Abstract
We report the use of swept-source microscope-integrated optical coherence tomography (SS-MIOCT), capable of live 4D (3D across time) intraoperative imaging, to directly visualize suture depth during lateral rectus resection. Key surgical steps visualized in this report included needle depth during partial and full thickness muscle passes along with scleral passes.
Introduction
Suturing steps during strabismus surgery require an accurate assessment of needle depth in tissue, which can be challenging from a standard, top-down view of the surgical field. If the needle is passed too superficially during muscle or scleral passes, the risk of postoperative muscle slip or detachment increases. Conversely, if the needle is passed too deeply, then the risk of scleral perforation increases. Rates of scleral perforation have been reported in 2.8% of strabismus surgeries, which may be an underestimation since many patients are evaluated for this complication only if the surgeon subjectively senses that the needle has advanced too deep.1,2 Scleral perforations have been associated with serious ocular sequelae, including retinal detachment and endophthalmitis.3
To avoid scleral perforation, surgeons generally aim for a sufficient but safe needle depth of approximately 25% of the scleral thickness.4 Achieving this suture depth is largely subjective and dependent on inferred visual cues, such as a disappearance of the needle into the sclera. We describe a case in which swept-source microscope-integrated optical coherence tomography (SS-MIOCT) was used to directly visualize and evaluate key suturing-related steps of strabismus surgery in real-time.
Methods
Informed consent to use SS-MIOCT was obtained from the patient under a Duke University Health System Institutional Review Board approved protocol. Instead of surgical loupes, a 4D (3D across time) capable SS-MIOCT system, consisting of a portable cart with a customized scanner integrated into the optical path of a Leica M844 ophthalmic microscope (Leica Microsystem, Buffalo IL), was used (see Supplemental Figure 1, showing SS-MIOCT layout). The 4D SS-MIOCT images were acquired, processed, rendered, and displayed in real-time on a monitor to allow an MIOCT technician to help verbally guide the surgeon’s needle to the target suture depth.5 This research SS-MIOCT system, developed at Duke, has been described previously.6 Briefly, the SS-MIOCT system used a source with λ0=1040nm, A-scan rate=100kHz, A/B-scan ratio=500, volume rate=2Hz, resolution=14×14×7.8μm [x,y,z], imaging range=12×12×7.4mm [x,y,z], and sensitivity=102dB.
Results
Case Report
An 82-year-old man presented to Duke Eye Center with a complaint of diplopia at distance despite 14 prism diopters (PD) of base-out prism ground into his spectacles. Previous treatments included bilateral small medial rectus recessions, re-recession of the left medial rectus, and several botulinum toxin injections, which the patient reported were no longer effective. Visual acuity with spectacles was 20/20 and 20/25 at distance in the right and left eyes, respectively, and 20/20 at near for both eyes. Esodeviation without spectacles measured 18 and 8PD at distance and near, respectively, with a small intermittent right hypertropia. With prism spectacles, he had an intermittent esotropia of 4PD at distance, an exophoria of 7PD at near, 40 seconds arc stereopsis, and homonymous diplopia on Worth 4-dot testing at distance. Ocular rotations were without limitation. He desired surgical intervention and was consented for left lateral rectus resection.
Following IV sedation and retrobulbar block, a limbal traction suture was used to adduct the eye. A conjunctival limbal peritomy was made, and Tenon’s capsule was divided above and below the muscle. A muscle hook was passed under the entire muscle insertion, rendering the muscle clearly visible, free from attachments anteriorly, and under gentle traction. Check ligaments were severed. A point 6mm posterior to the muscle insertion was marked with cautery. Next, a 6-0 double-armed polyglactin suture on a spatulated S-14 needle (Ethicon Inc., Somerville, NJ, USA) was interwoven through the muscle with partial and full thickness bites on both poles of the muscle. Each suture pass was visualized in real-time by SS-MIOCT (Figures 1 and 2) to ensure partial and full thickness depths in the rectus muscle. Sutures were then secured with double-locking surgeon’s knots. The muscle was clamped, cauterized, and transected just anterior to the suture line. Excess muscle was removed, and the muscle was reattached to the globe via partial thickness scleral passes at the original muscle insertion site using the previously placed 6-0 polyglactin sutures. These scleral passes were also visualized in real-time by SS-MIOCT (Figure 3), allowing for active forward guidance of the suture needle to an appropriate scleral depth. The conjunctiva was reapproximated.
