely to report being AA; in addition they lived in lower income and education areas (all p<0.05). There were very few statistically significant differences between Hispanic and non-Hispanic patients with respect to clinical prognostic factors.
Table-2.
Patient characteristics and disease-site specific prognostic factors according to clinical trial subgroup analysis
| Type of cancer | Percent of patients who are: | Percent of patients with disease-site specific prognostic factors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥65yo | Female | African American |
Hispanic | Education≤ U.S. median |
Income≤ U.S. median |
||||
| Adjuvant breast ER− and PR−*** | 7.5% | 100% | 12.7% | 6.2% | 34.2% | 50.0% | Tumor > 5 cm: 8.9% |
Post-menopausal: 47.5% |
≥ 4 nodes: 12.5%** |
| Adjuvant breast ER+ and/or PR+*** | 13.2% | 100% | 7.2% | 4.8% | 30.5% | 46.0% | Tumor > 5 cm: 8.7% |
Post-menopausal: 56.0% |
≥ 4 nodes: 22.6%** |
| Advanced breast ER+ and/or PR+ | 41.9% | 100% | 9.4% | 4.5% | 31.8% | 53.5% | Post-menopausal: 93.5% |
Her2negative: 88.4%** |
|
| Advanced colorectal | 39.7% | 41.2% | 13.0% | 7.8% | 44.3% | 60.3% | Performance status 2: 10.4% |
||
| Adjuvant gastric | 33.5% | 27.6% | 17.7% | 10.5% | 50.0% | 63.3% | ≥ 4 nodes: 42.0% |
Stage T3-T4: 67.7% |
|
| Adjuvant colorectal | 37.9% | 40.7% | 6.9% | 3.5% | 35.6% | 56.5% | N2–3 nodes: 30.9% |
Stage T3-T4: 85.2% |
Performance status 2: 2.9% |
| Advanced prostate | 66.7% | 0% | 20.1% | 3.6% | 39.9% | 55.9% | Performance status ≥ 2: 10.7% |
Severity = Extensive: 74.2% |
|
| Advanced prostate, hormone refractory | 70.7% | 0% | 13.7% | 5.2% | 36.3% | 51.8% | Extraskeletal metastasis: 52.9% |
Progression other than PSA: 81.1% |
Performance status ≥ 2: 9.2% |
| AML | 33.7% | 44.8% | 8.3% | 4.6% | 39.5% | 56.6% | Performance status ≥ 2: 21.9% |
||
| Advanced NSCLC | 41.4% | 33.5% | 12.4% | 2.7% | 45.8% | 62.2% | ≥ 5% weight loss: 39.3% |
Abnormal LDH: 37.6% |
Stage IV: 89.0% |
| Advanced aggressive NHL | 15.2% | 39.5% | 9.2% | 5.3% | 36.9% | 53.8% | IPI risk = High/High-Int.: 40.3% |
||
| Advanced indolent NHL | 11.9% | 42.4% | 4.4% | 2.6% | 32.6% | 49.9% | IPI risk = High/High-Int.: 13.0% |
||
| Multiple Myeloma | 17.3% | 40.9% | 17.8% | 3.6% | 37.3% | 55.3% | Stage III: 59.1% |
||
| Advanced GIST | 46.2% | 44.8% | 11.4% | 4.6% | 38.2% | 48.5% | Performance status 3: 3.3% |
Measurable disease: 94.4% |
|
| Total | 27.5% | 40.1%1 | 10.7% | 4.8% | 35.1% | 51.5% | |||
Total percent includes only the non-sex specific cancers
Percent of non-missing data
S0012 did not collect baseline information on the number of positive lymph nodes. In order to include patients from S0012 in the analyses, a dummy covariate was created to denote missing node involvement information. Similarly, since approximately 11% of the patients in the advanced breast cancer group had missing Her2 status, a dummy covariate was created to denote Her2 status missing information.
Table-3.
Patient characteristics and disease-site specific prognostic factors according to Hispanic ethnicity and clinical trial subgroup analysis (patients with complete data).
| Type of cancer | Percent of patients in high risk categories (% of all Hispanics vs. % of all non-Hispanics) | |||||||
|---|---|---|---|---|---|---|---|---|
| ≥65yo | Female1 | African American | Education≤ U.S. median | Income≤ U.S. median | Disease-site specific prognostic factors | |||
| Adjuvant breast ER− and PR− | 6 vs. 8 | 3 vs. 13* | 68 vs. 32* | 68 vs. 49* | Tumor>5 cm: 20 vs. 8* |
Post-menopausal: 40 vs. 48* |
≥ 4 nodes: 9 vs. 13 |
|
| Adjuvant breast ER+ and/or PR+ | 5 vs. 14* | 2 vs. 7* | 70 vs. 29* | 66 vs. 45* | Tumor>5 cm: 13 vs. 8* |
Post-menopausal: 51 vs. 56* |
≥ 4 nodes: 29 vs. 22* |
|
| Advanced breast ER+ and/or PR+ | 30 vs. 42 | 5 vs. 10 | 53 vs. 31* | 70 vs. 53* | Post-menopausal: 95 vs. 93 |
Her2negative: 92 vs. 88 |
||
| Advanced colorectal | 19 vs. 41* | 41 vs. 41 | 2 vs. 14* | 83 vs. 41* | 78 vs. 59* | Performance status 2: 11 vs. 10 |
||
| Adjuvant gastric | 20 vs. 35* | 42 vs. 26* | 2 vs. 20* | 72 vs. 47* | 72 vs. 62 | ≥4 nodes: 32 vs. 43 |
Stage T3-T4: 66 vs. 68 |
|
| Adjuvant colorectal | 28 vs. 38 | 43 vs. 41 | 0 vs. 7* | 74 vs. 34* | 76 vs. 56* | N2–3 nodes: 26 vs. 31 |
Stage T3-T4: 89 vs. 85 |
Performance status 2: 8 vs. 3* |
| Advanced prostate | 52 vs. 67* | 2 vs. 21* | 77 vs. 38* | 67 vs. 56* | Performance status ≥2: 13 vs. 11 |
Severity = Extensive: 79 vs. 74 |
||
| Advanced prostate, hormone refractory | 54 vs. 72* | 0 vs. 14* | 63 vs. 35* | 58 vs. 51 | Extraskeletal metastasis: 58 vs. 53 |
Progression other than PSA: 89 vs. 81 |
Performance status ≥2: 12 vs. 9 |
|
| AML | 16 vs. 35* | 47 vs. 45 | 0 vs. 9* | 71 vs. 38* | 76 vs. 56* | Performance status ≥2: 11 vs. 22 |
||
| Advanced NSCLC | 39 vs. 42 | 39 vs. 33 | 0 vs. 13* | 77 vs. 45* | 77 vs. 62 | ≥5% weight loss: 48 vs. 39 |
Abnormal LDH: 35 vs. 38 |
Stage IV: 81 vs. 89 |
| Advanced aggressive NHL | 8 vs. 16 | 32 vs. 40 | 0 vs. 10* | 62 vs. 35* | 73 vs. 53* | IPI risk=High/High-Int.: 35 vs. 41 |
||
| Advanced indolent NHL | 20 vs. 12 | 36 vs. 43 | 4 vs. 4 | 44 vs. 32 | 56 vs. 50 | IPI risk=High/High-Int.: 20 vs. 13 |
||
| Multiple Myeloma | 9 vs. 18 | 37 vs. 41 | 5 vs. 18* | 67 vs. 36* | 70 vs. 55 | Stage III: 65 vs. 59 |
||
| Advanced GIST | 21 vs. 47* | 46 vs. 45 | 0 vs. 12 | 71 vs. 37* | 64 vs. 48 | Performance status 3: 4 vs. 3 |
Measurable disease: 96 vs. 94 |
|
| Total1 | 17 vs. 28* | 41 vs. 40 | 2 vs. 11* | 70 vs. 33* | 68 vs. 51* | |||
Percent provided for the non-sex specific cancers only
Indicates a significant difference between Hispanic and non-Hispanic groups (p<0.05)
Outcomes by Ethnicity Status
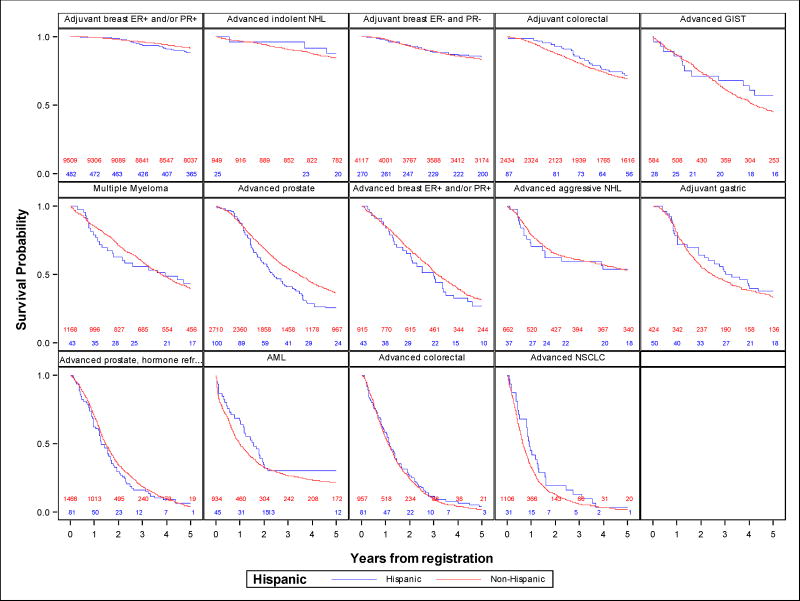
Figure-1 shows Kaplan-Meier OS curves comparing Hispanic and non-Hispanic patients. There were no clear differences according to Hispanic ethnicity in OS; the only exception was among patients with advanced prostate cancer where Hispanics had worse survival. Interestingly, no survival differences were observed among patients with hormone-refractory advanced prostate cancer. Multivariate Cox regression results showed a significant association between Hispanic ethnicity and worse OS in the advanced-stage prostate cancer group (HR=1.40, 95% CI: 