FIGURE 1. Live visualization of partial thickness suture placement into rectus muscle as seen with swept-source microscope-integrated optical coherence tomography (SS-MIOCT).
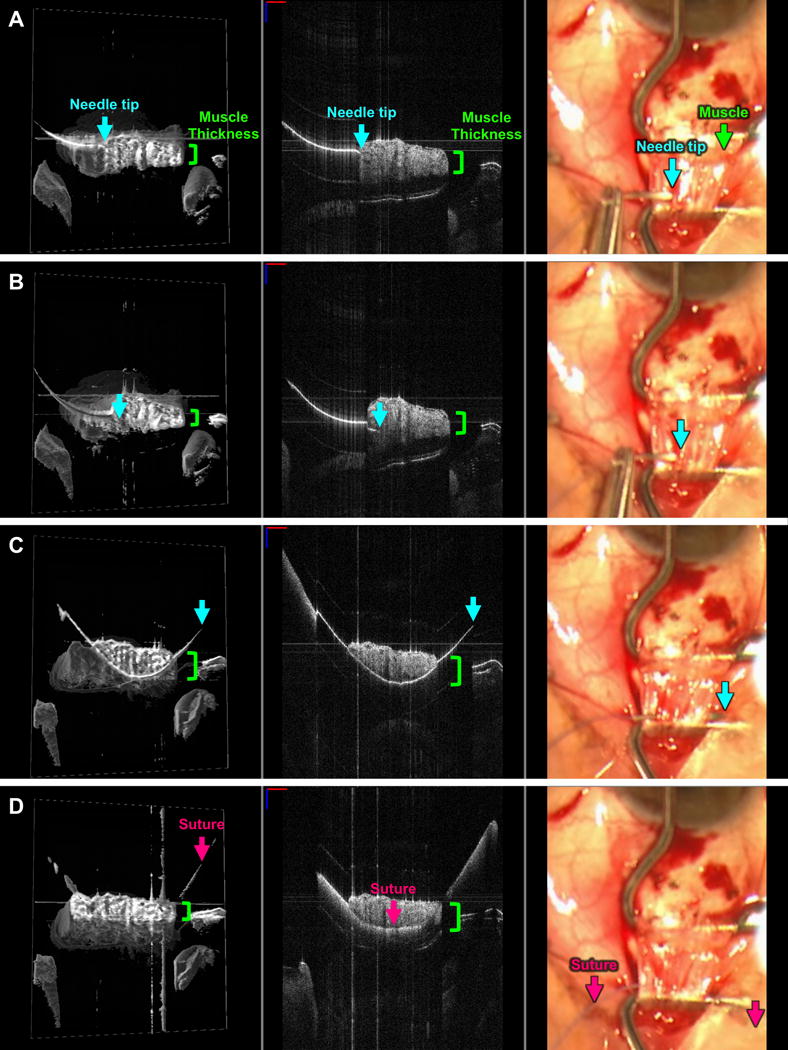
SS-MIOCT 3D volume (left) with a white box demarcating its corresponding 2D B-scan (middle) and the standard surgical microscope view (right) showing the needle tip (blue arrow) at the surface interface of the lateral rectus muscle (A), within – but not beyond – the thickness (green bracket) of the muscle (B), and exiting the muscle (C) with the lowest point of the needle remaining within the thickness of the muscle. The suture (pink arrow) does not extend beyond the thickness of the muscle (D). The depth of the needle and suture relative to the muscle is much more easily visualized in the 3D volumes and 2D B-scans compared to the standard surgical microscope views (A-D). The suture needle can artifactually appear discontinuous due to different refractive indices of air and tissue. In all SS-MIOCT images, the red scale bar in the 2D B-scan measures 1 mm laterally and the blue scale bar measures 1 mm axially.
FIGURE 2. Live visualization of a full thickness pass through one pole of the rectus muscle (locking bite) as seen with swept-source microscope-integrated optical coherence tomography (SS-MIOCT).