1.10–1.78, p=.006). Consistent with the OS results, Hispanic patients in the advanced-stage prostate cancer group also had worse PFS (HR=1.36, 95% CI: 1.09–1.70, p=.007) and CSS (HR=1.42, 95% CI: 1.08–1.87, p=.01). Hispanics had better CSS in early-stage ER and PR-negative breast cancer (HR=0.63, 95% CI: 0.43–0.93, p=.02). There were no other statistically significant differences in survival outcomes. Importantly, none of these results achieved statistical significance under a multiple testing p-value level of p=.0036. Results from the multivariable Cox models are illustrated in Figure-2. There was no evidence of a general trend towards better or worse outcomes for Hispanic patients across the panel of different analyses according to the global tests.
Figure-1. Kaplan-Meier survival curves for Hispanic vs. non-Hispanic patients.
Numbers along the bottom of each graph represent at-risk counts for Hispanic (in blue) and non-Hispanic (in red) patients at the corresponding time in years along the x-axis.
Figure-2. Multivariable analysis of OS, PFS and CSS according to Hispanic ethnicity by tumor site analysis.
Forest plots are shown for each survival outcome by cancer site category and represent Cox regression-based hazard ratios for Hispanic versus non-Hispanic ethnicity. Each horizontal bar represents the 95% confidence interval for the associated hazard ratio (HR; black dot). HRs to the left of the line of equal hazard (HR=1) indicate better survival for Hispanic patients, and HRs to the right of line of equal hazard indicate worse survival for Hispanic patients. Cancer groups are shown in order of the HR from the overall survival Cox regressions.
Sensitivity Analyses
When using non-Hispanic whites as reference category, the results remained grossly unchanged (Supplementary Figure-1). The exclusion of SES variables changed some of the results, highlighting the importance of social factors in cancer outcomes. Similar to the full model, Hispanic patients with advanced stage prostate cancer had worse outcomes. Hispanic patients with early stage ER and/or PR-positive breast cancer had worse PFS than their non-Hispanic counterparts. Hispanics with early stage ER and PR-negative breast cancer had better CSS than non-Hispanics. Otherwise, there were no statistically significant differences between Hispanic and non-Hispanic patients (data not shown). In no disease group was there evidence that the association of Hispanic ethnicity and outcome differed between categories of education or income using interaction tests. Sensitivity analyses evaluating a longer follow-up time of 10 years are shown in Supplementary Figure-2, indicating generally similar results. We examined different approaches to identifying cancer-specific deaths, finding no global pattern of better or worse cancer-specific deaths for Hispanic patients under any of three alternative scenarios (Supplementary Figure-3). Finally, we found no instances of statistically significant associations of Hispanic ethnicity and outcomes in the opposite directions between patients enrolled prior to and after the year 2000, and there was no statistically significant evidence that global patterns in the three outcomes (OS, PFS, and CSS) differed between patients registered prior to vs. after the year 2000 (data not shown).
DISCUSSION
In this large cohort of patients participating in therapeutic SWOG clinical trials we found little evidence that overall, progression-free, or cancer-specific survival differed between Hispanics and other ethnic groups; indeed there were no differences after adjusting for multiple comparisons. These results contrast with recent cancer statistics indicating that stage adjusted mortality rates among Hispanics are different compared to non-Hispanic whites6. The mortality rate for males (per 100,000 and age adjusted to the 2000 U.S. standard population) is estimated to be 148 for Hispanics and 211 for non-Hispanic whites; among females mortality rates are 99.4 and 149.2 respectively.
This differential mortality favoring Hispanics has been reported across tumor types and across other chronic diseases and is known as the Hispanic paradox. This term refers to the epidemiological finding that Hispanics in the U.S. tend to have substantially better health outcomes than the average population in spite of what their aggregate socioeconomic indicators would predict7, 8, 9, 10. In the current study, using a homogenous population of clinical trial participants, after controlling for important clinical factors, we demonstrate similar survival outcomes among Hispanics compared to non-Hispanics. Importantly, the patients included in our cohort were treated with similar protocols and were followed prospectively in a uniform fashion. Our findings suggest that current therapies provide similar benefits regardless of Hispanic ethnicity. This finding is important, because cancer among Hispanics accounted for 126,000 new cancer cases in 20156. Moreover, an increasing number of cancer cases among Hispanics is expected in the future since Hispanics in the U.S. are younger on average and cancers are more common in older patients5, 11.
The only group of patients where we observed a detriment in outcomes was the advanced prostate cancer group, although this difference was not statistically significant under multiple testing. Also of interest is the absence of any pattern of statistically significant associations for patients with advanced hormone refractory prostate cancer. Of note, this patient group only included registrations beginning the year 1999, whereas patients with advanced prostate cancer we enrolled from 1989 through 2008. Although we found no general pattern of temporal trends in the association of ethnicity and outcomes, temporal trends could be influential in this disease setting. Differential outcomes according to race/ethnicity have been reported in prostate cancer patients, it has been described that AA patients have mortality rate double that of white men,12, 13;14. In a similar analysis to the one presented here, Albain et al. reported that, after adjusting for important covariates, AA prostate cancer patients despite being treated similarly had a 19% increased risk of dying compared to whites3.
Relatively few studies have reported the mortality rates among Hispanic prostate cancer patients. A recent study using the SEER-Medicare database reported similar all-cause and prostate-specific mortality rates for Hispanics when compared with non-Hispanic whites15. In the models before adjusting for multiple comparisons, we observed that among patients with advanced prostate cancer, Hispanics had an increased risk of dying. It is possible that comorbidities, obesity or differences in the volume of the disease could have had an effect otcome.6, 12, 16,17, 18. While the implications of worse outcomes among Hispanic patients with prostate cancer could be relevant considering that prostate cancer is the most frequent cancer in Hispanic males (22% of the cases.); we must emphasize that after adjusting for multiple comparisons this difference was no longer of statistical significance.
In our sensitivity analysis excluding SES-related variables, Hispanics with ER and/or PR-positive early stage breast cancer had significantly worse PFS compared to non-Hispanics. While the benefit of adjuvant endocrine therapy among these patients is unquestionable, the therapy is prolonged and costly and this could have a differential effect among Hispanics; known to have overall lower income and educational levels compared to whites. Early discontinuation of endocrine therapy is a frequent problem19, 20, in the largest study to date evaluating adherence in this group of patients, a 32% rate of early discontinuation was observed21. Early discontinuation and non-adherence have been associated with worse outcomes and patients of ethnic minorities and disadvantaged income and educational levels are particularly vulnerable22, 23. Unfortunately in our analysis we are not able to take into account adherence to adjuvant endocrine therapies.
Despite the strengths of our unique analysis, we acknowledge that the cohort of patients may not represent the characteristics of the overall Hispanic population. Just 5% of patients overall were Hispanic, which is actually greater than the proportion of Hispanics in the population for the age distribution represented by clinical trial patients29. Nonetheless this low rate presents potential issues with respect to both selection bias and statistical power. It is well known that clinical trial participants tend to be younger, healthier, have fewer comorbidities and belong to higher SES and educational levels compared with non-participants24,25,26. Different studies have characterized clinical trial participants, with some suggesting that ethnic minorities are underrepresented3, 27. In addition, it is clear that clinical factors such as performance status, number of comorbid conditions and cancer stage also determine clinical trial participation28. We cannot exclude the possibility that Hispanics participating in SWOG clinical trials have biological and socio-demographic characteristics different from the general Hispanic population. However, although these factors likely influence overall estimates of survival outcomes, they are less likely to influence relative differences by ethnicity. Finally, the analysis of cancer-specific survival was limited by the absence of specific cause-of-death information for many patients. Despite these limitations, our study demonstrates for the first time that, when treated homogenously, Hispanics have similar outcomes compared to non-Hispanics. Efforts should continue to promote and engage ethnic minorities to participate in clinical trials. Only by including large and representative samples of patients in clinical trials will we be able to ensure generalizability of the obtained results to the entire population.