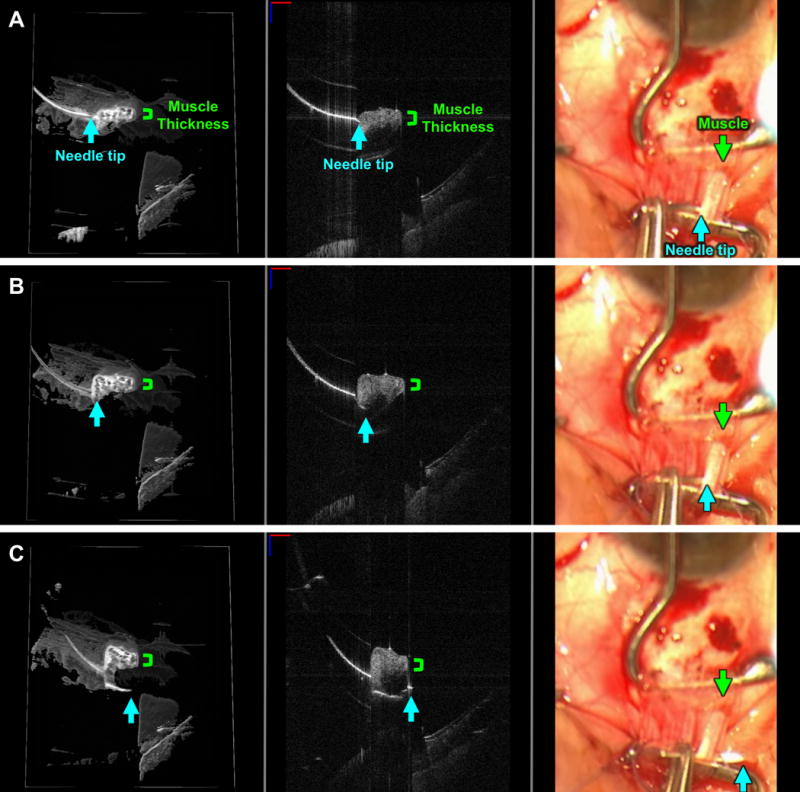
SS-MIOCT 3D volume (left) with a white box demarcating its corresponding 2D B-scan (middle) and the standard surgical microscope view (right) showing the needle tip (blue arrow) at the surface interface of the lateral rectus muscle (A), progressing beyond the thickness (green bracket) of the muscle (B), and exiting on the other side of muscle (C). The depth of the needle relative to the muscle is much more easily visualized in the 3D volumes and 2D B-scans compared to the standard surgical microscope views (A-C). The suture needle can artifactually appear discontinuous due to different refractive indices of air and tissue. In all SS-MIOCT images, the red scale bar in the 2D B-scan measures 1 mm laterally and the blue scale bar measures 1 mm axially.
FIGURE 3. Live visualization of a scleral pass as seen with swept-source microscope-integrated optical coherence tomography (SS-MIOCT).
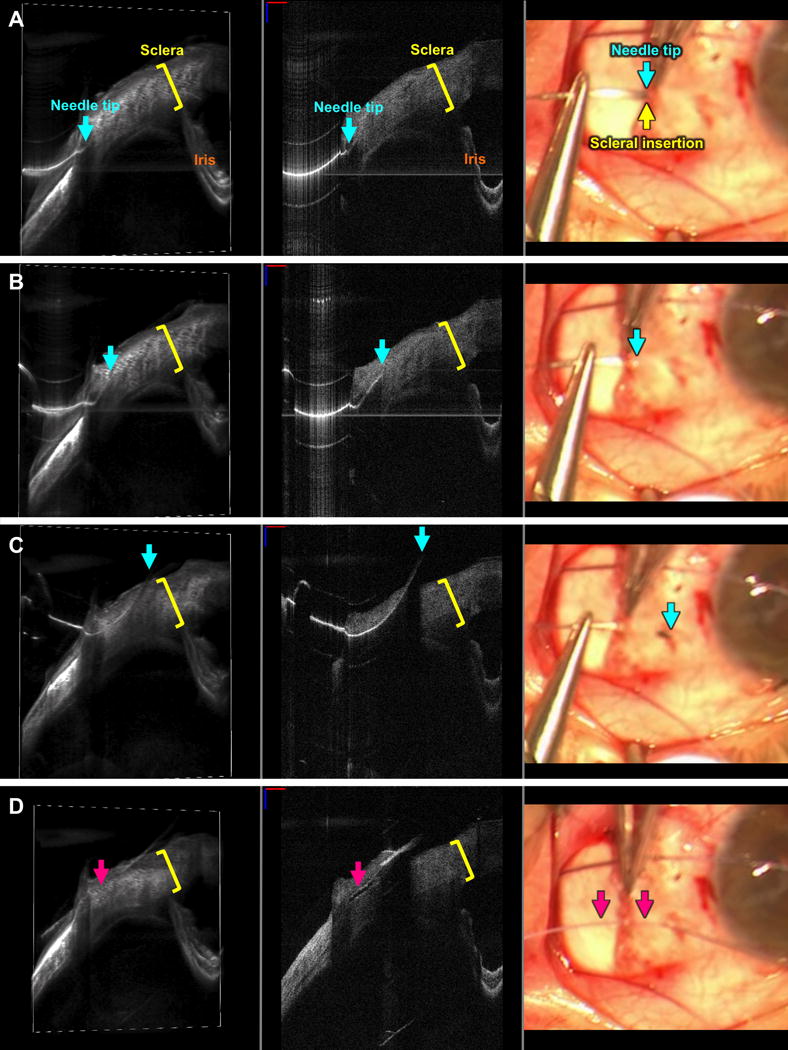
SS-MIOCT 3D volume (left) with a white box demarcating its corresponding 2D B-scan (middle) and the standard surgical microscope view (right) showing the needle tip (blue arrow) at the muscle insertion site (green arrow) at the surface interface with the sclera (A), within the thickness (yellow bracket) of the sclera (B), and exiting the sclera (C) with the lowest point of the needle and the suture (D) roughly 40% deep within the thickness of the sclera. The depth of the needle and suture relative to the sclera is much more easily visualized in the 3D volumes and 2D B-scans compared to the standard surgical microscope views (A-D). The suture needle can artifactually appear discontinuous due to different refractive indices of air and tissue. In all SS-MIOCT images, the red scale bar in the 2D B-scan measures 1 mm laterally and the blue scale bar measures 1 mm axially.
The patient reported doing very well and was happy with results at his postoperative month four visit. With updated spectacles including prism only for his vertical phoria, he was orthophoric at distance, with a near exophoria of 2PD.
Discussion