In summary, in this study we report for the first time in a large cohort of clinical trial participants outcomes according to Hispanic ethnicity. After adjusting for multiple comparisons, no notable differences in survival outcomes by ethnicity were evident, demonstrating that Hispanic ethnicity was not a predictor of survival among SWOG clinical trial participants.
Supplementary Material
CONDENSED ABSTRACT.
In this large study evaluating outcomes among cancer patients participating in phase II/III SWOG clinical trials (n=29,338) and receiving uniform treatment and follow-up, we observed similar survival outcomes between Hispanic and non-Hispanic patients.
Acknowledgments
FUNDING
Research reported in this study was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number UG1CA189974; NCORP Cancer Care Delivery Award and The Hope Foundation. Mariana Chavez-MacGregor is supported by the Cancer Prevention and Research Institute of Texas (CIPRIT) grant RP140020-P2 and by Susan G. Komen Breast Cancer Foundation grant SAC150061. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other supporters. We thank the many SWOG investigators who conducted the trials and provided the data used in this analysis.
Footnotes
Author contributions
Conceptualization: MCM, DLH
Methodology: MCM, JMU, AM, SR, DHL
Software: JMU, AM
Validation: JMU, AM
Formal analysis: JMU, AM
Investigation: MCM, JMU, AM, SR, DHL
Resources: MCM, DLH
Data curation: MCM, JMU, AM, SR, DHL
Writing-original: MCM
Writing-review and editing: MCM, JMU, AM, SR, DHL
Visualization: MCM, JMU, AM
Supervision: MCM, JMU, DLH
Projection administration: MCM, DHL
Funding acquisition: MCM
CONFLICT OF INTEREST
None of the authors have any potential conflicts of interest
References
- 1.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bureau UC. 2011–2013 ACS 3-year estimates. 2015. [Google Scholar]
- 5.Colby SL, O J. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. US Census Bureau; 2015. [Google Scholar]
- 6.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65:457–480. doi: 10.3322/caac.21314. [DOI] [PubMed] [Google Scholar]
- 7.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11:496–518. [PubMed] [Google Scholar]
- 8.Ho A, Shih M, Simon P. Hispanic paradox. Am J Public Health. 2007;97:392. doi: 10.2105/AJPH.2006.105320. author reply 392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 2):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 10.Smith DP, Bradshaw BS. Rethinking the Hispanic paradox: death rates and life expectancy for US non-Hispanic White and Hispanic populations. Am J Public Health. 2006;96:1686–1692. doi: 10.2105/AJPH.2003.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ennis SR, R-V M, Albert NG. The Hispanic Population: 2010: 2010 US Census Briefs. US Census Bureau; 2011. [Google Scholar]
- 12.Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106:1276–1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 13.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 14.Marlow NM, Halpern MT, Pavluck AL, Ward EM, Chen AY. Disparities associated with advanced prostate cancer stage at diagnosis. J Health Care Poor Underserved. 2010;21:112–131. doi: 10.1353/hpu.0.0253. [DOI] [PubMed] [Google Scholar]
- 15.Chhatre S, Bruce Malkowicz S, Sanford Schwartz J, Jayadevappa R. Understanding the Racial and Ethnic Differences in Cost and Mortality Among Advanced Stage Prostate Cancer Patients (STROBE) Medicine (Baltimore) 2015;94:e1353. doi: 10.1097/MD.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He T, Mullins CD. Age-related racial disparities in prostate cancer patients: A systematic review. Ethn Health. 2016:1–12. doi: 10.1080/13557858.2016.1235682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23:1665–1671. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 18.Laurent V, Guerard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 21.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 26.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncol. 2015:1–3. doi: 10.1001/jamaoncol.2015.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 28.Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106:2452–2458. doi: 10.1002/cncr.21907. [DOI] [PubMed] [Google Scholar]
- 29.Unger JM, Coltman CA, Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24:141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.