Assessing needle depth during strabismus surgery presents a challenge, as the needle tip cannot easily be visualized through muscle or sclera in the standard surgical field view. Using SS-MIOCT, we visualized the precise depth of the needle in both of these structures and provided real-time guidance and feedback to the surgeon. This enhanced visualization of partial and full thickness muscle and scleral passes using SS-MIOCT can ensure proper anchoring and locking of muscle passes and allow for strong but sufficiently superficial scleral passes. Use of this technology could help significantly decrease postoperative complications such as muscle slip or detachment along with infection and visual loss associated with inadvertent scleral perforations. Recent studies have used OCT to detect rectus muscle insertions, but our report is, to our knowledge, the first to demonstrate intraoperative OCT for strabismus surgery.7,8
One advantage of the surgical microscope over surgical loupes is the ability to integrate adjunctive visualization technologies such as SS-MIOCT; however, we acknowledge that strabismus surgeons typically use surgical loupes. While loupes provide a wider field of view and more maneuverability than the surgical microscope, SS-MIOCT guidance may be of particular value in eyes at increased risk for scleral perforation such as those with high myopia, previous strabismus surgeries, thin sclera, or recession versus resection surgery.1,9,10 Other nuances to recognize with SS-MIOCT include needle discontinuities due to passage through different refractive indices (such as air to muscle), potentially challenging visualization when the eye is rotated away from the microscope (such as large recessions), and a separate SS-MIOCT display in the current implementation. The separate display can be addressed by continued advances in integrated heads-up display technology that projects the SS-MIOCT data into the surgical microscope oculars.11
The attending surgeon had over 20 years of experience assessing needle depth subjectively; however, this may be more challenging for novice surgeons. One study reported 100% of scleral perforations occurred by residents and fellows versus 0% by attending surgeons.12 By providing direct visualization of the needle depth, SS-MIOCT could help improve speed and confidence with these maneuvers and potentially decrease rates of complications for both experienced and novice surgeons.
Supplementary Material
SUPPLEMENTAL FIGURE 1. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) layout.
VIDEO. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) 3D volumes (left) with white boxes demarcating their corresponding 2D B-scans (right) during a scleral pass. This is followed by 4D (3D across time) SS-MIOCT visualization of partial thickness suturing into rectus muscle.
Footnotes
Author Conflicts of Interest: At the time of this work, Dr. Izatt was Chairman and Chief Scientific Advisor for Bioptigen Inc., and had corporate, equity, and intellectual property interests (including royalties) in this company. Dr. Toth receives financial support from Alcon, Bioptigen, and Genentech and has an intraoperative imaging patent with Duke University. Dr. Kuo has an imaging algorithm patent licensed by Duke to Bioptigen, which is unrelated to the present work. For the remaining authors no conflicts were declared.
National meetings where material was presented: Association for Research in Vision and Ophthalmology Annual Meeting on Sunday, May 1 2016 in Seattle, WA.
Institution at which the study was conducted: Duke University Eye Center
References
- 1.Dang Y, Racu C, Isenberg SJ. Scleral penetrations and perforations in strabismus surgery and associated risk factors. J AAPOS. 2004;8:325–31. doi: 10.1016/j.jaapos.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Olitsky SE, Coats DK. Complications of Strabismus Surgery. Middle East Afr J Ophthalmol. 2015;22:271–8. doi: 10.4103/0974-9233.159692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salamon SM, Friberg TR, Luxenberg MN. Endophthalmitis after strabismus surgery. Am J Ophthalmol. 1982;93:39–41. doi: 10.1016/0002-9394(82)90696-1. [DOI] [PubMed] [Google Scholar]
- 4.Buckley EG. Atlas of Ophthalmic Surgery. St. Louis: Mosby; 1995. [Google Scholar]
- 5.Viehland C, Keller B, Carrasco-Zevallos OM, et al. Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT. Biomed Opt Express. 2016;7:1815–29. doi: 10.1364/BOE.7.001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn P, Migacz J, O’Donnell R, et al. Preclinical evaluation and intraoperative human retinal imaging with a high-resolution microscope-integrated spectral domain optical coherence tomography device. Retina. 2013;33:1328–37. doi: 10.1097/IAE.0b013e3182831293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo CS, Smith D, Kraft SP. The accuracy of anterior segment optical coherence tomography (AS-OCT) in localizing extraocular rectus muscles insertions. J AAPOS. 2015;19:233–6. doi: 10.1016/j.jaapos.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Pihlblad MS, Erenler F, Sharma A, Manchandia A, Reynolds JD. Anterior Segment Optical Coherence Tomography of the Horizontal and Vertical Extraocular Muscles With Measurement of the Insertion to Limbus Distance. J Pediatr Ophthalmol Strabismus. 2016;53:141–5. doi: 10.3928/01913913-20160405-05. [DOI] [PubMed] [Google Scholar]
- 9.Awad AH, Mullaney PB, Al-Hazmi A, et al. Recognized globe perforation during strabismus surgery: incidence, risk factors, and sequelae. J AAPOS. 2000;4:150–3. [PubMed] [Google Scholar]
- 10.Haugen OH, Kjeka O. Localized, extreme scleral thinning causing globe rupture during strabismus surgery. J AAPOS. 2005;9:595–6. doi: 10.1016/j.jaapos.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Carrasco-Zevallos O, Keller B, et al. Novel microscope-integrated stereoscopic heads-up display for intrasurgical optical coherence tomography. Biomed Opt Express. 2016;7:1711–26. doi: 10.1364/BOE.7.001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surachatkumtonekul T, Phamonvaechavan P, Kumpanardsanyakorn S, Wongpitoonpiya N, Nimmannit A. Scleral penetrations and perforations in strabismus surgery: incidence, risk factors and sequelae. J Med Assoc Thai. 2009;92:1463–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) layout.
VIDEO. Swept-source microscope-integrated optical coherence tomography (SS-MIOCT) 3D volumes (left) with white boxes demarcating their corresponding 2D B-scans (right) during a scleral pass. This is followed by 4D (3D across time) SS-MIOCT visualization of partial thickness suturing into rectus muscle.


